Endocrine Disruptor-Induced Bone Damage Due to Hormone Dysregulation: A Review
Abstract
:1. Introduction
2. Method
3. Osteogenesis
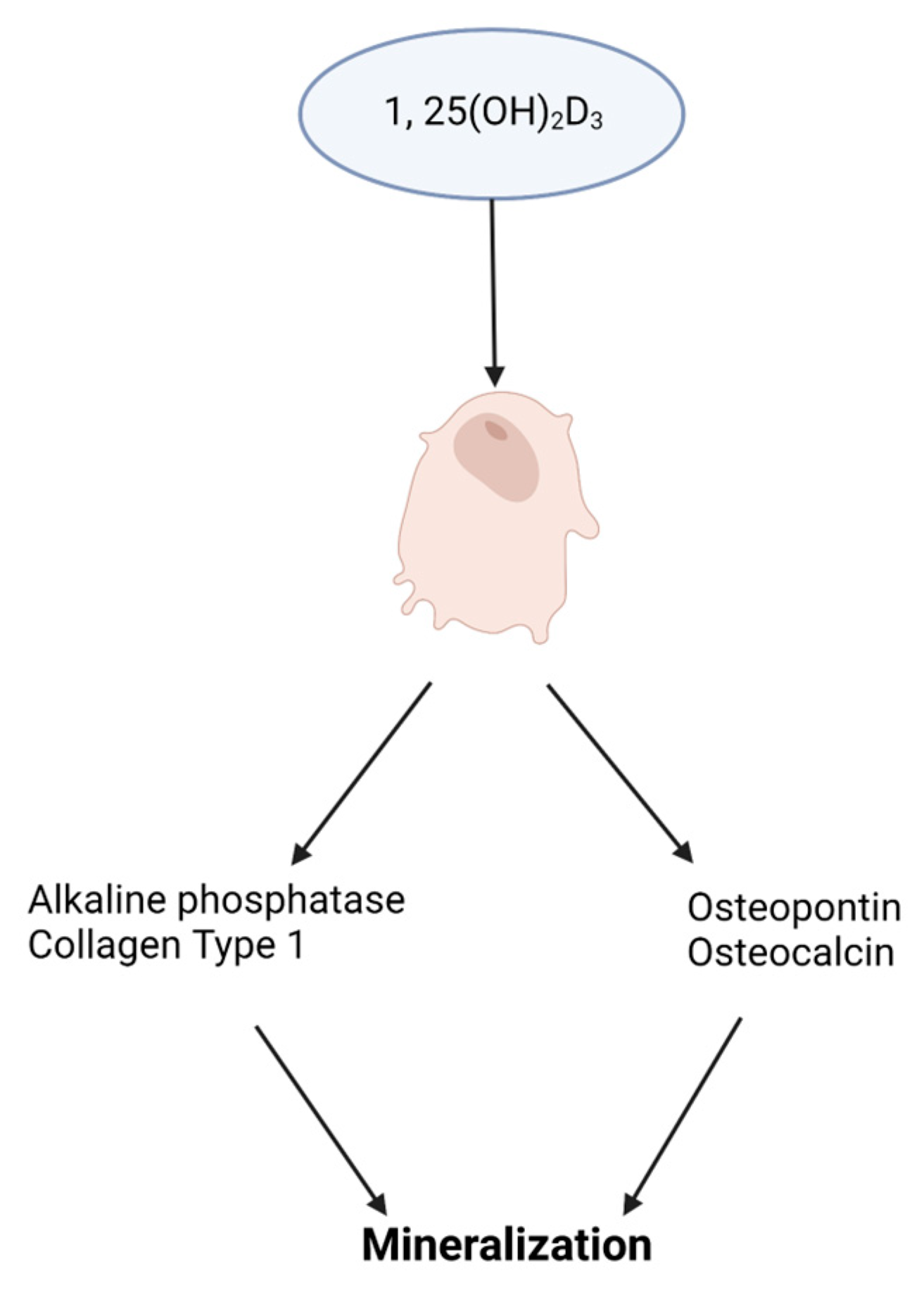
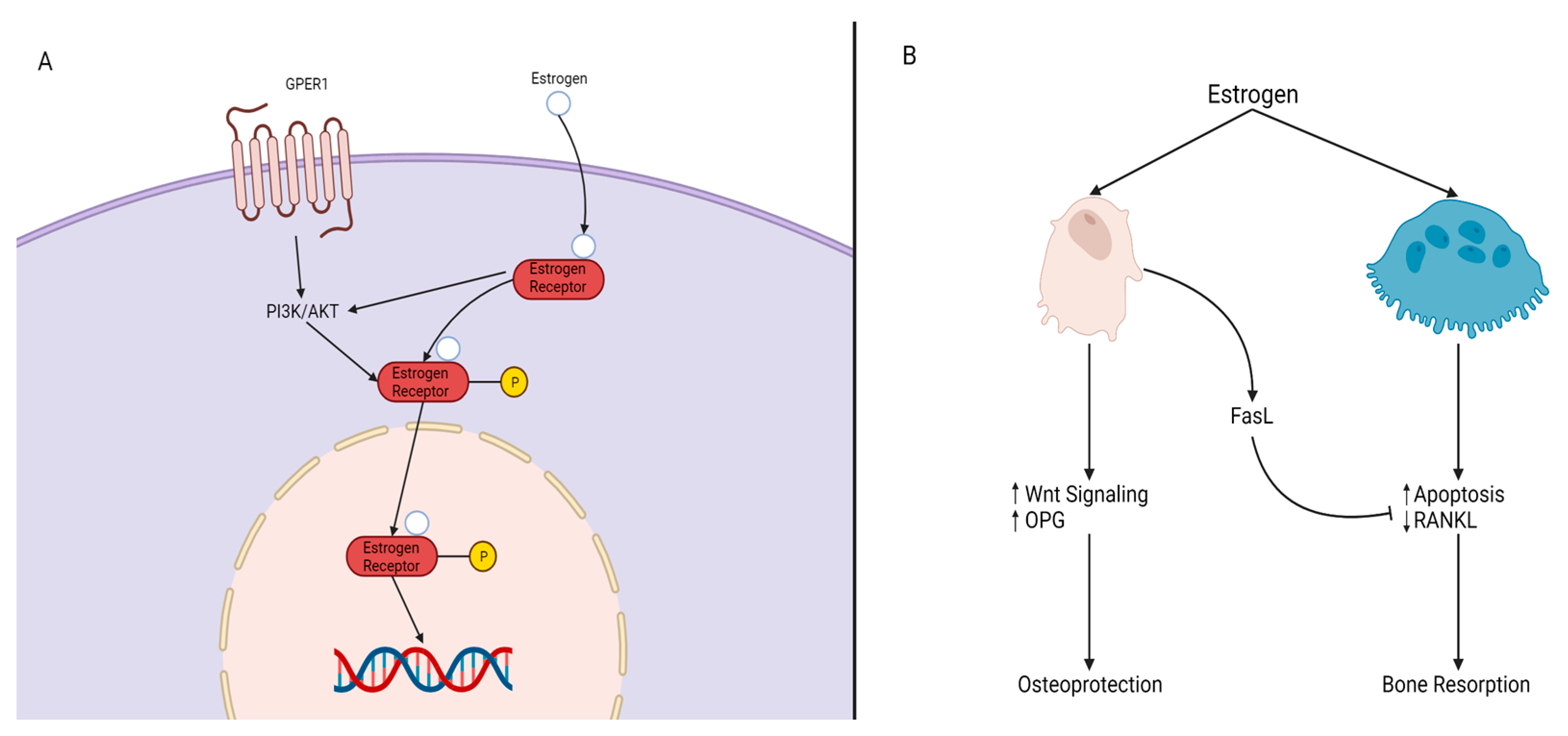
4. Osteogenesis and Its Hormone Regulation
