Heterogeneity of Lipopolysaccharide as Source of Variability in Bioassays and LPS-Binding Proteins as Remedy
Abstract
:1. Introduction
2. LPS Heterogeneity
2.1. Chemical Heterogeneity of LPS
2.2. LPS Supramolecular Structures and Intermolecular Interactions
3. LPS Recognition and Immunological Impact
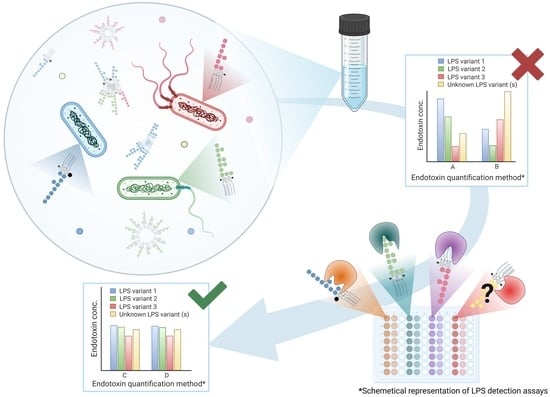
4. LPS Detection: Methods, Challenges, and Future Options
4.1. Limitations of Detection Assays
4.2. Low Endotoxin Recovery and Endotoxin Potency
4.3. LPS-Binding Molecules
| Origin | Binding Molecule | LPS Strain (Serotype) | LPS Target | KD Values | Citation |
|---|---|---|---|---|---|
| Microorganisms | |||||
| Bacteriophage | Bacteriophage Sf6 tailspike protein | Shigella flexneri | O-antigen | - | [197] |
| Bacteriophage | Phage P22 tailspike protein | S. enterica typhimurium, S. enteritidis | O-antigen | - | [198,199] |
| Virus | SARS-CoV-2 spike protein | E. coli | Lipid A | 47 nM | [200] |
| Gram positive bacteria (Bacillus polymyxa) | Polymyxin B (PMB) | E. coli (O55:B5), S. minnesota (Re 595) | Lipid A | Lipid A 5.6 nM, LPS 25.4 nM | [189] |
| Gram positive bacteria (Bacillus polymyxa) | Polymyxin B (PMB) | E. coli (K12) | Lipid A | 0.71 µM | [201] |
| Gram positive bacteria (Bacillus polymyxa) | Synthetic polymyxin MIPS-9451 | E. coli (O111:B4, O26:B6), S. enterica (abortus equi, enteritidis, minnesota), Serratia marcescens, Helicobacter pylori, Porphyromonas gingivalis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus mirabilis, Proteus vulgaris, Campylobacter jejuni, Bordetella pertussis | Lipid A | 0.14 µM– 7.2 µM | [176] |
| Insects | |||||
| Honey bee | Melittin | E. coli (O111:B4), E. coli (F583) | Lipid A | 0.3 µM | [181] |
| Hornet | Masroparan-1 (MP-1) | E. coli (O55:B5), E. coli (O111:B4), S. minnesota (Re 595) | Lipid A | Lipid A 456 nM, LPS 484 nM | [196] |
| Fly | Attacin | E. coli (K12) | Lipid A and inner core | - | [202] |
| Rove beetle (Paederus) | Sarcotoxin Pd | E. coli, Klebsiella pneumoniae | - | - | [180] |
| Larvae (Papilio xuthus) | Papiliocin | E. coli (O111:B4) | - | 63 nM | [171,179] |
| Larvae (Papilio xuthus) | N- terminal helix of papiliocin (PapN) | - | Phosphate of lipid A | - | [177] |
| Arthropods | |||||
| Horseshoe crab | Factor B | E. coli (O111:B4) S. minnesota R595 | - | 3.49 nM 10.3 nM | [183,203] |
| Horseshoe crab | Factor C | S. minnesota R595 (Re) | - | 0.758 nM | [183,203] |
| Horseshoe crab | Factor C | E. coli (K12) | Lipid A | 0.76 nM | [184] |
| Horseshoe crab | Tachyplesin I (TP I) | - | Phosphate groups and KDO | under 100 μM | [204] |
| Horseshoe crab (Tachypleus tridentatus) | Tachypleus antilipopolysaccharide LPS factor (TALF) | E. coli (O111:B4) | Lipid A | - | [182] |
| Shrimp (Penaeus monodon) | Shrimp anti-lipopolysaccharide factor (SALF) | - | - | - | [172] |
| Shrimp (Rimicaris sp.) | G-type lysozyme (LysG1) | E. coli, Psedoalteromonas hodoensiswas | Lipid A | - | [193] |
| Horseshoe crab (Carcinoscorpius rotundicauda) | Derived from factor C Sushi-1 Sushi-3 | E. coli (K-12) | Lipid A | Sushi1 0.14 nM Sushi3 0.39 nM | [201,205] |
| Horseshoe crab (Achypleus tridentatus, Limulus polyphemus) | LALF31–52 | E. coli (O111:B4) | Lipid A | 47.8 µM | [206] |
| Horseshoe crab | A synthetic cyclic peptide derived from LALF CLP-19 | E. coli (O111:B4) | Lipid A | 8.26 μM | [206] |
| Mammals | |||||
| Human | Human lysozyme (HL) | Klebsiella pneumoniae O1 | O-antigen | 0.41 mM | [207] |
| Human | Interleukin-8 | Aggregatibacter actinomycetemcomitans | - | 1.2–17 μM | [187] |
| Human | Human β-defensin 114 (DEFB114) | E. coli (O111:B4) | - | 0.44 µM | [208] |
| Human | Human β-defensin 126 (DEFB126) | E. coli (O111:B4) | - | 54.16 nM | [209] |
| Human | CD14 | E. coli (O55:B5) | Lipid A | 8.7 µM | [185] |
| Human | CD14 | S. minnesota (Re595) | Lipid A | 29 nM | [210] |
| Human | MD-2 | E. coli (O55:B5) | Lipid A | 3.2 µM | [185] |
| Human | TLR4 | E. coli (O55:B5) | Lipid A | 14.1 µM | [185] |
| Human | LBP | S.minnesota (Re595) | Lipid A | 3.5 nM | [210] |
| Human | rLBP | E. coli (J5) | Lipid A | 1.05 nM | [211] |
| Human | 5I-histidine-rich polypeptide (Histatin 5) | Porphyromonas gingivalis | - | 1.5 µM | [212] |
| Human | Lactoferrin | E. coli (various serotypes), Pseudomonas aeruginosa, Klebsiella pneumoniae, Neisseria meningitides, Neisseria gonorrhoeae, Haemophilus influenzae, Branhamella catarrhalis, Shigella flexneri, Helicobacter pylori | Lipid A | 2 nM | [213] |
| Human | BPI21 | S. minnesota (R595) | Lipid A | 3.75 nM | [214,215] |
| Human | rBPI23 rBPI55 CAP57 | E. coli (J5) lipid A, S. minnesota (Ra), E. coli (O113), S. abortus | Lipid A | 1.7 nM–5.2 nM | [211] |
| Human | BNEP (derived from BPI) | LPS E. coli (O55:B5); Lipid A S. minnesota (Re 595) | Lipid A | LPS 25.8 nM Lipid A 11.8 nM | [189] |
| Human | LL-37 (derived from CAP-18) | LPS E. coli (O111:B4) | - | 77.5 nM | [216,217] |
| Human | Derived from high mobility group box 1 (HMGB1) HPep1 HPep6 | E. coli (O111:B4), S. minnesota, S. typhimurium | O-Antigen Lipid A | - | [188,218] |
| Human | Serum amyloid P component (SAP) | S. minnesota (R595) | Lipid A | 3.9 nM | [214,215] |
| Bovine, human | Lactoferrin | E. coli (O55:B5) | Lipid A | Human 3.6 nM Bovine 4.5 nM Low-affinity binding site: Human 390 nM | [186] |
| Bovine | Fragment of lactoferrin (LF); Fragment of lactoferrin = lactosmart | E. coli (O26:B6) Pseudomonas aeruginosa, Shigella flexneri | Phosphate group, KDO and lipid A | 0.049 nM LPS/ lactosmart, 32 nM LPS/LF | [192] |
| Bovine | Derived from neutrophil granules BAC7(1–35) | E. coli (O111:B4) | Lipid A | - | [219] |
| Rabbit | Cationic protein 18 (CAP18) | S. minnesota (R595) | Lipid A | 0.58 nM | [214,215] |
| Sheep leukocytes | Sheep myeloid antimicrobial peptide-29 (SMAP-29) or (SC5) | - | - | - | [194] |
| Porcine (pig) leukocytes | Protegrin-1 (PG-1) | Neisseria meningitidis | Phosphate head groups and lipid A | - | [220] |
| Birds | |||||
| Chicken | Fowlicidin-1 | - | - | - | [221] |
| Chicken | Lysozyme | S. minnesota (R595) | Phosphate groups of lipid A | - | [178] |
| Amphibians | |||||
| Frog (Xenopus laevis) | Magainin 2 | E. coli, Acinetobacter calcoaceticus | - | - | [222] |
5. Recommendations for a More Efficient Workflow with LPS
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A






References
- Banerjee, S.; van der Heijden, M.G.A. Soil Microbiomes and One Health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- Schmid, A.K.; Allers, T.; DiRuggiero, J. SnapShot: Microbial Extremophiles. Cell 2020, 180, 818.e1. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Billod, J.-M.; Martín-Santamaría, S.; Silipo, A.; Molinaro, A. Gram-Negative Extremophile Lipopolysaccharides: Promising Source of Inspiration for a New Generation of Endotoxin Antagonists. Eur. J. Org. Chem. 2017, 2017, 4055–4073. [Google Scholar] [CrossRef]
- Merino, N.; Aronson, H.S.; Bojanova, D.P.; Feyhl-Buska, J.; Wong, M.L.; Zhang, S.; Giovannelli, D. Living at the Extremes: Extremophiles and the Limits of Life in a Planetary Context. Front. Microbiol. 2019, 10, 780. [Google Scholar] [CrossRef]
- Rietschel, E.T.; Kirikae, T.; Schade, F.U.; Mamat, U.; Schmidt, G.; Loppnow, H.; Ulmer, A.J.; Zähringer, U.; Seydel, U.; Di Padova, F.; et al. Bacterial Endotoxin: Molecular Relationships of Structure to Activity and Function. FASEB J. 1994, 8, 217–225. [Google Scholar] [CrossRef]
- Kolmos, H.J. Panum’s Studies on “Putrid Poison” 1856. Dan. Med. Bull. 2006, 53, 450–452. [Google Scholar]
- Pfeiffer, R. Untersuchungen Über Das Choleragift. Z. Für Hyg. Und Infekt. 1892, 11, 393–412. [Google Scholar] [CrossRef]
- ICH Guideline Q4B—Annex 14 to Note for Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions on Bacterial Endotoxins Tests. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-ich-guideline-q4b-annex-14-note-evaluation-recommendation-pharmacopoeial-texts-use-ich-regions_en.pdf (accessed on 12 April 2023).
