Cross-Kingdom Regulation of Plant-Derived miRNAs in Modulating Insect Development
Abstract
:1. Introduction
| miRNA | Host Plant | Target Organisms and Function | Reference |
|---|---|---|---|
| miR172 | Brassica oleracea | The stomach, intestine, serum feces, blood, spleen, liver, and kidney of mouse | [13] |
| miR168a | Rice | Human/mouse low-density lipoprotein receptor adapter protein 1 | [17] |
| miR159 | Arabidopsis thaliana Glycine max | Human TCF7 that encodes a Wnt signaling transcription factor | [18] |
| miR5338 | Rape | Mfn1 in the mouse prostate | [19] |
| miR7267-3p | Ginger | The Lactobacillus rhamnosus monooxygenase ycnE of human/mouse | [24] |
| miR396a-5p | Ginger | Inhibition of expression of Nsp12 in mouse | [25] |
| miR206 | Sedr | Involved in hippo signaling pathway-fly, Wnt signaling pathway, and N-Glycan biosynthesis of honeybee | [26] |
| miR159a | Arabidopsis thaliana | Target the basic juvenile hormone-suppressible protein 1 gene of Plutella xylostella | [27] |
| agomir-7703-5p | Arabidopsis thaliana | Inhibition of the expression of phenoloxidase subunit 2 gene of Plutella xylostella | [27] |
| sbi-miR1-3p, sbi-miR2-3p, sbi-miR3-5p, sbi-miR5-5p, sbi-miR166-3p, sbi-miR390-5p, sbi-miR396-5p, sbi-miR2927-5p, sbi-miR6230-3p, sbi-miR6230-3p, sbi-5163-3p, hvu-miR3-3p, hvu-miR2-5p | Sorghum or barley | involved in detoxification of Schizaphis graminum and Sipha flava, such as metabolism of xenobiotics by P450s | [28] |
| miR162a | Cole (Brassica campestris) flower | Suppressing endogenous mTOR expression of Apis mellifera and Drosophila | [29] |
| Csu-novel-260 | Insect-resistant genetically engineered rice | Suppressing the expression of the disembodied gene expression of Chilo suppressalis | [30] |
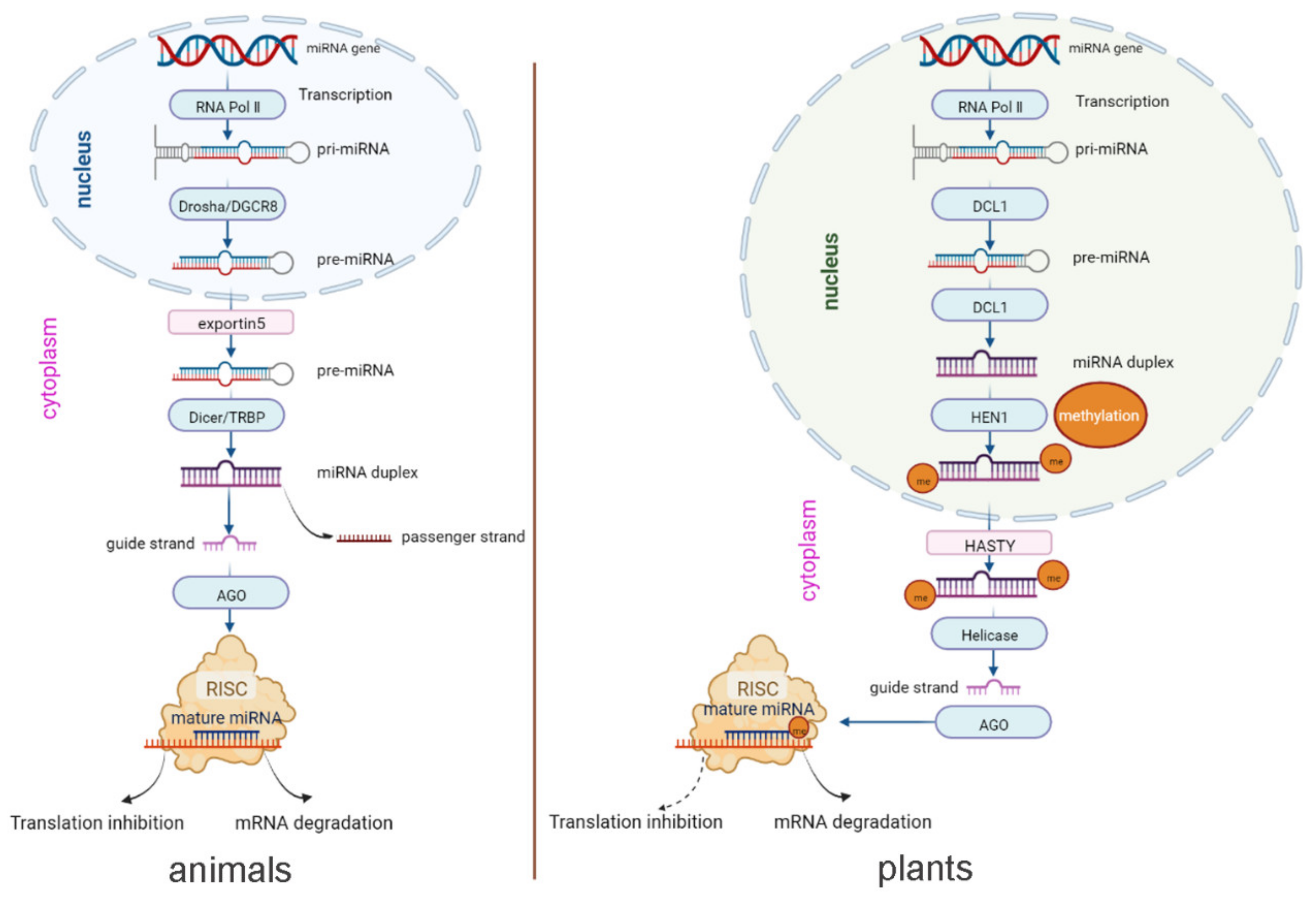
2. Comparison of miRNA Biogenesis and Mechanisms in Plants and Insects
3. Coevolution between Insects and Plants
4. The Coevolution of miRNAs and miRNA Targets
5. Regulation of Insect Development by Plant-Derived miRNAs
6. Insect-Derived miRNAs Targeting Plant Defense Response
7. The Cross-Kingdom Transport Mechanism of Plant-Derived miRNAs
8. Controversy Regarding the Modulation of Insect Development by Plant-Derived miRNAs
9. Conclusions and Future Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kataria, P.; Surela, N.; Chaudhary, A.; Das, J. MiRNA: Biological Regulator in Host-Parasite Interaction during Malaria Infection. Int. J. Environ. Res. Public Health 2022, 19, 2395. [Google Scholar] [CrossRef]
- Wang, J.; Mei, J.; Ren, G. Plant microRNAs: Biogenesis, Homeostasis, and Degradation. Front. Plant Sci. 2019, 10, 360. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.; Yang, Y.; Liu, J.; Li, H.; Li, R.; Cao, C.; Shi, L.; Wu, W.; He, K. A Timely Review of Cross-Kingdom Regulation of Plant-Derived MicroRNAs. Front. Genet. 2021, 12, 613197. [Google Scholar] [CrossRef]