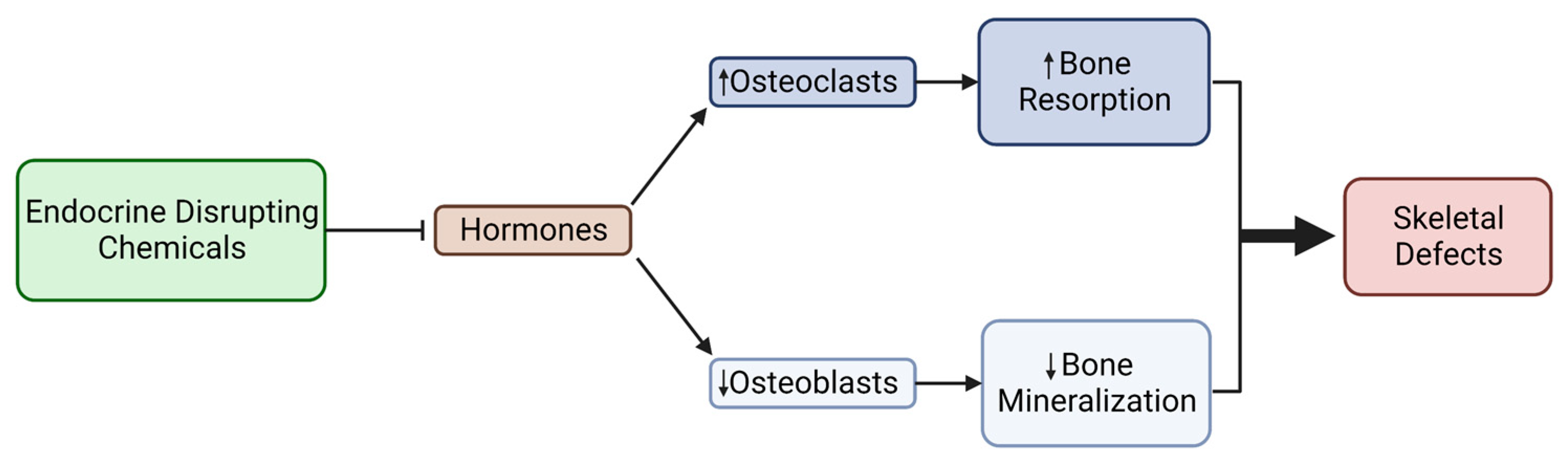
5. Mechanisms of Endocrine Disruption and Bone Damage
6. Conclusions
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Congenital Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/birth-defects (accessed on 26 April 2023).