- Stromberg, L.R.; Mendez, H.M.; Mukundan, H. Detection Methods for Lipopolysaccharides: Past and Present. In Escherichia coli—Recent Advances on Physiology, Pathogenesis and Biotechnological Applications; InTech: London, UK, 2017; pp. 141–168. [Google Scholar] [CrossRef]
- Stenutz, R.; Weintraub, A.; Widmalm, G. The Structures of Escherichia coli O-Polysaccharide Antigens. FEMS Microbiol. Rev. 2006, 30, 382–403. [Google Scholar] [CrossRef]
- Bengoechea, J.A.; Najdenski, H.; Skurnik, M. Lipopolysaccharide O Antigen Status of Yersinia enterocolitica O:8 Is Essential for Virulence and Absence of O Antigen Affects the Expression of Other Yersinia Virulence Factors. Mol. Microbiol. 2004, 52, 451–469. [Google Scholar] [CrossRef]
- Schromm, A.B.; Brandenburg, K.; Loppnow, H.; Moran, A.P.; Koch, M.H.J.; Rietschel, E.T.; Seydel, U. Biological Activities of Lipopolysaccharides Are Determined by the Shape of Their Lipid A Portion. Eur. J. Biochem. 2000, 267, 2008–2013. [Google Scholar] [CrossRef]
- King, J.D.; Kocíncová, D.; Westman, E.L.; Lam, J.S. Lipopolysaccharide Biosynthesis in Pseudomonas aeruginosa. Innate Immun. 2009, 15, 261–312. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide Endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Furevi, A.; Perepelov, A.V.; Guo, X.; Cao, H.; Wang, Q.; Reeves, P.R.; Knirel, Y.A.; Wang, L.; Widmalm, G. Structure and Genetics of Escherichia Coli O Antigens. FEMS Microbiol. Rev. 2020, 44, 655–683. [Google Scholar] [CrossRef] [PubMed]
- Lukáčová, M.; Barák, I.; Kazár, J. Role of Structural Variations of Polysaccharide Antigens in the Pathogenicity of Gram-Negative Bacteria. Clin. Microbiol. Infect. 2008, 14, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Pupo, E.; Lindner, B.; Brade, H.; Schromm, A.B. Intact Rough- and Smooth-form Lipopolysaccharides from Escherichia Coli Separated by Preparative Gel Electrophoresis Exhibit Differential Biologic Activity in Human Macrophages. FEBS J. 2013, 280, 1095–1111. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.-E.; Lindberg, B.; Lindberg, A.A.; Wollin, R. Structural Studies on the Hexose Region of the Core in Lipopolysaccharides from Enterobacteriaceae. Eur. J. Biochem. 1981, 115, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Amor, K.; Heinrichs, D.E.; Frirdich, E.; Ziebell, K.; Johnson, R.P.; Whitfield, C. Distribution of Core Oligosaccharide Types in Lipopolysaccharides from Escherichia coli. Infect. Immun. 2000, 68, 1116–1124. [Google Scholar] [CrossRef]
- Krauss, J.H.; Seydel, U.; Weckesser, J.; Mayer, H. Structural Analysis of the Nontoxic Lipid A of Rhodobacter capsulatus 37b4. Eur. J. Biochem. 1989, 180, 519–526. [Google Scholar] [CrossRef]
- Masoud, H.; Perry, M.B.; Richards, J.C. Characterization of the Lipopolysaccharide of Moraxella catarrhalis. Eur. J. Biochem. 1994, 220, 209–216. [Google Scholar] [CrossRef]
- Masoud, H.; Urbanik-Sypniewska, T.; Lindner, B.; Weckesser, J.; Mayer, H. The Structure of the Lipid A Component of Sphaerotilus natans. Arch. Microbiol. 1991, 156, 167–175. [Google Scholar] [CrossRef]
- Plötz, B.M.; Lindner, B.; Stetter, K.O.; Holst, O. Characterization of a Novel Lipid A Containing D-Galacturonic Acid That Replaces Phosphate Residues. J. Biol. Chem. 2000, 275, 11222–11228. [Google Scholar] [CrossRef] [PubMed]
- Komaniecka, I.; Choma, A.; Lindner, B.; Holst, O. The Structure of a Novel Neutral Lipid A from the Lipopolysaccharide of Bradyrhizobium elkanii Containing Three Mannose Units in the Backbone. Chem. Eur. J. 2010, 16, 2922–2929. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, D.V.; Kannenberg, E.L.; Sherrier, D.J.; Buhr, R.J.; Carlson, R.W. The Lipopolysaccharide Lipid A Long-Chain Fatty Acid Is Important for Rhizobium leguminosarum Growth and Stress Adaptation in Free-Living and Nodule Environments. Mol. Plant-Microbe Interact. 2017, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Pither, M.D.; Mantova, G.; Scaglione, E.; Pagliuca, C.; Colicchio, R.; Vitiello, M.; Chernikov, O.V.; Hua, K.-F.; Kokoulin, M.S.; Silipo, A.; et al. The Unusual Lipid A Structure and Immunoinhibitory Activity of LPS from Marine Bacteria Echinicola Pacifica KMM 6172T and Echinicola Vietnamensis KMM 6221T. Microorganisms 2021, 9, 2552. [Google Scholar] [CrossRef] [PubMed]
- Zaehringer, U.; Knirel, Y.; Lindner, B.; Sonesson, A.; Marre, R.; Rietschel, E.T. The Lipopolysaccaride of Legionella pneumophila Serogroup 1 (Strain Philadelphia1): Chemical structure and biological significance. Prog. Clin. Biol. Res. 1995, 1, 113–139. [Google Scholar]
- Gunn, J.S. Bacterial Modification of LPS and Resistance to Antimicrobial Peptides. J. Endotoxin Res. 2001, 7, 57–62. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Reynolds, C.M.; Trent, M.S.; Bishop, R.E. Lipid A Modification Systems in Gram-Negative Bacteria. Annu. Rev. Biochem. 2007, 76, 295–329. [Google Scholar] [CrossRef]
- Cardoso, P.G.; Macedo, G.C.; Azevedo, V.; Oliveira, S.C. Brucella Spp. Noncanonical LPS: Structure, Biosynthesis, and Interaction with Host Immune System. Microb. Cell Fact. 2006, 5, 13. [Google Scholar] [CrossRef]
- Onishi, H.R.; Pelak, B.A.; Gerckens, L.S.; Silver, L.L.; Kahan, F.M.; Chen, M.-H.; Patchett, A.A.; Galloway, S.M.; Hyland, S.A.; Anderson, M.S.; et al. Antibacterial Agents That Inhibit Lipid A Biosynthesis. Science 1996, 274, 980–982. [Google Scholar] [CrossRef]
- Phillips, N.J.; Adin, D.M.; Stabb, E.V.; McFall-Ngai, M.J.; Apicella, M.A.; Gibson, B.W. The Lipid A from Vibrio fischeri Lipopolysaccharide. J. Biol. Chem. 2011, 286, 21203–21219. [Google Scholar] [CrossRef]
- Irvine, K.L.; Gangloff, M.; Walsh, C.M.; Spring, D.R.; Gay, N.J.; Bryant, C.E. Identification of Key Residues That Confer Rhodobacter sphaeroides LPS Activity at Horse TLR4/MD-2. PLoS ONE 2014, 9, e98776. [Google Scholar] [CrossRef]
- El Hamidi, A.; Novikov, A.; Karibian, D.; Perry, M.B.; Caroff, M. Structural Characterization of Bordetella parapertussis Lipid A. J. Lipid Res. 2009, 50, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Tawab, A.; Akbar, N.; Hasssan, M.; Habib, F.; Ali, A.; Rahman, M.; Jabbar, A.; Rauf, W.; Iqbal, M. Mass Spectrometric Analysis of Lipid A Obtained from the Lipopolysaccharide of Pasteurella multocida. RSC Adv. 2020, 10, 30917–30933. [Google Scholar] [CrossRef] [PubMed]