- Li, M.; Yu, B. Recent advances in the regulation of plant miRNA biogenesis. RNA Biol. 2021, 18, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A. The lin-4 microRNA: The ultimate micromanager. Cell Cycle 2014, 13, 1060–1061. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhou, S. Post-transcriptional regulation of insect metamorphosis and oogenesis. Cell. Mol. Life Sci. 2020, 77, 1893–1909. [Google Scholar] [CrossRef]
- Carthew, R.W.; Agbu, P.; Giri, R. MicroRNA function in Drosophila melanogaster. Semin. Cell Dev. Biol. 2017, 65, 29–37. [Google Scholar] [CrossRef]
- Ashby, R.; Forêt, S.; Searle, I.; Maleszka, R. MicroRNAs in Honey Bee Caste Determination. Sci. Rep. 2016, 6, 18794. [Google Scholar] [CrossRef]
- Zhang, Q.; Dou, W.; Taning, C.N.T.; Smagghe, G.; Wang, J.J. Regulatory roles of microRNAs in insect pests: Prospective targets for insect pest control. Curr. Opin. Biotechnol. 2021, 70, 158–166. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, Y.; Sun, B.; Shao, Y.; Jing, A.; Wang, J.; Xiao, Z. Assessing the survival of exogenous plant microRNA in mice. Food Sci. Nutr. 2014, 2, 380–388. [Google Scholar] [CrossRef]
- Zhu, W.J.; Liu, Y.; Cao, Y.N.; Peng, L.X.; Yan, Z.Y.; Zhao, G. Insights into Health-Promoting Effects of Plant MicroRNAs: A Review. J. Agric. Food Chem. 2021, 69, 14372–14386. [Google Scholar] [CrossRef]
- Majumdar, R.; Galewski, P.J.; Eujayl, I.; Minocha, R.; Vincill, E.; Strausbaugh, C.A. Regulatory Roles of Small Non-coding RNAs in Sugar Beet Resistance against Beet Curly Top Virus. Front. Plant Sci. 2021, 12, 780877. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, F.; Dong, L.; Wu, H.; Xu, J.; Li, H.; Wang, J.; Zhou, Z.; Liu, C.; Wang, Y.; et al. SIDT1-dependent absorption in the stomach mediates host uptake of dietary and orally administered microRNAs. Cell Res. 2021, 31, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.R.; Fong, M.Y.; Somlo, G.; Wu, J.; Swiderski, P.; Wu, X.; Wang, S.E. Cross-kingdom inhibition of breast cancer growth by plant miR159. Cell Res. 2016, 26, 217–228. [Google Scholar] [CrossRef]
- Chen, X.; Wu, R.Z.; Zhu, Y.Q.; Ren, Z.M.; Tong, Y.L.; Yang, F.; Dai, G.H. Study on the inhibition of Mfn1 by plant-derived miR5338 mediating the treatment of BPH with rape bee pollen. BMC Complement. Altern. Med. 2018, 18, 38. [Google Scholar] [CrossRef]
- Zhang, Y.; Wiggins, B.E.; Lawrence, C.; Petrick, J.; Ivashuta, S.; Heck, G. Analysis of plant-derived miRNAs in animal small RNA datasets. BMC Genom. 2012, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Labandeira, C.C. A paleobiologic perspective on plant-insect interactions. Curr. Opin. Plant Biol. 2013, 16, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Sattar, S.; Addo-Quaye, C.; Song, Y.; Anstead, J.A.; Sunkar, R.; Thompson, G.A. Expression of small RNA in Aphis gossypii and its potential role in the resistance interaction with melon. PLoS ONE 2012, 7, e48579. [Google Scholar] [CrossRef]