- World Health Organization. Birth Defects Surveillance: A Manual for Programme Managers, 2nd ed.; World Health Organization: Geneva, Switzerland; Available online: https://www.who.int/publications-detail-redirect/9789240015395 (accessed on 31 March 2023).
- Källén, B. Genetic and Non-genetic Factors in the Origin of Congenital Malformations. In Epidemiology of Human Congenital Malformations; Springer: Cham, Switzerland, 2014; pp. 5–8. [Google Scholar] [CrossRef]
- Breeland, G.; Sinkler, M.A.; Menezes, R.G. Embryology, Bone Ossification. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539718/ (accessed on 31 March 2023).
- Sera, S.R.; Zur Nieden, N.I. microRNA Regulation of Skeletal Development. Curr. Osteoporos. Rep. 2018, 4, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Chen, H.; Ghori, F.Y. Genetic and Transcriptional Control of Bone Formation. Oral Maxillofac. Surg. Clin. N. Am. 2011, 3, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.W.; Sim, K.B.; Kim, S.D. Development and Growth of the Normal Cranial Vault: An Embryologic Review. J. Korean Neurosurg. Soc. 2016, 59, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Guilak, F.; Nuttall, M.E.; Sathishkumar, S.; Vidal, M.; Bunnell, B.A. In Vitro Differentiation Potential of Mesenchymal Stem Cells. Transfus. Med. Hemotherapy 2008, 35, 228–238. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A Transcriptional Activator of Osteoblast Differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Zur Nieden, N.I.; Kempka, G.; Rancourt, D.E.; Ahr, H.J. Induction of Chondro-, Osteo- and Adipogenesis in Embryonic Stem Cells by Bone Morphogenetic Protein-2: Effect of Cofactors on Differentiating Lineages. BMC Dev. Biol. 2005, 5, 21. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 2007, 12, 3068–3092. [Google Scholar] [CrossRef]
- Zur Nieden, N.I.; Price, F.D.; Davis, L.A.; Everitt, R.E.; Rancourt, D.E. Gene Profiling on Mixed Embryonic Stem Cell Populations Reveals a Biphasic Role for β-Catenin in Osteogenic Differentiation. Mol. Endocrinol. 2007, 3, 674–685. [Google Scholar] [CrossRef]
- Darbre, P.D. Overview of Air Pollution and Endocrine Disorders. Int. J. Gen. Med. 2018, 11, 191–207. [Google Scholar] [CrossRef]
- Yaglova, N.V.; Yaglov, V.V. Endocrine Disruptors as a New Etiologic Factor of Bone Tissue Diseases (Review). Sovrem. Tekhnologii Med. 2021, 2, 84–94. [Google Scholar] [CrossRef]
- Sinha, R.; Yen, P.M. Cellular Action of Thyroid Hormone. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.E., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK285568/ (accessed on 22 March 2023).
- Goel, R.; Raju, R.; Maharudraiah, J.; Kumar, G.S.S.; Ghosh, K.; Kumar, A.; Lakshmi, T.P.; Sharma, J.; Sharma, R.; Balakrishnan, L.; et al. A Signaling Network of Thyroid-Stimulating Hormone. J. Proteom. Bioinform. 2011, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Bassett, J.H.D.; Williams, G.R. Role of Thyroid Hormones in Skeletal Development and Bone Maintenance. Endocr. Rev. 2016, 37, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Lademann, F.; Weidner, H.; Tsourdi, E.; Kumar, R.; Rijntjes, E.; Köhrle, J.; Hofbauer, L.C.; Rauner, M. Disruption of BMP Signaling Prevents Hyperthyroidism-Induced Bone Loss in Male Mice. J. Bone Miner. Res. 2020, 35, 2058–2069. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xing, W.; Pourteymoor, S.; Mohan, S. Effects of Thyroxine (T4), 3,5,3’-Triiodo-L-Thyronine (T3) and Their Metabolites on Osteoblast Differentiation. Calcif. Tissue Int. 2016, 99, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Lademann, F.; Tsourdi, E.; Hofbauer, L.C.; Rauner, M. Thyroid Hormone Actions and Bone Remodeling—The Role of the Wnt Signaling Pathway. Exp. Clin. Endocrinol. Diabetes 2020, 128, 450–454. [Google Scholar] [CrossRef]
- O’Shea, P.J.; Kim, D.W.; Logan, J.G.; Davis, S.; Walker, R.L.; Meltzer, P.S.; Cheng, S.Y.; Williams, G.R. Advanced Bone Formation in Mice with a Dominant-Negative Mutation in the Thyroid Hormone Receptor β Gene Due to Activation of Wnt/β-Catenin Protein Signaling. J. Biol. Chem. 2012, 287, 17812–17822. [Google Scholar] [CrossRef]
- Tsourdi, E.; Rijntjes, E.; Köhrle, J.; Hofbauer, L.C.; Rauner, M. Hyperthyroidism and Hypothyroidism in Male Mice and Their Effects on Bone Mass, Bone Turnover, and the Wnt Inhibitors Sclerostin and Dickkopf-1. Endocrinology 2015, 156, 3517–3527. [Google Scholar] [CrossRef]
- Zhang, R.; Oyajobi, B.O.; Harris, S.E.; Chen, D.; Tsao, C.; Deng, H.-W.; Zhao, M. Wnt/β-Catenin Signaling Activates Bone Morphogenetic Protein 2 Expression in Osteoblasts. Bone 2013, 52, 145–156. [Google Scholar] [CrossRef]