- Christian, A.; Zähringer, U. Chemical Structure of Lipid A—The Primary Immunomodulatory Center of Bacterial Lipopolysaccharide. Trends Glycosci. Glycotechnol. 2002, 14, 69–86. [Google Scholar]
- Miura, K.; Ueno, H.; Numa, Y.; Morita, S.; Nishimoto, M. Effects of Fatty Acid from Deep-Sea Microorganisms on Lipid Bilayer Membrane Fluidity under High Pressure: Comparison of Branched-Chain and Polyunsaturated Fatty Acid. E3S Web Conf. 2021, 322, 01019. [Google Scholar] [CrossRef]
- Carillo, S.; Pieretti, G.; Lindner, B.; Romano, I.; Nicolaus, B.; Lanzetta, R.; Parrilli, M.; Corsaro, M. The Lipid A from the Haloalkaliphilic Bacterium Salinivibrio Sharmensis Strain BAGT. Mar. Drugs 2013, 11, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Crisafi, F.; La Cono, V.; Yakimov, M.M.; Molinaro, A.; Silipo, A. The Structure of the Lipid A of Gram-Negative Cold-Adapted Bacteria Isolated from Antarctic Environments. Mar. Drugs 2020, 18, 592. [Google Scholar] [CrossRef]
- Carty, S.M.; Sreekumar, K.R.; Raetz, C.R.H. Effect of Cold Shock on Lipid A Biosynthesis in Escherichia coli. J. Biol. Chem. 1999, 274, 9677–9685. [Google Scholar] [CrossRef]
- Kawahara, K.; Tsukano, H.; Watanabe, H.; Lindner, B.; Matsuura, M. Modification of the Structure and Activity of Lipid A in Yersinia pestis Lipopolysaccharide by Growth Temperature. Infect. Immun. 2002, 70, 4092–4098. [Google Scholar] [CrossRef]
- Pither, M.D.; Sun, M.-L.; Speciale, I.; Silipo, A.; Zhang, Y.-Z.; Molinaro, A.; Di Lorenzo, F. Structural Determination of the Lipid A from the Deep-Sea Bacterium Zunongwangia profunda SM-A87: A Small-Scale Approach. Glycoconj. J. 2022, 39, 565–578. [Google Scholar] [CrossRef]
- Ernst, R.K.; Moskowitz, S.M.; Emerson, J.C.; Kraig, G.M.; Adams, K.N.; Harvey, M.D.; Ramsey, B.; Speert, D.P.; Burns, J.L.; Miller, S.I. Unique Lipid A Modifications in Pseudomonas aeruginosa Isolated from the Airways of Patients with Cystic Fibrosis. J. Infect. Dis. 2007, 196, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, J.A.; Lynch, J.B.; Flores Ramos, S.; Zhou, L.; Apicella, M.A.; Yew, J.Y.; Ruby, E.G. Acidic pH Promotes Lipopolysaccharide Modification and Alters Colonization in a Bacteria–Animal Mutualism. Mol. Microbiol. 2019, 112, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, H.S.; Kalb, S.R.; Cotter, R.J.; Raetz, C.R.H. Role of Mg2+ and pH in the Modification of Salmonella Lipid A after Endocytosis by Macrophage Tumour Cells. Mol. Microbiol. 2005, 55, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, L.A.; Herrera, C.M.; Fernandez, L.; Hankins, J.V.; Trent, M.S.; Hancock, R.E.W. The PmrCAB Operon Mediates Polymyxin Resistance in Acinetobacter baumannii ATCC 17978 and Clinical Isolates through Phosphoethanolamine Modification of Lipid A. Antimicrob. Agents Chemother. 2011, 55, 3743–3751. [Google Scholar] [CrossRef]
- Masoud, H.; Weintraub, S.T.; Wang, R.; Cotter, R.; Holt, S.C. Investigation of the Structure of Lipid A from Actinobacillus actinomycetemcomitans Strain Y4 and Human Clinical Isolate PO 1021-7. Eur. J. Biochem. 1991, 200, 775–781. [Google Scholar] [CrossRef]
- Shimoyama, A.; Di Lorenzo, F.; Yamaura, H.; Mizote, K.; Palmigiano, A.; Pither, M.D.; Speciale, I.; Uto, T.; Masui, S.; Sturiale, L.; et al. Lipopolysaccharide from Gut-Associated Lymphoid-Tissue-Resident Alcaligenes faecalis: Complete Structure Determination and Chemical Synthesis of Its Lipid A. Angew. Chem. Int. Ed. 2021, 60, 10023–10031. [Google Scholar] [CrossRef]
- Weintraub, A.; Zähringer, U.; Wollenweber, H.-W.; Seydel, U.; Rietschel, E.T. Structural Characterization of the Lipid A Component of Bacteroides fragilis Strain NCTC 9343 Lipopolysaccharide. Eur. J. Biochem. 1989, 183, 425–431. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Pither, M.D.; Martufi, M.; Scarinci, I.; Guzmán-Caldentey, J.; Łakomiec, E.; Jachymek, W.; Bruijns, S.C.M.; Santamaría, S.M.; Frick, J.-S.; et al. Pairing Bacteroides vulgatus LPS Structure with Its Immunomodulatory Effects on Human Cellular Models. ACS Cent. Sci. 2020, 6, 1602–1616. [Google Scholar] [CrossRef]
- Malgorzata-Miller, G.; Heinbockel, L.; Brandenburg, K.; Van Der Meer, J.W.M.; Netea, M.G.; Joosten, L.A.B. Bartonella quintana Lipopolysaccharide (LPS): Structure and Characteristics of a Potent TLR4 Antagonist for in-Vitro and in-Vivo Applications. Sci. Rep. 2016, 6, 34221. [Google Scholar] [CrossRef]
- Ieranò, T.; Cescutti, P.; Leone, M.R.; Luciani, A.; Rizzo, R.; Raia, V.; Lanzetta, R.; Parrilli, M.; Maiuri, L.; Silipo, A.; et al. The Lipid A of Burkholderia multivorans C1576 Smooth-Type Lipopolysaccharide and Its pro-Inflammatory Activity in a Cystic Fibrosis Airways Model. Innate Immun. 2010, 16, 354–365. [Google Scholar] [CrossRef]
- Cullen, T.W.; Trent, M.S. A Link between the Assembly of Flagella and Lipooligosaccharide of the Gram-Negative Bacterium Campylobacter jejuni. Proc. Natl. Acad. Sci. USA 2010, 107, 5160–5165. [Google Scholar] [CrossRef] [PubMed]
- Rund, S.; Lindner, B.; Brade, H.; Holst, O. Structural Analysis of the Lipopolysaccharide from Chlamydia trachomatis Serotype L2. J. Biol. Chem. 1999, 274, 16819–16824. [Google Scholar] [CrossRef] [PubMed]
- Stewart, I.; Schluter, P.J.; Shaw, G.R. Cyanobacterial Lipopolysaccharides and Human Health—A Review. Environ. Health 2006, 5, 7. [Google Scholar] [CrossRef]
- Sweet, C.R.; Watson, R.E.; Landis, C.A.; Smith, J.P. Temperature-Dependence of Lipid a Acyl Structure in Psychrobacter Cryohalolentis and Arctic Isolates of Colwellia hornerae and Colwellia piezophila. Mar. Drugs 2015, 13, 4701–4720. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.H.; Guan, Z.; Ingram, B.O.; Six, D.A.; Song, F.; Wang, X.; Zhao, J. Discovery of New Biosynthetic Pathways: The Lipid A Story. J. Lipid Res. 2009, 50, S103–S108. [Google Scholar] [CrossRef]