- Li, C.; Wong, A.Y.P.; Wang, S.; Jia, Q.; Chuang, W.P.; Bendena, W.G.; Tobe, S.S.; Yang, S.H.; Chung, G.; Chan, T.F.; et al. miRNA-Mediated Interactions in and between Plants and Insects. Int. J. Mol. Sci. 2018, 19, 3239. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xu, F.; Zhang, X.; Mu, J.; Sayed, M.; Hu, X.; Lei, C.; Sriwastva, M.; Kumar, A.; Sundaram, K.; et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol. Ther. 2021, 29, 2424–2440. [Google Scholar] [CrossRef]
- Gharehdaghi, L.; Bakhtiarizadeh, M.R.; He, K.; Harkinezhad, T.; Tahmasbi, G.; Li, F. Diet-derived transmission of MicroRNAs from host plant into honey bee Midgut. BMC Genom. 2021, 22, 587. [Google Scholar] [CrossRef]
- Zhang, L.L.; Jing, X.D.; Chen, W.; Wang, Y.; Lin, J.H.; Zheng, L.; Dong, Y.H.; Zhou, L.; Li, F.F.; Yang, F.Y.; et al. Host Plant-Derived miRNAs Potentially Modulate the Development of a Cosmopolitan Insect Pest, Plutella xylostella. Biomolecules 2019, 9, 602. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Dou, Y.; Yu, B.; Liu, Y.; Heng-Moss, T.M.; Lu, G.; Wachholtz, M.; Bradshaw, J.D.; Twigg, P.; et al. Insect and plant-derived miRNAs in greenbug (Schizaphis graminum) and yellow sugarcane aphid (Sipha flava) revealed by deep sequencing. Gene 2017, 599, 68–77. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, M.; Fu, Z.; Zhou, Z.; Kong, Y.; Liang, H.; Lin, Z.; Luo, J.; Zheng, H.; Wan, P.; et al. Plant microRNAs in larval food regulate honeybee caste development. PLoS Genet. 2017, 13, e1006946. [Google Scholar] [CrossRef]
- Wen, N.; Chen, J.; Chen, G.; Du, L.; Chen, H.; Li, Y.; Peng, Y.; Yang, X.; Han, L. The overexpression of insect endogenous microRNA in transgenic rice inhibits the pupation of Chilo suppressalis and Cnaphalocrocis medinalis. Pest Manag. Sci. 2021, 77, 3990–3999. [Google Scholar] [CrossRef]
- Moran, Y.; Agron, M.; Praher, D.; Technau, U. The evolutionary origin of plant and animal microRNAs. Nat. Ecol. Evol. 2017, 1, 27. [Google Scholar] [CrossRef]
- Samad, A.F.A.; Kamaroddin, M.F.; Sajad, M. Cross-Kingdom Regulation by Plant microRNAs Provides Novel Insight into Gene Regulation. Adv. Nutr. 2021, 12, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.W.; Byers, K.J.; Bradshaw, H.D., Jr. The genetic control of flower-pollinator specificity. Curr. Opin. Plant Biol. 2013, 16, 422–428. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Raven, P.H. Butterflies and Plants: A Study in Coevolution. Evolution 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Beran, F.; Petschenka, G. Sequestration of Plant Defense Compounds by Insects: From Mechanisms to Insect-Plant Coevolution. Annu. Rev. Entomol. 2022, 67, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Marquis, R.J.; Salazar, D.; Baer, C.; Reinhardt, J.; Priest, G.; Barnett, K. Ode to Ehrlich and Raven or how herbivorous insects might drive plant speciation. Ecology 2016, 97, 2939–2951. [Google Scholar] [CrossRef]
- Anderson, J.T.; Mitchell-Olds, T. Ecological genetics and genomics of plant defenses: Evidence and approaches. Funct. Ecol. 2011, 25, 312–324. [Google Scholar] [CrossRef]
- de Castro, É.C.P.; Zagrobelny, M.; Cardoso, M.Z.; Bak, S. The arms race between heliconiine butterflies and Passiflora plants—New insights on an ancient subject. Biol. Rev. Camb. Philos. Soc. 2018, 93, 555–573. [Google Scholar] [CrossRef]