- Martin, T.J.; Sims, N.A.; Seeman, E. Physiological and Pharmacological Roles of PTH and PTHrP in Bone Using Their Shared Receptor, PTH1R. Endocr. Rev. 2021, 42, a011148. [Google Scholar] [CrossRef]
- Pacifici, R. Role of Gut Microbiota in the Skeletal Response to PTH. J. Clin. Endocrinol. Metab. 2021, 106, 636–645. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Parathyroid Hormone: Anabolic and Catabolic Actions on the Skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef] [PubMed]
- Cheloha, R.W.; Gellman, S.H.; Vilardaga, J.P.; Gardella, T.J. PTH Receptor-1 Signalling—Mechanistic Insights and Therapeutic Prospects. Nat. Rev. Endocrinol. 2015, 12, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhao, X.; Yang, C.; Crane, J.; Xian, L.; Lu, W.; Wan, M.; Cao, X. Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling. J. Bone Miner. Res. 2012, 9, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Hanai, J.I.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M.; et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017, 25, 661–672. [Google Scholar] [CrossRef]
- Haussler, M.R.; Livingston, S.; Sabir, Z.L.; Haussler, C.A.; Jurutka, P.W. Vitamin D Receptor Mediates a Myriad of Biological Actions Dependent on Its 1,25-Dihydroxyvitamin D Ligand: Distinct Regulatory Themes Revealed by Induction of Klotho and Fibroblast Growth Factor-23. JBMR Plus 2020, 5, e10432. [Google Scholar] [CrossRef]
- Oelzner, P.; Petrow, P.K.; Wolf, G.; Bräuer, R. 1,25-Dihydroxyvitamin D3 Prevents Bone Loss of the Secondary Spongiosa in Arthritic Rats by an Increase of Bone Formation and Mineralization and Inhibition of Bone Resorption. BMC Musculoskelet. Disord. 2014, 15, 345. [Google Scholar] [CrossRef]
- Sparks, N.R.L.; Martinez, I.K.C.; Soto, C.H.; zur Nieden, N.I. Low Osteogenic Yield in Human Pluripotent Stem Cells Associates with Differential Neural Crest Promoter Methylation. Stem Cells 2018, 36, 349–362. [Google Scholar] [CrossRef]
- Sparks, N.R.L.; Walker, L.M.; Sera, S.R.; Madrid, J.V.; Hanna, M.; Dominguez, E.C.; zur Nieden, N.I. Sidestream Smoke Extracts from Harm-Reduction and Conventional Camel Cigarettes Inhibit Osteogenic Differentiation via Oxidative Stress and Differential Activation of Intrinsic Apoptotic Pathways. Antioxidants 2022, 11, 2474. [Google Scholar] [CrossRef]
- zur Nieden, N.I.; Kempka, G.; Ahr, H.J. In Vitro Differentiation of Embryonic Stem Cells into Mineralized Osteoblasts. Differentiation 2003, 71, 18–27. [Google Scholar] [CrossRef]
- Lou, Y.R.; Toh, T.C.; Tee, Y.H.; Yu, H. 25-Hydroxyvitamin D3 Induces Osteogenic Differentiation of Human Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 42816. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The Role of Estrogen and Androgen Receptors in Bone Health and Disease. Nat. Rev. 2013, 9, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Faltas, C.L.; LeBron, K.A.; Holz, M.K. Unconventional Estrogen Signaling in Health and Disease. Endocrinology 2020, 161, bqaa030. [Google Scholar] [CrossRef]
- Thomas, S.; Jaganathan, B.G. Signaling Network Regulating Osteogenesis in Mesenchymal Stem Cells. Cell Commun. Signal. 2022, 16, 47–61. [Google Scholar] [CrossRef]
- Wan, M.; Yang, C.; Li, J.; Wu, X.; Yuan, H.; Ma, H.; He, X.; Nie, S.; Chang, C.; Cao, X. Parathyroid Hormone Signaling through Low-Density Lipoprotein-Related Protein 6. Genes Dev. 2008, 22, 2968–2979. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Suh, W.I.; Kang, N.K.; Lee, B.; Chang, Y.K. MAPK/ERK and JNK Pathways Regulate Lipid Synthesis and Cell Growth of Chlamydomonas Reinhardtii under Osmotic Stress, Respectively. Sci. Rep. 2018, 8, 13857. [Google Scholar] [CrossRef]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen Prevents Bone Loss via Estrogen Receptor α and Induction of Fas Ligand in Osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef]
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schüler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M.; et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017, 7, 6460. [Google Scholar] [CrossRef]
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Aurilia, C.; Marini, F.; Giusti, F.; Brandi, M.L. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int. J. Mol. Sci. 2023, 24, 3772. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Overview of Endocrine Disruption. Available online: https://www.epa.gov/endocrine-disruption/overview-endocrine-disruption (accessed on 31 March 2023).