- Phillips, N.J.; Schilling, B.; McLendon, M.K.; Apicella, M.A.; Gibson, B.W. Novel Modification of Lipid A of Francisella tularensis. Infect. Immun. 2004, 72, 5340–5348. [Google Scholar] [CrossRef]
- Barker, J.H.; Weiss, J.; Apicella, M.A.; Nauseef, W.M. Basis for the Failure of Francisella tularensis Lipopolysaccharide to Prime Human Polymorphonuclear Leukocytes. Infect. Immun. 2006, 74, 3277–3284. [Google Scholar] [CrossRef]
- Garcia-Vello, P.; Di Lorenzo, F.; Lamprinaki, D.; Notaro, A.; Speciale, I.; Molinaro, A.; Juge, N.; De Castro, C. Structure of the O-Antigen and the Lipid A from the Lipopolysaccharide of Fusobacterium nucleatum ATCC 51191. ChemBioChem 2021, 22, 1252–1260. [Google Scholar] [CrossRef]
- Helander, I.M.; Lindner, B.; Brade, H.; Altmann, K.; Lindberg, A.A.; Rietschel, E.T. Chemical Structure of the Lipopolysaccharide Haemophilus influenzae Strain I-69 Rd-/B+. Eur. J. Biochem. 1988, 177, 483–492. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Palmigiano, A.; Paciello, I.; Pallach, M.; Garozzo, D.; Bernardini, M.-L.; La Cono, V.; Yakimov, M.M.; Molinaro, A.; Silipo, A. The Deep-Sea Polyextremophile Halobacteroides lacunaris TB21 Rough-Type LPS: Structure and Inhibitory Activity towards Toxic LPS. Mar. Drugs 2017, 15, 201. [Google Scholar] [CrossRef]
- Tran, A.X.; Stead, C.M.; Trent, M.S. Remodeling of Helicobacter pylori Lipopolysaccharide. J. Endotoxin Res. 2005, 11, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yun, J.; Liu, L.; Li, Y.; Wang, X. Identification of Two Genes Encoding for the Late Acyltransferases of Lipid A in Klebsiella pneumoniae. Curr. Microbiol. 2016, 73, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Black, I.M.; Heiss, C.; Jain, M.; Muszyński, A.; Carlson, R.W.; Gabriel, D.W.; Azadi, P. Structure of Lipopolysaccharide from Liberibacter crescens Is Low Molecular Weight and Offers Insight into Candidatus Liberibacter Biology. Int. J. Mol. Sci. 2021, 22, 11240. [Google Scholar] [CrossRef] [PubMed]
- Krasikova, I.N.; Kapustina, N.V.; Isakov, V.V.; Dmitrenok, A.S.; Dmitrenok, P.S.; Gorshkova, N.M.; Solov’eva, T.F. Detailed Structure of Lipid A Isolated from Lipopolysaccharide from the Marine Proteobacterium Marinomonas vaga ATCC 27119T. Eur. J. Biochem. 2004, 271, 2895–2904. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Ren, D.; Zhang, W.; Merkel, T.J.; Gu, X.-X. Moraxella Catarrhalis Activates Murine Macrophages through Multiple Toll Like Receptors and Has Reduced Clearance in Lungs from TLR4 Mutant Mice. PLoS ONE 2012, 7, e37610. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, C.Q.; St. Michael, F.; Stupak, J.; Li, J.; Cox, A.D.; Richards, J.C. Functional Characterization of Lpt3 and Lpt6, the Inner-Core Lipooligosaccharide Phosphoethanolamine Transferases from Neisseria meningitidis. J. Bacteriol. 2010, 192, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, M.; Weis, J.J.; Toshchakov, V.; Salkowski, C.A.; Cody, M.J.; Ward, D.C.; Qureshi, N.; Michalek, S.M.; Vogel, S.N. Signaling by Toll-like Receptor 2 and 4 Agonists Results in Differential Gene Expression in Murine Macrophages. Infect. Immun. 2001, 69, 1477–1482. [Google Scholar] [CrossRef]
- Sidorczyk, Z.; Zähringer, U.; Rietschel, E.T. Chemical Structure of the Lipid A Component of the Lipopolysaccharide from a Proteus mirabilis Re-Mutant. Eur. J. Biochem. 1983, 22, 15–22. [Google Scholar] [CrossRef]
- Huang, J.X.; Azad, M.A.K.; Yuriev, E.; Baker, M.A.; Nation, R.L.; Li, J.; Cooper, M.A.; Velkov, T. Molecular Characterization of Lipopolysaccharide Binding to Human α-1-Acid Glycoprotein. J. Lipids 2012, 2012, 475153. [Google Scholar] [CrossRef]
- Sweet, C.R.; Alpuche, G.M.; Landis, C.A.; Sandman, B.C. Endotoxin Structures in the Psychrophiles Psychromonas marina and Psychrobacter cryohalolentis Contain Distinctive Acyl Features. Mar. Drugs 2014, 12, 4126–4147. [Google Scholar] [CrossRef]
- Caroff, M.; Novikov, A. Lipopolysaccharides: Structure, Function and Bacterial Identification. OCL 2020, 27, 31. [Google Scholar] [CrossRef]
- Otten, E.G.; Werner, E.; Crespillo-Casado, A.; Boyle, K.B.; Dharamdasani, V.; Pathe, C.; Santhanam, B.; Randow, F. Ubiquitylation of Lipopolysaccharide by RNF213 during Bacterial Infection. Nature 2021, 594, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Aldapa-Vega, G.; Moreno-Eutimio, M.A.; Berlanga-Taylor, A.J.; Jiménez-Uribe, A.P.; Nieto-Velazquez, G.; López-Ortega, O.; Mancilla-Herrera, I.; Cortés-Malagón, E.M.; Gunn, J.S.; Isibasi, A.; et al. Structural Variants of Salmonella typhimurium Lipopolysaccharide Induce Less Dimerization of TLR4/MD-2 and Reduced pro-Inflammatory Cytokine Production in Human Monocytes. Mol. Immunol. 2019, 111, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Makimura, Y.; Asai, Y.; Sugiyama, A.; Ogawa, T. Chemical Structure and Immunobiological Activity of Lipid A from Serratia marcescens LPS. J. Med. Microbiol. 2007, 56, 1440–1446. [Google Scholar] [CrossRef]
- Barrau, C.; Di Lorenzo, F.; Menes, R.J.; Lanzetta, R.; Molinaro, A.; Silipo, A. The Structure of the Lipid a from the Halophilic Bacterium Spiribacter salinus M19-40T. Mar. Drugs 2018, 16, 124. [Google Scholar] [CrossRef]
- Herrera, C.M.; Crofts, A.A.; Henderson, J.C.; Pingali, S.C.; Davies, B.W.; Stephen, M. The Vibrio cholerae VprA-VprB Two-Component System Controls Virulence through Endotoxin Modification. MBio 2014, 5, e02283-14. [Google Scholar] [CrossRef]
- Chandler, C.E.; Harberts, E.M.; Pelletier, M.R.; Thaipisuttikul, I.; Jones, J.W.; Hajjar, A.M.; Sahl, J.W.; Goodlett, D.R.; Pride, A.C.; Rasko, D.A.; et al. Early Evolutionary Loss of the Lipid A Modifying Enzyme PagP Resulting in Innate Immune Evasion in Yersinia pestis. Proc. Natl. Acad. Sci. USA 2020, 117, 22984–22991. [Google Scholar] [CrossRef]
- de Oliveira Magalhães, P.; Lopes, A.M.; Mazzola, P.G.; Rangel-Yagui, C.; Penna, T.C.V.; Pessoa, A. Methods of Endotoxin Removal from Biological Preparations: A Review. J. Pharm. Pharm. Sci. 2007, 10, 388–404. [Google Scholar]
- Santos, N.C.; Silva, A.C.; Castanho, M.A.R.B.; Martins-Silva, J.; Saldanha, C. Evaluation of Lipopolysaccharide Aggregation by Light Scattering Spectroscopy. ChemBioChem 2003, 4, 96–100. [Google Scholar] [CrossRef]