- Jacobsen, D.J.; Raguso, R.A. Lingering Effects of Herbivory and Plant Defenses on Pollinators. Curr. Biol. 2018, 28, R1164–R1169. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Yuan, J.; Zhao, Z.; Lu, J. MicroRNA duplication accelerates the recruitment of new targets during vertebrate evolution. RNA 2018, 24, 787–802. [Google Scholar] [CrossRef]
- Barbash, S.; Shifman, S.; Soreq, H. Global coevolution of human microRNAs and their target genes. Mol. Biol. Evol. 2014, 31, 1237–1247. [Google Scholar] [CrossRef]
- Felippes, F.F.; Schneeberger, K.; Dezulian, T.; Huson, D.H.; Weigel, D. Evolution of Arabidopsis thaliana microRNAs from random sequences. RNA 2008, 14, 2455–2459. [Google Scholar] [CrossRef]
- Hu, H.Y.; He, L.; Fominykh, K.; Yan, Z.; Guo, S.; Zhang, X.; Taylor, M.S.; Tang, L.; Li, J.; Liu, J.; et al. Evolution of the human-specific microRNA miR-941. Nat. Commun. 2012, 3, 1145. [Google Scholar] [CrossRef]
- Liu, T.; Fang, C.; Ma, Y.; Shen, Y.; Li, C.; Li, Q.; Wang, M.; Liu, S.; Zhang, J.; Zhou, Z.; et al. Global investigation of the co-evolution of MIRNA genes and microRNA targets during soybean domestication. Plant J. 2016, 85, 396–409. [Google Scholar] [CrossRef]
- Barik, S.; Kumar, A.; Sarkar Das, S.; Yadav, S.; Gautam, V.; Singh, A.; Singh, S.; Sarkar, A.K. Coevolution Pattern and Functional Conservation or Divergence of miR167s and Their Targets across Diverse Plant Species. Sci. Rep. 2015, 5, 14611. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Lv, X.; Xu, B.; Zhang, H.; Yan, J.; Li, H.; Wu, L. Evolution of an X-Linked miRNA Family Predominantly Expressed in Mammalian Male Germ Cells. Mol. Biol. Evol. 2019, 36, 663–678. [Google Scholar] [CrossRef]
- Weiberg, A.; Wang, M.; Lin, F.M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.D.; Jin, H. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef]
- Claycomb, J.; Abreu-Goodger, C.; Buck, A.H. RNA-mediated communication between helminths and their hosts: The missing links. RNA Biol. 2017, 14, 436–441. [Google Scholar] [CrossRef]
- Seidel, E.; Le, V.T.K.; Bar-On, Y.; Tsukerman, P.; Enk, J.; Yamin, R.; Stein, N.; Schmiedel, D.; Oiknine Djian, E.; Weisblum, Y.; et al. Dynamic Co-Evolution of Host and Pathogen: HCMV Downregulates the Prevalent Allele MICA∗008 to Escape Elimination by NK Cells. Cell Rep. 2015, 10, 968–982. [Google Scholar] [CrossRef]
- Alshehri, B. Plant-derived xenomiRs and cancer: Cross-kingdom gene regulation. Saudi J. Biol. Sci. 2021, 28, 2408–2422. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.S.K.; Law, S.T.S.; Li, C.; Qu, Z.; Bendena, W.G.; Tobe, S.S.; Hui, J.H.L. Diversity of Insect Sesquiterpenoid Regulation. Front. Genet. 2020, 11, 1027. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, W.; Zhao, H.; Gao, L.; Fan, Y.; Zhou, S. The microRNAs let-7 and miR-278 regulate insect metamorphosis and oogenesis by targeting the juvenile hormone early-response gene Krüppel-homolog 1. Development 2018, 145, dev170670. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guan, R.; Guo, H.; Miao, X. New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests. Plant Cell Environ. 2015, 38, 2277–2285. [Google Scholar] [CrossRef]