- Koh, H.K.; Sebelius, K.G. Ending the Tobacco Epidemic. JAMA 2012, 308, 767–768. [Google Scholar] [CrossRef] [PubMed]
- Al-Bashaireh, A.M.; Haddad, L.G.; Weaver, M.; Chengguo, X.; Kelly, D.L.; Yoon, S. The Effect of Tobacco Smoking on Bone Mass: An Overview of Pathophysiologic Mechanisms. J. Osteoporos. 2018, 2018, 1206235. [Google Scholar] [CrossRef] [PubMed]
- Law, M.R.; Hackshaw, A.K. A Meta-Analysis of Cigarette Smoking, Bone Mineral Density and Risk of Hip Fracture: Recognition of a Major Effect. BMJ 1997, 315, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Turan, S. Endocrine Disrupting Chemicals and Bone. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101495. [Google Scholar] [CrossRef]
- Kapoor, D.; Jones, T.H. Smoking and Hormones in Health and Endocrine Disorders. Eur. J. Endocrinol. 2005, 152, 491–499. [Google Scholar] [CrossRef]
- Tziomalos, K.; Charsoulis, F. Endocrine Effects of Tobacco Smoking. Clin. Endocrinol. 2004, 61, 664–674. [Google Scholar] [CrossRef]
- Rune, G.M.; Frotscher, M. Neurosteroid Synthesis in the Hippocampus: Role in Synaptic Plasticity. Neuroscience 2005, 136, 833–842. [Google Scholar] [CrossRef]
- Yuan, L.; Ni, J. The Association between Tobacco Smoke Exposure and Vitamin D Levels among US General Population, 2001–2014: Temporal Variation and Inequalities in Population Susceptibility. Environ. Sci. Pollut. Res. 2022, 29, 32773–32787. [Google Scholar] [CrossRef]
- Ren, W.; Gu, Y.; Zhu, L.; Wang, L.; Chang, Y.; Yan, M.; Han, B.; He, J. The Effect of Cigarette Smoking on Vitamin D Level and Depression in Male Patients with Acute Ischemic Stroke. Compr. Psychiatry 2016, 65, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Eisa, N.H.; Reddy, S.V.; Elmansi, A.M.; Kondrikova, G.; Kondrikov, D.; Shi, X.M.; Novince, C.M.; Hamrick, M.W.; McGee-Lawrence, M.E.; Isales, C.M.; et al. Kynurenine Promotes RANKL-Induced Osteoclastogenesis In Vitro by Activating the Aryl Hydrocarbon Receptor Pathway. Int. J. Mol. Sci. 2020, 21, 7931. [Google Scholar] [CrossRef]
- Karmach, O.; Madrid, J.V.; Dasgupta, S.; Volz, D.C.; zur Nieden, N.I. Embryonic Exposure to Cigarette Smoke Extract Impedes Skeletal Development and Evokes Craniofacial Defects in Zebrafish. Int. J. Mol. Sci. 2022, 23, 9904. [Google Scholar] [CrossRef]
- Liu, J.; Clark, L.P.; Bechle, M.J.; Hajat, A.; Kim, S.Y.; Robinson, A.L.; Sheppard, L.; Szpiro, A.A.; Marshall, J.D. Disparities in Air Pollution Exposure in the United States by Race/Ethnicity and Income, 1990–2010. Environ. Health Perspect. 2021, 129, 127005. [Google Scholar] [CrossRef]
- Prada, D.; López, G.; Solleiro-Villavicencio, H.; Garcia-Cuellar, C.; Baccarelli, A.A. Molecular and Cellular Mechanisms Linking Air Pollution and Bone Damage. Environ. Res. 2020, 185, 109465. [Google Scholar] [CrossRef]
- World Health Organization. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease. Available online: https://apps.who.int/iris/handle/10665/250141. (accessed on 31 March 2023).
- Ranzani, O.T.; Milà, C.; Kulkarni, B.; Kinra, S.; Tonne, C. Association of Ambient and Household Air Pollution with Bone Mineral Content Among Adults in Peri-urban South India. JAMA Netw. Open 2020, 3, e1918504. [Google Scholar] [CrossRef] [PubMed]
- Center for Diseases Control. Particle Pollution. Available online: https://www.cdc.gov/air/particulate_matter.html (accessed on 31 March 2023).