- Gorbet, M.B.; Sefton, M.V. Endotoxin: The Uninvited Guest. Biomaterials 2005, 26, 6811–6817. [Google Scholar] [CrossRef]
- Bergstrand, A.; Svanberg, C.; Langton, M.; Nydén, M. Aggregation Behavior and Size of Lipopolysaccharide from Escherichia coli O55:B5. Colloids Surf. B Biointerfaces 2006, 53, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.J.; Chorover, J. ATR-FTIR Study of Lipopolysaccharides at Mineral Surfaces. Colloids Surf. B Biointerfaces 2008, 62, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Tan, M.; Ho, B.; Ding, J.L.; Wohland, T. Determination of Critical Micelle Concentrations and Aggregation Numbers by Fluorescence Correlation Spectroscopy: Aggregation of a Lipopolysaccharide. Anal. Chim. Acta 2006, 556, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Ribi, E.; Anacker, R.L.; Brown, R.; Haskins, W.T.; Malmgren, B.; Milner, K.C.; Rudbach, J.A. Reaction of Endotoxin and Surfactants. J. Bacteriol. 1966, 92, 1493–1509. [Google Scholar] [CrossRef] [PubMed]
- Bello, G.; Eriksson, J.; Terry, A.; Edwards, K.; Lawrence, M.J.; Barlow, D.; Harvey, R.D. Characterization of the Aggregates Formed by Various Bacterial Lipopolysaccharides in Solution and upon Interaction with Antimicrobial Peptides. Langmuir 2015, 31, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Gontsarik, M.; Amenitsch, H.; Salentinig, S. Human Antimicrobial Peptide Triggered Colloidal Transformations in Bacteria Membrane Lipopolysaccharides. Small 2022, 18, 2104211. [Google Scholar] [CrossRef]
- Parikh, S.J.; Chorover, J. Infrared Spectroscopy Studies of Cation Effects on Lipopolysaccharides in Aqueous Solution. Colloids Surf. B Biointerfaces 2007, 55, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H.; Gornicec, J.; Neuper, T.; Parigiani, M.A.; Wallner, M.; Duschl, A.; Horejs-Hoeck, J. Biological Activity of Masked Endotoxin. Sci. Rep. 2017, 7, 44750. [Google Scholar] [CrossRef]
- Petsch, D.; Deckwer, W.-D.; Anspach, F.B. Proteinase K Digestion of Proteins Improves Detection of Bacterial Endotoxins by the Limulus Amebocyte Lysate Assay: Application for Endotoxin Removal from Cationic Proteins. Anal. Biochem. 1998, 259, 42–47. [Google Scholar] [CrossRef]
- Kaca, W.; Roth, R.I.; Levin, J. Hemoglobin, a Newly Recognized Lipopolysaccharide (LPS)-Binding Protein That Enhances LPS Biological Activity. J. Biol. Chem. 1994, 269, 25078–25084. [Google Scholar] [CrossRef]
- Esparza, G.A.; Teghanemt, A.; Zhang, D.; Gioannini, T.L.; Weiss, J.P. Endotoxin·albumin Complexes Transfer Endotoxin Monomers to MD-2 Resulting in Activation of TLR4. Innate Immun. 2012, 18, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Bello, G.; Bodin, A.; Lawrence, M.J.; Barlow, D.; Mason, A.J.; Barker, R.D.; Harvey, R.D. The Influence of Rough Lipopolysaccharide Structure on Molecular Interactions with Mammalian Antimicrobial Peptides. BBA—Biomembr. 2016, 1858, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS Signaling in C3H/HeJ and C57BL/10ScCr Mice: Mutations in TLR4 Gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting Edge: Toll-Like Receptor 4 (TLR4)-Deficient Mice Are Hyporesponsive to Lipopolysaccharide: Evidence for TLR4 as the Lps Gene Product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Noh, J.; Yoon, S.R.; Kim, T.; Choi, I.; Jung, H. Toll-Like Receptors in Natural Killer Cells and Their Application for Immunotherapy. J. Immunol. Res. 2020, 2020, 2045860. [Google Scholar] [CrossRef]
- Otte, J.-M.; Rosenberg, I.M.; Podolsky, D.K. Intestinal Myofibroblasts in Innate Immune Responses of the Intestine. Gastroenterology 2003, 124, 1866–1878. [Google Scholar] [CrossRef]
- Nagai, Y.; Akashi, S.; Nagafuku, M.; Ogata, M.; Iwakura, Y.; Akira, S.; Kitamura, T.; Kosugi, A.; Kimoto, M.; Miyake, K. Essential Role of MD-2 in LPS Responsiveness and TLR4 Distribution. Nat. Immunol. 2002, 3, 667–672. [Google Scholar] [CrossRef]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.-S.; Lee, H.; Lee, J.-O. The Structural Basis of Lipopolysaccharide Recognition by the TLR4–MD-2 Complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Michelini, S.; Barbero, F.; Prinelli, A.; Steiner, P.; Weiss, R.; Verwanger, T.; Andosch, A.; Lütz-Meindl, U.; Puntes, V.F.; Drobne, D.; et al. Gold Nanoparticles (AuNPs) Impair LPS-Driven Immune Responses by Promoting a Tolerogenic-like Dendritic Cell Phenotype with Altered Endosomal Structures. Nanoscale 2021, 13, 7648–7666. [Google Scholar] [CrossRef]
- Scott, A.J.; Oyler, B.L.; Goodlett, D.R.; Ernst, R.K. Lipid A Structural Modifications in Extreme Conditions and Identification of Unique Modifying Enzymes to Define the Toll-like Receptor 4 Structure-Activity Relationship. BBA—Mol. Cell Biol. Lipids 2017, 1862, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J.C.; Su, T.; Horng, T.; Chow, A.; Akira, S.; Medzhitov, R. TRAM Couples Endocytosis of Toll-like Receptor 4 to the Induction of Interferon-β. Nat. Immunol. 2008, 9, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Palsson-McDermott, E.M.; O’Neill, L.A.J. Signal Transduction by the Lipopolysaccharide Receptor, Toll-like Receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Vanaja, S.K.; Russo, A.J.; Behl, B.; Banerjee, I.; Yankova, M.; Deshmukh, S.D.; Rathinam, V.A.K. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 2016, 165, 1106–1119. [Google Scholar] [CrossRef]
- Barker, J.H.; Weiss, J.P. Detecting Lipopolysaccharide in the Cytosol of Mammalian Cells: Lessons from MD-2/TLR4. J. Leukoc. Biol. 2019, 106, 127–132. [Google Scholar] [CrossRef]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-Negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef]
- Zamyatina, A.; Heine, H. Lipopolysaccharide Recognition in the Crossroads of TLR4 and Caspase-4/11 Mediated Inflammatory Pathways. Front. Immunol. 2020, 11, 585146. [Google Scholar] [CrossRef]