- Han, W.H.; Wang, J.X.; Zhang, F.B.; Liu, Y.X.; Wu, H.; Wang, X.W. Small RNA and Degradome Sequencing Reveal Important MicroRNA Function in Nicotiana tabacum Response to Bemisia tabaci. Genes 2022, 13, 361. [Google Scholar] [CrossRef]
- Gordon, K.H.; Waterhouse, P.M. RNAi for insect-proof plants. Nat. Biotechnol. 2007, 25, 1231–1232. [Google Scholar] [CrossRef]
- Weiberg, A.; Wang, M.; Bellinger, M.; Jin, H. Small RNAs: A new paradigm in plant-microbe interactions. Annu. Rev. Phytopathol. 2014, 52, 495–516. [Google Scholar] [CrossRef]
- Wang, M.; Thomas, N.; Jin, H. Cross-kingdom RNA trafficking and environmental RNAi for powerful innovative pre- and post-harvest plant protection. Curr. Opin. Plant Biol. 2017, 38, 133–141. [Google Scholar] [CrossRef]
- Ballaré, C.L. Jasmonate-induced defenses: A tale of intelligence, collaborators and rascals. Trends Plant Sci. 2011, 16, 249–257. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Bos, J.I. Effector proteins that modulate plant—Insect interactions. Curr. Opin. Plant Biol. 2011, 14, 422–428. [Google Scholar] [CrossRef]
- Pavlidi, N.; Vontas, J.; Van Leeuwen, T. The role of glutathione S-transferases (GSTs) in insecticide resistance in crop pests and disease vectors. Curr. Opin. Insect Sci. 2018, 27, 97–102. [Google Scholar] [CrossRef]
- Basu, S.; Varsani, S.; Louis, J. Altering Plant Defenses: Herbivore-Associated Molecular Patterns and Effector Arsenal of Chewing Herbivores. Mol. Plant Microbe Interact. 2018, 31, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Diezel, C.; von Dahl, C.C.; Gaquerel, E.; Baldwin, I.T. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol. 2009, 150, 1576–1586. [Google Scholar] [CrossRef]
- Malhotra, E.V.; Jain, R.; Tyagi, S.; Venkat Raman, K.; Bansal, S.; Pattanayak, D. Identification of dynamic microRNA associated with systemic defence against Helicoverpa armigera infestation in Cajanus scarabaeoides. Pest Manag. Sci. 2022, 78, 3144–3154. [Google Scholar] [CrossRef] [PubMed]
- Duxbury, M.S.; Ashley, S.W.; Whang, E.E. RNA interference: A mammalian SID-1 homologue enhances siRNA uptake and gene silencing efficacy in human cells. Biochem. Biophys. Res. Commun. 2005, 331, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Winston, W.M.; Sutherlin, M.; Wright, A.J.; Feinberg, E.H.; Hunter, C.P. Caenorhabditis elegans SID-2 is required for environmental RNA interference. Proc. Natl. Acad. Sci. USA 2007, 104, 10565–10570. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Ratajczak, J. Extracellular microvesicles/exosomes: Discovery, disbelief, acceptance, and the future? Leukemia 2020, 34, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Ivashuta, S.; Zhang, Y.; Wiggins, B.E.; Ramaseshadri, P.; Segers, G.C.; Johnson, S.; Meyer, S.E.; Kerstetter, R.A.; McNulty, B.C.; Bolognesi, R.; et al. Environmental RNAi in herbivorous insects. RNA 2015, 21, 840–850. [Google Scholar] [CrossRef]