- US EPA. OAR. In Particulate Matter (PM) Basics. Overviews and Factsheets. Available online: https://www.epa.gov/pm-pollution/particulate-matter-pm-basics (accessed on 31 March 2023).
- WHO. Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 31 March 2023).
- Alver, K.; Meyer, H.E.; Falch, J.A.; Søgaard, A.J. Outdoor Air Pollution, Bone Density and Self-Reported Forearm Fracture: The Oslo Health Study. Osteoporos. Int. 2010, 21, 1751–1760. [Google Scholar] [CrossRef]
- Hong, H.; Zhao, H.; Huang, L.; Zhong, D.; Shi, D. Bone Developmental Toxicity of Organophosphorus Flame Retardants TDCIPP and TPhP in Marine Medaka Oryzias Melastigma. Ecotoxicol. Environ. Saf. 2021, 223, 112605. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yang, L.; Han, J.; Zou, Y.; Wang, Y.; Feng, C.; Zhou, B. Early-life exposure to tris (1,3-dichloro-2-propyl) phosphate caused multigenerational neurodevelopmental toxicity in zebrafish via altering maternal thyroid hormones transfer and epigenetic modifications. Environ. Pollut. 2021, 285, 117471. [Google Scholar] [CrossRef]
- Gosavi, R.A.; Knudsen, G.A.; Birnbaum, L.S.; Pedersen, L.C. Mimicking of estradiol binding by flame retardants and their metabolites: A crystallographic analysis. Environ. Health Perspect. 2013, 121, 1194–1199. [Google Scholar] [CrossRef]
- What Is a Pesticide? Available online: https://www.epa.gov/minimum-risk-pesticides/what-pesticide (accessed on 11 April 2023).
- New Research Highlights Health Effects of Pesticides on Mothers and Their Children. Available online: https://www.niehs.nih.gov/research/programs/geh/geh_newsletter/2022/6/articles/new_research_highlights_health_effects_of_pesticides_on_mothers_and_their_children.cfm (accessed on 11 April 2023).
- Pesticides & Human Health. Available online: https://www.pesticidereform.org/pesticides-human-health/ (accessed on 11 April 2023).
- Bretveld, R.W.; Thomas, C.M.; Scheepers, P.T.; Zielhuis, G.A.; Roeleveld, N. Pesticide exposure: The hormonal function of the female reproductive system disrupted? Reprod. Biol. Endocrinol. 2006, 4, 30. [Google Scholar] [CrossRef]
- Andersen, H.R.; Vinggaard, A.M.; Rasmussen, T.H.; Gjermandsen, I.M.; Bonefeld-Jørgensen, E.C. Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro. Toxicol. Appl. Pharmacol. 2002, 179, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Leemans, M.; Couderq, S.; Demeneix, B.; Fini, J.B. Pesticides with Potential Thyroid Hormone-Disrupting Effects: A Review of Recent Data. Front. Endocrinol. 2019, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Mnif, W.; Hassine, A.I.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor pesticides: A review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, J.; Pizzorno, L. Environmental Toxins Are a Major Cause of Bone Loss. Integr. Med. 2021, 20, 10–17. [Google Scholar]
- Hsu, C.Y.; Chen, L.R.; Chen, K.H. Osteoporosis in Patients with Chronic Kidney Diseases: A Systemic Review. Int. J. Mol. Sci. 2020, 21, 6846. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, J.; Kim, H.J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Moon, H.B.; Kim, S.; et al. Association between Several Persistent Organic Pollutants and Thyroid Hormone Levels in Cord Blood Serum and Bloodspot of the Newborn Infants of Korea. PLoS ONE 2015, 10, 0125213. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Shi, Z.; Strong, M.J.; Miller, D.F.; Rusch, D.B.; Buechlein, A.M.; Flemington, E.K.; McLachlan, J.A.; Nephew, K.P.; Burow, M.E.; et al. Effects of the endocrine-disrupting chemical DDT on self-renewal and differentiation of human mesenchymal stem cells. Environ. Health Perspect. 2015, 123, 42–48. [Google Scholar] [CrossRef]
- DDE. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/dde.pdf (accessed on 11 April 2023).
- Dichlorodiphenyltrichloroethane (DDT). Available online: https://www.cdc.gov/biomonitoring/pdf/ddt_factsheet.pdf (accessed on 21 March 2023).
- Balte, P.P.; Kühr, J.; Kruse, H.; Karmaus, W.J.J. Body Burden of Dichlorodiphenyl Dichloroethene (DDE) and Childhood Pulmonary Function. Int. J. Environ. Res. Public Health 2017, 14, 1376. [Google Scholar] [CrossRef]
- Torres-Avilés, N.A.; Albores-García, D.; Luna, A.L.; Moreno-Galván, M.; Salgado-Bustamante, M.; Portales-Pérez, D.P.; Calderón-Aranda, E.S. Exposure to p,p’-DDE Induces Morphological Changes and Activation of the PKCα-p38-C/EBPβ Pathway in Human Promyelocytic HL-60 Cells. Biomed. Res. Int. 2016, 2016, 1375606. [Google Scholar] [CrossRef]
- Imidacloprid Technical Fact Sheet. Available online: http://npic.orst.edu/factsheets/archive/imidacloprid.html (accessed on 11 April 2023).