- Deng, M.; Tang, Y.; Li, W.; Wang, X.; Zhang, R.; Zhang, X.; Zhao, X.; Liu, J.; Tang, C.; Liu, Z.; et al. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity 2018, 49, 740–753.e7. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Matikainen, S.; Nyman, T.A.; Cypryk, W. Function and Regulation of Noncanonical Caspase-4/5/11 Inflammasome. J. Immunol. 2020, 204, 3063–3069. [Google Scholar] [CrossRef]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.-A.; Fernández-Peña, C.; Talavera, A.; Kichko, T.; et al. TRPA1 Channels Mediate Acute Neurogenic Inflammation and Pain Produced by Bacterial Endotoxins. Nat. Commun. 2014, 5, 3125. [Google Scholar] [CrossRef] [PubMed]
- Mazgaeen, L.; Gurung, P. Recent Advances in Lipopolysaccharide Recognition Systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.N.; Diamond, G.; Verghese, M.W.; Randell, S.H. CD14-Dependent Lipopolysaccharide-Induced β-Defensin-2 Expression in Human Tracheobronchial Epithelium. J. Biol. Chem. 2000, 275, 29731–29736. [Google Scholar] [CrossRef] [PubMed]
- Håversen, L.; Ohlsson, B.G.; Hahn-Zoric, M.; Hanson, L.Å.; Mattsby-Baltzer, I. Lactoferrin Down-Regulates the LPS-Induced Cytokine Production in Monocytic Cells via NF-κB. Cell. Immunol. 2002, 220, 83–95. [Google Scholar] [CrossRef]
- Hardie, E.M.; Kruse-Elliott, K. Endotoxic Shock. J. Vet. Intern. Med. 1990, 4, 258–266. [Google Scholar] [CrossRef]
- Duerr, C.U.; Zenk, S.F.; Chassin, C.; Pott, J.; Gütle, D.; Hensel, M.; Hornef, M.W. O-Antigen Delays Lipopolysaccharide Recognition and Impairs Antibacterial Host Defense in Murine Intestinal Epithelial Cells. PLOS Pathog. 2009, 5, e1000567. [Google Scholar] [CrossRef]
- Rapicavoli, J.N.; Blanco-Ulate, B.; Muszyński, A.; Figueroa-Balderas, R.; Morales-Cruz, A.; Azadi, P.; Dobruchowska, J.M.; Castro, C.; Cantu, D.; Roper, M.C. Lipopolysaccharide O-Antigen Delays Plant Innate Immune Recognition of Xylella fastidiosa. Nat. Commun. 2018, 9, 390. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J. Sepsis and Septic Shock. Nat. Rev. Dis. Prim. 2016, 2, 16045. [Google Scholar] [CrossRef]
- Mann, P.B.; Wolfe, D.; Latz, E.; Golenbock, D.; Preston, A.; Harvill, E.T. Comparative Toll-Like Receptor 4-Mediated Innate Host Defense to Bordetella Infection. Infect. Immun. 2005, 73, 8144–8152. [Google Scholar] [CrossRef]
- Gorman, A.; Golovanov, A.P. Lipopolysaccharide Structure and the Phenomenon of Low Endotoxin Recovery. Eur. J. Pharm. Biopharm. 2022, 180, 289–307. [Google Scholar] [CrossRef]
- Gangloff, S.C.; Hijiya, N.; Haziot, A.; Goyert, S.M. Lipopolysaccharide Structure Influences the Macrophage Response via CD14-Independent and CD14-Dependent Pathways. Clin. Infect. Dis. 1999, 28, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Lien, E.; Golenbock, D.T. MD-2-Mediated Ionic Interactions between Lipid A and TLR4 Are Essential for Receptor Activation. J. Biol. Chem. 2010, 285, 8695–8702. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, B.; Benaoudia, S.; Wallet, P.; Magnotti, F.; Provost, A.; Michal, F.; Martin, A.; Di Lorenzo, F.; Py, B.F.; Molinaro, A.; et al. Human Caspase-4 Detects Tetra-Acylated LPS and Cytosolic Francisella and Functions Differently from Murine Caspase-11. Nat. Commun. 2018, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Arenas, J.; Pupo, E.; Phielix, C.; David, D.; Zariri, A.; Zamyatina, A.; Tommassen, J.; van der Ley, P. Shortening the Lipid A Acyl Chains of Bordetella Pertussis Enables Depletion of Lipopolysaccharide Endotoxic Activity. Vaccines 2020, 8, 594. [Google Scholar] [CrossRef]
- Ingalls, R.R.; Rice, P.A.; Qureshi, N.; Takayama, K.; Lin, K.; Golenbock, D.T. The Inflammatory Cytokine Response to Chlamydia Trachomatis Infection Is Endotoxin Mediated. Infect. Immun. 1995, 63, 3125–3130. [Google Scholar] [CrossRef]
- Jarvis, B.W.; Lichenstein, H.; Qureshi, N. Diphosphoryl Lipid A from Rhodobacter sphaeroides Inhibits Complexes That Form in Vitro between Lipopolysaccharide (LPS)-Binding Protein, Soluble CD14, and Spectrally Pure LPS. Infect. Immun. 1997, 65, 3011–3016. [Google Scholar] [CrossRef]
- Lembo-Fazio, L.; Billod, J.-M.; Di Lorenzo, F.; Paciello, I.; Pallach, M.; Vaz-Francisco, S.; Holgado, A.; Beyaert, R.; Fresno, M.; Shimoyama, A.; et al. Bradyrhizobium Lipid A: Immunological Properties and Molecular Basis of Its Binding to the Myeloid Differentiation Protein-2/Toll-Like Receptor 4 Complex. Front. Immunol. 2018, 9, 1888. [Google Scholar] [CrossRef]
- Muroi, M.; Tanamoto, K. Structural Regions of MD-2 That Determine the Agonist-Antagonist Activity of Lipid IVa. J. Biol. Chem. 2006, 281, 5484–5491. [Google Scholar] [CrossRef]
- Matsuura, M.; Takahashi, H.; Watanabe, H.; Saito, S.; Kawahara, K. Immunomodulatory Effects of Yersinia Pestis Lipopolysaccharides on Human Macrophages. Clin. Vaccine Immunol. 2010, 17, 49–55. [Google Scholar] [CrossRef]
- Rebeil, R.; Ernst, R.K.; Gowen, B.B.; Miller, S.I.; Hinnebusch, B.J. Variation in Lipid A Structure in the Pathogenic Yersiniae. Mol. Microbiol. 2004, 52, 1363–1373. [Google Scholar] [CrossRef]
- Maeshima, N.; Fernandez, R.C. Recognition of Lipid A Variants by the TLR4-MD-2 Receptor Complex. Front. Cell. Infect. Microbiol. 2013, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Mandell, L.; Moran, A.P.; Cocchiarella, A.; Houghton, J.; Taylor, N.; Fox, J.G.; Wang, T.C.; Kurt-Jones, E.A. Intact Gram-Negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus Bacteria Activate Innate Immunity via Toll-Like Receptor 2 but Not Toll-Like Receptor 4. Infect. Immun. 2004, 72, 6446–6454. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Van Deuren, M.; Kullberg, B.J.; Cavaillon, J.M.; Van Der Meer, J.W.M. Does the Shape of Lipid A Determine the Interaction of LPS with Toll-like Receptors? Trends Immunol. 2002, 23, 135–139. [Google Scholar] [CrossRef]
- Rangarajan, M.; Aduse-Opoku, J.; Paramonov, N.; Hashim, A.; Bostanci, N.; Fraser, O.P.; Tarelli, E.; Curtis, M.A. Identification of a Second Lipopolysaccharide in Porphyromonas Gingivalis W50. J. Bacteriol. 2008, 190, 2920–2932. [Google Scholar] [CrossRef] [PubMed]
- EDQM European Pharmacopoeia to Put an End to the Rabbit Pyrogen Test. Available online: https://www.edqm.eu/en/-/european-pharmacopoeia-to-put-an-end-to-the-rabbit-pyrogen-test (accessed on 14 April 2023).