- Terra, W.R. The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch. Insect Biochem. Physiol. 2001, 47, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Snow, J.W.; Hale, A.E.; Isaacs, S.K.; Baggish, A.L.; Chan, S.Y. Ineffective delivery of diet-derived microRNAs to recipient animal organisms. RNA Biol. 2013, 10, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, H.; Yang, X.; Chen, G.; Du, L.; Chen, H.; Li, Y.; Peng, Y.; Han, L. Consumption of miRNA-Mediated Insect-Resistant Transgenic Rice Pollen Does Not Harm Apis mellifera Adults. J. Agric. Food Chem. 2021, 69, 4234–4242. [Google Scholar] [CrossRef] [PubMed]
- Masood, M.; Everett, C.P.; Chan, S.Y.; Snow, J.W. Negligible uptake and transfer of diet-derived pollen microRNAs in adult honey bees. RNA Biol. 2016, 13, 109–118. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, D.; Xiang, Z.; He, N. Nonfunctional ingestion of plant miRNAs in silkworm revealed by digital droplet PCR and transcriptome analysis. Sci. Rep. 2015, 5, 12290. [Google Scholar] [CrossRef]
- Yu, B.; Yang, Z.; Li, J.; Minakhina, S.; Yang, M.; Padgett, R.W.; Steward, R.; Chen, X. Methylation as a crucial step in plant microRNA biogenesis. Science 2005, 307, 932–935. [Google Scholar] [CrossRef]
- Tosar, J.P.; Rovira, C.; Naya, H.; Cayota, A. Mining of public sequencing databases supports a non-dietary origin for putative foreign miRNAs: Underestimated effects of contamination in NGS. RNA 2014, 20, 754–757. [Google Scholar] [CrossRef]
- Mar-Aguilar, F.; Arreola-Triana, A.; Mata-Cardona, D.; Gonzalez-Villasana, V.; Rodríguez-Padilla, C.; Reséndez-Pérez, D. Evidence of transfer of miRNAs from the diet to the blood still inconclusive. PeerJ 2020, 8, e9567. [Google Scholar] [CrossRef]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef]
- Gogoi, A.; Sarmah, N.; Kaldis, A.; Perdikis, D.; Voloudakis, A. Plant insects and mites uptake double-stranded RNA upon its exogenous application on tomato leaves. Planta 2017, 246, 1233–1241. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V. Exogenous RNAs for Gene Regulation and Plant Resistance. Int. J. Mol. Sci. 2019, 20, 2282. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, X.; Wang, Z.; Wang, Y.; Liu, Z.; Wang, H.; Xu, B. Cross-Kingdom Regulation of Plant-Derived miRNAs in Modulating Insect Development. Int. J. Mol. Sci. 2023, 24, 7978. https://doi.org/10.3390/ijms24097978
Chi X, Wang Z, Wang Y, Liu Z, Wang H, Xu B. Cross-Kingdom Regulation of Plant-Derived miRNAs in Modulating Insect Development. International Journal of Molecular Sciences. 2023; 24(9):7978. https://doi.org/10.3390/ijms24097978
Chicago/Turabian StyleChi, Xuepeng, Zhe Wang, Ying Wang, Zhenguo Liu, Hongfang Wang, and Baohua Xu. 2023. "Cross-Kingdom Regulation of Plant-Derived miRNAs in Modulating Insect Development" International Journal of Molecular Sciences 24, no. 9: 7978. https://doi.org/10.3390/ijms24097978