- Tariba Lovaković, B.; Kašuba, V.; Sekovanić, A.; Orct, T.; Jančec, A.; Pizent, A. Effects of Sub-Chronic Exposure to Imidacloprid on Reproductive Organs of Adult Male Rats: Antioxidant State, DNA Damage, and Levels of Essential Elements. Antioxidants 2021, 10, 1965. [Google Scholar] [CrossRef]
- Mikolić, A.; Karačonji, I.B. Imidacloprid as reproductive toxicant and endocrine disruptor: Investigations in laboratory animals. Arch. Ind. Hyg. Toxicol. 2018, 69, 103–108. [Google Scholar] [CrossRef]
- Mzid, M.; Badraoui, R.; Khedir, S.B.; Sahnoun, Z.; Rebai, T. Protective effect of ethanolic extract of Urtica urens L. against the toxicity of imidacloprid on bone remodeling in rats and antioxidant activities. Biomed. Pharmacother. 2017, 91, 1022–1041. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Wang, G.; Wang, X.Y.; Liu, M.; Chuai, M.; Lee, K.K.; He, X.S.; Lu, D.X.; Yang, X. Imidacloprid Exposure Suppresses Neural Crest Cells Generation during Early Chick Embryo Development. J. Agric. Food Chem. 2016, 64, 4705–4715. [Google Scholar] [CrossRef]
- Abnosi, M.H.; Mehranjani, M.S.; Shariatzadeh, M.A.; Dehdehi, L. Para-Nonylphenol Impairs Osteogenic Differentiation of Rat Bone Marrow Mesenchymal Stem Cells by Influencing the Osteoblasts Mineralization. Iran J. Basic Med. Sci. 2012, 15, 1131–1139. [Google Scholar] [CrossRef]
- Hussein, A.M.; Sina, M. P-Nonylphenol Impairment of Osteogenic Differentiation of Mesenchymal Stem Cells Was Found to Be Due to Oxidative Stress and Down-Regulation of RUNX2 and BMP. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1336–1346. [Google Scholar] [CrossRef]
- Zhang, H.; Taya, K.; Nagaoka, K.; Yoshida, M.; Watanabe, G. 4-Nitrophenol (PNP) Inhibits the Expression of Estrogen Receptor β and Disrupts Steroidogenesis during the Ovarian Development in Female Rats. Environ. Pollut. 2017, 229, 685–692. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Zeng, F.; Fu, X.; Xu, W.; Yu, J. Influence of nonylphenol exposure on basic growth, development, and thyroid tissue structure in F1 male rats. PeerJ 2019, 7, e7039. [Google Scholar] [CrossRef]
- Healthline. What Is BPA and Is It Cause for Concern? Available online: https://www.healthline.com/nutrition/what-is-bpa (accessed on 31 March 2023).
- García-Recio, E.; Costela-Ruiz, V.J.; Illescas-Montes, R.; Melguizo-Rodríguez, L.; García-Martínez, O.; Ruiz, C.; de Luna-Bertos, E. Modulation of Osteogenic Gene Expression by Human Osteoblasts Cultured in the Presence of Bisphenols BPF, BPS, or BPAF. Int. J. Mol. Sci. 2023, 24, 4256. [Google Scholar] [CrossRef]
- Wang, T.; Xu, F.; Song, L.; Li, J.; Wang, Q. Bisphenol A exposure prenatally delays bone development and bone mass accumulation in female rat offspring via the ERβ/HDAC5/TGFβ signaling pathway. Toxicology 2021, 458, 152830. [Google Scholar] [CrossRef]
- Johns, L.E.; Ferguson, K.K.; Cantonwine, D.E.; McElrath, T.F.; Mukherjee, B.; Meeker, J.D. Urinary BPA and Phthalate Metabolite Concentrations and Plasma Vitamin D Levels in Pregnant Women: A Repeated Measures Analysis. Environ. Health Perspect. 2017, 125, 087026. [Google Scholar] [CrossRef]
- Brandi, M.L.; Bandinelli, S.; Iantomasi, T.; Giusti, F.; Talluri, E.; Sini, G.; Nannipieri, F.; Battaglia, S.; Giusti, R.; Egan, C.G.; et al. Association between vitamin D and bisphenol A levels in an elderly Italian population: Results from the InCHIANTI study. Endocr. Connect. 2022, 11, e210571. [Google Scholar] [CrossRef]
- Agas, D.; Lacava, G.; Sabbieti, M.G. Bone and bone marrow disruption by endocrine-active substances. J. Cell. Physiol. 2018, 234, 192–213. [Google Scholar] [CrossRef]
- Koskela, A.; Koponen, J.; Lehenkari, P.; Viluksela, M.; Korkalainen, M.; Tuukkanen, J. Perfluoroalkyl substances in human bone: Concentrations in bones and effects on bone cell differentiation. Sci. Rep. 2017, 7, 6841. [Google Scholar] [CrossRef] [PubMed]