- Kawabata, S.; Shibata, T. New Insights into the Hemolymph Coagulation Cascade of Horseshoe Crabs Initiated by Autocatalytic Activation of a Lipopolysaccharide-Sensitive Zymogen. Dev. Comp. Immunol. 2022, 135, 104491. [Google Scholar] [CrossRef] [PubMed]
- Koshiba, T.; Hashii, T.; Kawabata, S. A Structural Perspective on the Interaction between Lipopolysaccharide and Factor C, a Receptor Involved in Recognition of Gram-Negative Bacteria. J. Biol. Chem. 2007, 282, 3962–3967. [Google Scholar] [CrossRef] [PubMed]
- Levin, J. Discovery and Early Development of the Limulus Test; Williams, K.L., Ed.; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-17147-6. [Google Scholar]
- Wang, C.; Nelson, T.; Chen, D.; Ellis, J.C.; Abbott, N.L. Understanding Lipopolysaccharide Aggregation and Its Influence on Activation of Factor C. J. Colloid Interface Sci. 2019, 552, 540–553. [Google Scholar] [CrossRef]
- Iwanaga, S.; Miyata, T.; Tokunaga, F.; Muta, T. Molecular Mechanism of Hemolymph Clotting System in Limulus. Thromb. Res. 1992, 68, 1–32. [Google Scholar] [CrossRef]
- Iwanaga, S.; Kawabata, S.-I.; Muta, T. New Types of Clotting Factors and Defense Molecules Found in Horseshoe Crab Hemolymph: Their Structures and Functions. J. Biochem. 1998, 123, 1–15. [Google Scholar] [CrossRef]
- Ketchum, P.A.; Novitsky, T.J. Assay of Endotoxin by Limulus Amebocyte Lysate. In Septic Shock Methods and Protocols; Humana Press: Totowa, NJ, USA, 2000; Volume 36, pp. 3–12. [Google Scholar]
- Novitsky, T.J. Limulus Amebocyte Lysate (LAL) Detection of Endotoxin in Human Blood. J. Endotoxin Res. 1994, 1, 253–263. [Google Scholar] [CrossRef]
- Sandle, T. Endotoxin and Pyrogen Testing. In Pharmaceutical Microbiology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 131–145. [Google Scholar]
- Iwanaga, S.; Morita, T.; Harada, T.; Nakamura, S.; Niwa, M.; Takada, K.; Kimura, T.; Sakakibara, S. Chromogenic Substrates for Horseshoe Crab Clotting Enzyme. Pathophysiol. Haemost. Thromb. 1978, 7, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, Y.; Qiu, F. Low Endotoxin Recovery and Its Impact on Endotoxin Detection. Biopolymers 2021, 112, e23470. [Google Scholar] [CrossRef]
- Roslansky, P.F.; Novitsky, T.J. Sensitivity of Limulus Amebocyte Lysate (LAL) to LAL-Reactive Glucans. J. Clin. Microbiol. 1991, 29, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Reich, J.; Lang, P.; Grallert, H.; Motschmann, H. Masking of Endotoxin in Surfactant Samples: Effects on Limulus-Based Detection Systems. Biologicals 2016, 44, 417–422. [Google Scholar] [CrossRef]
- Reich, J.; Weyer, F.A.; Tamura, H.; Nagaoka, I.; Motschmann, H. Low Endotoxin Recovery—Masking of Naturally Occuring Endotoxin. Int. J. Mol. Sci. 2019, 20, 838. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.C. Endotoxemia: Methods of Detection and Clinical Correlates. Clin. Microbiol. Rev. 1995, 8, 268–292. [Google Scholar] [CrossRef]
- Grallert, H.; Leopoldseder, S.; Schuett, M.; Kurze, P.; Buchberger, B. EndoLISA®: A Novel and Reliable Method for Endotoxin Detection. Nat. Methods 2011, 8, iii–v. [Google Scholar] [CrossRef]
- Bu, R.; Deng, X.; Cao, Y.; Jin, J.; Mai, B.; Meng, K.; Liu, X.; Chi, J.C.; Zhang, Y.; Qiu, F. Effect of Different Sample Treatment Methods on Low Endotoxin Recovery Phenomenon. J. Microbiol. Methods 2021, 186, 106241. [Google Scholar] [CrossRef]
- Maloney, T.; Phelan, R.; Simmons, N. Saving the Horseshoe Crab: A Synthetic Alternative to Horseshoe Crab Blood for Endotoxin Detection. PLoS Biol. 2018, 16, e2006607. [Google Scholar] [CrossRef]
- Loverock, B.; Simon, B.; Burgenson, A.; Baines, A.; Lonza, W. A Recombinant Factor C Procedure for the Detection of Gram-Negative Bacterial Endotoxin. United States Pharmacop. Conv.-Pharmacop. Forum 2010, 36, 321–329. [Google Scholar]
- Stang, K.; Fennrich, S.; Krajewski, S.; Stoppelkamp, S.; Burgener, I.A.; Wendel, H.-P.; Post, M. Highly Sensitive Pyrogen Detection on Medical Devices by the Monocyte Activation Test. J. Mater. Sci. Mater. Med. 2014, 25, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.C.; Jacobs, D.M. Binding of Polymyxin B to the Lipid A Portion of Bacterial Lipopolysaccharides. Immunochemistry 1976, 13, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Goode, A.; Yeh, V.; Bonev, B.B. Interactions of Polymyxin B with Lipopolysaccharide-Containing Membranes. Faraday Discuss. 2021, 232, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Appelmelk, B.J.; Su, D.; Verweij-van Vught, A.M.J.J.; Thijs, B.G.; MacLaren, D.M. Polymyxin B-Horseradish Peroxidase Conjugates as Tools in Endotoxin Research. Anal. Biochem. 1992, 207, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.B.; Barclay, G.R. Endotoxin-Polymyxin Complexes in an Improved Enzyme-Linked Immunosorbent Assay for IgG Antibodies in Blood Donor Sera to Gram-Negative Endotoxin Core Glycolipids. Vox Sang. 1987, 52, 272–280. [Google Scholar] [CrossRef]
- Trautmann, M.; Held, T.K.; Susa, M.; Karajan, M.A.; Wulf, A.; Cross, A.S.; Marre, R. Bacterial Lipopolysaccharide (LPS)-Specific Antibodies in Commercial Human Immunoglobulin Preparations: Superior Antibody Content of an IgM- Enriched Product. Clin. Exp. Immunol. 1998, 111, 81–90. [Google Scholar] [CrossRef]
- Borton, L.K.; Coleman, K.P. Material-Mediated Pyrogens in Medical Devices: Applicability of the in Vitro Monocyte Activation Test. ALTEX 2018, 207, 453–463. [Google Scholar] [CrossRef]
- Dawson, M.E. Endotoxin Standards and CSE Potency. LAL Updat. 1993, 11, 1–5. [Google Scholar]
- Chen, J.; Vinther, A. Low Endotoxin Recovery in Common Biologics Products, PDA Annual Meeting; PDA Annual Meeting: Orlando, FL, USA, 2013. [Google Scholar]
- Tsuchiya, M. Possible Mechanism of Low Endotoxin Recovery. Am. Pharm. Rev. 2014, 17, 1–5. [Google Scholar]
- Tsuchiya, M. Sample Treatments That Solve Low Endotoxin Recovery Issues. PDA J. Pharm. Sci. Technol. 2019, 73, 433–442. [Google Scholar] [CrossRef]
- Wespel, M.; Geiss, M.; Nägele, M.; Combé, S.; Reich, J.; Studts, J.; Stolzenberger, J. The Impact of Endotoxin Masking on the Removal of Endotoxin during Manufacturing of a Biopharmaceutical Drug Product. J. Chromatogr. A 2022, 1671, 462995. [Google Scholar] [CrossRef] [PubMed]
- Correa, W.; Brandenburg, K.; Zähringer, U.; Ravuri, K.; Khan, T.; Von Wintzingerode, F. Biophysical Analysis of Lipopolysaccharide Formulations for an Understanding of the Low Endotoxin Recovery (LER) Phenomenon. Int. J. Mol. Sci. 2017, 18, 2737. [Google Scholar] [CrossRef] [PubMed]
- Rudbach, J.A.; Johnson, A.G. Restoration of Endotoxin Activity Following Alteration by Plasma. Nature 1964, 202, 811–812. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Lee, E.; Shin, S.; Jeong, K.; Lee, J.-Y.; Bae, S.-Y.; Kim, S.-H.; Lee, J.; Kim, S.R.; Lee, D.G.; et al. Structure and Function of Papiliocin with Antimicrobial and Anti-Inflammatory Activities Isolated from the Swallowtail Butterfly, Papilio xuthus. J. Biol. Chem. 2011, 286, 41296–41311. [Google Scholar] [CrossRef]
- Lin, M.-C.; Pan, C.-Y.; Hui, C.-F.; Chen, J.-Y.; Wu, J.-L. Shrimp Anti-Lipopolysaccharide Factor (SALF), an Antimicrobial Peptide, Inhibits Proinflammatory Cytokine Expressions through the MAPK and NF-κB Pathways in LPS-Induced HeLa Cells. Peptides 2013, 40, 42–48. [Google Scholar] [CrossRef]