- Tsang, H.; Cheung, T.Y.; Kodithuwakku, S.P.; Chai, J.; Yeung, W.S.; Wong, C.K.; Lee, K.F. Perfluorooctanoate suppresses spheroid attachment on endometrial epithelial cells through peroxisome proliferator-activated receptor alpha and down-regulation of Wnt signaling. Reprod Toxicol. 2013, 42, 164–171. [Google Scholar] [CrossRef]
- Di Nisio, A.; Rocca, M.S.; De Toni, L.; Sabovic, I.; Guidolin, D.; Dall’Acqua, S.; Acquasaliente, L.; De Filippis, V.; Plebani, M.; Foresta, C. Endocrine disruption of vitamin D activity by perfluoro-octanoic acid (PFOA). Sci. Rep. 2020, 10, 16789. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Tao, Y.; Li, N.; Ji, M.; Zhang, X.; Chen, Y.; He, Z.; Yu, K.; Yu, Z. TCDD Inhibited the Osteogenic Differentiation of Human Fetal Palatal Mesenchymal Cells through AhR and BMP-2/TGF-β/Smad Signaling. Toxicology 2020, 431, 152353. [Google Scholar] [CrossRef]
- Miki, Y.; Hata, S.; Ono, K.; Suzuki, T.; Ito, K.; Kumamoto, H.; Sasano, H. Roles of Aryl Hydrocarbon Receptor in Aromatase-Dependent Cell Proliferation in Human Osteoblasts. Int. J. Mol. Sci. 2017, 18, 2159. [Google Scholar] [CrossRef]
- Watson, A.L.T.D.; Nordberg, R.C.; Loboa, E.G.; Kullman, S.W. Evidence for Aryl Hydrocarbon Receptor-Mediated Inhibition of Osteoblast Differentiation in Human Mesenchymal Stem Cells. Toxicol. Sci. 2019, 167, 145–156. [Google Scholar] [CrossRef]
- Jämsä, T.; Viluksela, M.; Tuomisto, J.T.; Tuomisto, J.; Tuukkanen, J. Effects of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin on Bone in Two Rat Strains with Different Aryl Hydrocarbon Receptor Structures. JBMR 2001, 16, 1812–1820. [Google Scholar] [CrossRef]
- Singh, S.U.; Casper, R.F.; Fritz, P.C.; Sukhu, B.; Ganss, B.; Girard, B.; Savouret, J.F.; Tenenbaum, H.C. Inhibition of Dioxin Effects on Bone Formation in Vitro by a Newly Described Aryl Hydrocarbon Receptor Antagonist, Resveratrol. J. Endocrinol. 2000, 167, 183–195. [Google Scholar] [CrossRef]
- Reale, C.; Porreca, I.; Russo, F.; Marotta, M.; Roberto, L.; Russo, N.A.; Carchia, E.; Mallardo, M.; De Felice, M.; Ambrosino, C. Genetic background and window of exposure contribute to thyroid dysfunction promoted by low-dose exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice. Sci. Rep. 2018, 8, 16324. [Google Scholar] [CrossRef]


| Literature Search Strategy | |
|---|---|
| A. | PubMed and Google Scholar combinations (name of hormone + bone-related term or toxicant + hormone + bone-related term) of the following terms: hormones, thyroid hormone, parathyroid hormone, vitamin D, estrogen, osteogenesis, bone, bone development, bone damage, bone birth defects, bone toxicology, endocrine disruptors, endocrine-disrupting chemicals, tobacco, cigarette smoke, air pollution, flame retardants, bisphenol A, PFAS, TCDD, DDT, para-nonylphenol, and pesticides. |
| B. | English language papers were screened from publication date 1 January 2000 to 30 April 2023. Publications included original research articles, reviews, and book chapters consisting of in vitro studies, in vivo animal studies, human studies, and meta-analyses. Non-English and unavailable full-text articles were excluded. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwobi, N.; Sparks, N.R. Endocrine Disruptor-Induced Bone Damage Due to Hormone Dysregulation: A Review. Int. J. Mol. Sci. 2023, 24, 8263. https://doi.org/10.3390/ijms24098263
Iwobi N, Sparks NR. Endocrine Disruptor-Induced Bone Damage Due to Hormone Dysregulation: A Review. International Journal of Molecular Sciences. 2023; 24(9):8263. https://doi.org/10.3390/ijms24098263
Chicago/Turabian StyleIwobi, Nneamaka, and Nicole R. Sparks. 2023. "Endocrine Disruptor-Induced Bone Damage Due to Hormone Dysregulation: A Review" International Journal of Molecular Sciences 24, no. 9: 8263. https://doi.org/10.3390/ijms24098263