- Tani, T.; Shoji, H.; Guadagni, G.; Perego, A. Extracorporeal Removal of Endotoxin: The Polymyxin B-Immobilized Fiber Cartridge. In Contributions to Nephrology; S. Karger AG: Basel, Switzerland, 2010; Volume 167, pp. 35–44. ISBN 9783805594844. [Google Scholar] [CrossRef]
- Zuo, M.Y.; Chen, L.J.; Jiang, H.; Tan, L.; Luo, Z.F.; Wang, Y.M. Detecting Endotoxin with a Flow Cytometry-Based Magnetic Aptasensor. Anal. Biochem. 2014, 466, 38–43. [Google Scholar] [CrossRef]
- Thakur, M.; Dan, A. Poly- l -Lysine-Functionalized Green-Light-Emitting Carbon Dots as a Fluorescence Turn-on Sensor for Ultrasensitive Detection of Endotoxin. ACS Appl. Bio Mater. 2021, 4, 3410–3422. [Google Scholar] [CrossRef]
- McInerney, M.P.; Roberts, K.D.; Thompson, P.E.; Li, J.; Nation, R.L.; Velkov, T.; Nicolazzo, J.A. Quantitation of Polymyxin–Lipopolysaccharide Interactions Using an Image-Based Fluorescent Probe. J. Pharm. Sci. 2016, 105, 1006–1010. [Google Scholar] [CrossRef]
- Durai, P.; Lee, Y.; Kim, J.; Jeon, D.; Kim, Y. Biophysical Studies Reveal Key Interactions between Papiliocin-Derived PapN and Lipopolysaccharide in Gram-Negative Bacteria. J. Microbiol. Biotechnol. 2018, 28, 671–678. [Google Scholar] [CrossRef]
- Brandenburg, K.; Koch, M.H.J.; Seydel, U. Biophysical Characterisation of Lysozyme Binding to LPS Re and Lipid A. Eur. J. Biochem. 1998, 258, 686–695. [Google Scholar] [CrossRef]
- Krishnan, M.; Choi, J.; Jang, A.; Choi, S.; Yeon, J.; Jang, M.; Lee, Y.; Son, K.; Shin, S.Y.; Jeong, M.S.; et al. Molecular Mechanism Underlying the TLR4 Antagonistic and Antiseptic Activities of Papiliocin, an Insect Innate Immune Response Molecule. Proc. Natl. Acad. Sci. USA 2022, 119, e2115669119. [Google Scholar] [CrossRef] [PubMed]
- Memarpoor-Yazdi, M.; Zare-Zardini, H.; Asoodeh, A. A Novel Antimicrobial Peptide Derived from the Insect Paederus dermatitis. Int. J. Pept. Res. Ther. 2013, 19, 99–108. [Google Scholar] [CrossRef]
- Bhunia, A.; Domadia, P.N.; Bhattacharjya, S. Structural and Thermodynamic Analyses of the Interaction between Melittin and Lipopolysaccharide. BBA—Biomembr. 2007, 1768, 3282–3291. [Google Scholar] [CrossRef] [PubMed]
- Kloczewiak, M.; Black, K.M.; Loiselle, P.; Cavaillon, J.-M.; Wainwright, N.; Warren, H.S. Synthetic Peptides That Mimic The Binding Site Of Horseshoe Crab Antilipopolysaccharide Factor. J. Infect. Dis. 1994, 170, 1490–1497. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Takahashi, T.; Shibata, T.; Ikeda, S.; Koshiba, T.; Mizumura, H.; Oda, T.; Kawabata, S. Factor B Is the Second Lipopolysaccharide-Binding Protease Zymogen in the Horseshoe Crab Coagulation Cascade. J. Biol. Chem. 2015, 290, 19379–19386. [Google Scholar] [CrossRef]
- Ariki, S.; Koori, K.; Osaki, T.; Motoyama, K.; Inamori, K.; Kawabata, S. A Serine Protease Zymogen Functions as a Pattern-Recognition Receptor for Lipopolysaccharides. Proc. Natl. Acad. Sci. USA 2004, 101, 953–958. [Google Scholar] [CrossRef]
- Shin, H.J.; Lee, H.; Park, J.D.; Hyun, H.C.; Sohn, H.O.; Lee, D.W.; Kim, Y.S. Kinetics of Binding of LPS to Recombinant CD14, TLR4, and MD-2 Proteins. Mol. Cells 2007, 24, 119–124. [Google Scholar]
- Elass-Rochard, E.; Roseanu, A.; Legrand, D.; Trif, M.; Salmon, V.; Motas, C.; Montreuil, J.; Spik, G. Lactoferrin-Lipopolysaccharide Interaction: Involvement of the 28-34 Loop Region of Human Lactoferrin in the High-Affinity Binding to Escherichia Coli 055B5 Lipopolysaccharide. Biochem. J. 1995, 312, 839–845. [Google Scholar] [CrossRef]
- Ahlstrand, T.; Kovesjoki, L.; Maula, T.; Oscarsson, J.; Ihalin, R. Aggregatibacter Actinomycetemcomitans LPS Binds Human Interleukin-8. J. Oral Microbiol. 2018, 11, 1549931. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.H.; Oh, Y.J.; Kim, E.S.; Choi, J.E.; Shin, J.-S. High Mobility Group Box 1 Protein Binding to Lipopolysaccharide Facilitates Transfer of Lipopolysaccharide to CD14 and Enhances Lipopolysaccharide-Mediated TNF-α Production in Human Monocytes. J. Immunol. 2008, 180, 5067–5074. [Google Scholar] [CrossRef]
- Jiang, Z.; Hong, Z.; Guo, W.; Xiaoyun, G.; Gengfa, L.; Yongning, L.; Guangxia, X. A Synthetic Peptide Derived from Bactericidal/Permeability-Increasing Protein Neutralizes Endotoxin in Vitro and in Vivo. Int. Immunopharmacol. 2004, 4, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O.; Giacometti, A.; Ghiselli, R.; Bergnach, C.; Orlando, F.; Silvestri, C.; Mocchegiani, F.; Licci, A.; Skerlavaj, B.; Rocchi, M.; et al. LL-37 Protects Rats against Lethal Sepsis Caused by Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2006, 50, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Deris, Z.Z.; Swarbrick, J.D.; Roberts, K.D.; Azad, M.A.K.; Akter, J.; Horne, A.S.; Nation, R.L.; Rogers, K.L.; Thompson, P.E.; Velkov, T.; et al. Probing the Penetration of Antimicrobial Polymyxin Lipopeptides into Gram-Negative Bacteria. Bioconjug. Chem. 2014, 25, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Vijayan, V.; Ahmedi, S.; Pant, P.; Manzoor, N.; Singh, T.P.; Sharma, P.; Sharma, S. Lactosmart: A Novel Therapeutic Molecule for Antimicrobial Defense. Front. Microbiol. 2021, 12, 672589. [Google Scholar] [CrossRef]
- Luo, J.-C.; Zhang, J.; Sun, L. A G-Type Lysozyme from Deep-Sea Hydrothermal Vent Shrimp Kills Selectively Gram-Negative Bacteria. Molecules 2021, 26, 7624. [Google Scholar] [CrossRef]
- Skerlavaj, B.; Benincasa, M.; Risso, A.; Zanetti, M.; Gennaro, R. SMAP-29: A Potent Antibacterial and Antifungal Peptide from Sheep Leukocytes. FEBS Lett. 1999, 463, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Richard, G.; Roger MacKenzie, C.; Henry, K.A.; Vinogradov, E.; Christopher Hall, J.; Hussack, G. Antibody Binding to the O-Specific Antigen of Pseudomonas Aeruginosa O6 Inhibits Cell Growth. Antimicrob. Agents Chemother. 2020, 64, e02168-19. [Google Scholar] [CrossRef]
- Yibin, G.; Jiang, Z.; Hong, Z.; Gengfa, L.; Liangxi, W.; Guo, W.; Yongling, L. A Synthesized Cationic Tetradecapeptide from Hornet Venom Kills Bacteria and Neutralizes Lipopolysaccharide in Vivo and in Vitro. Biochem. Pharmacol. 2005, 70, 209–219. [Google Scholar] [CrossRef]
- Kunstmann, S.; Engström, O.; Wehle, M.; Widmalm, G.; Santer, M.; Barbirz, S. Increasing the Affinity of an O-Antigen Polysaccharide Binding Site in Shigella flexneri Bacteriophage Sf6 Tailspike Protein. Chem.—A Eur. J. 2020, 26, 7263–7273. [Google Scholar] [CrossRef]
- Baxa, U.; Cooper, A.; Weintraub, A.; Pfeil, W.; Seckler, R. Enthalpic Barriers to the Hydrophobic Binding of Oligosaccharides to Phage P22 Tailspike Protein. Biochemistry 2001, 40, 5144–5150. [Google Scholar] [CrossRef]
- Andres, D.; Baxa, U.; Hanke, C.; Seckler, R.; Barbirz, S. Carbohydrate Binding of Salmonella Phage P22 Tailspike Protein and Its Role during Host Cell Infection. Biochem. Soc. Trans. 2010, 38, 1386–1389. [Google Scholar] [CrossRef] [PubMed]
- Petruk, G.; Puthia, M.; Petrlova, J.; Samsudin, F.; Strömdahl, A.C.; Cerps, S.; Uller, L.; Kjellström, S.; Bond, P.J.; Schmidtchen, A. SARS-CoV-2 Spike Protein Binds to Bacterial Lipopolysaccharide and Boosts Proinflammatory Activity. J. Mol. Cell Biol. 2020, 12, 916–932. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.S.; Ng, M.L.P.; Yau, Y.H.; Chong, P.K.W.; Ho, B.; Ding, J.L. Definition of Endotoxin Binding Sites in Horseshoe Crab Factor C Recombinant Sushi Proteins and Neutralization of Endotoxin by Sushi Peptides. FASEB J. 2000, 14, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.; Nyström, T.; de Cock, H.; Bennich, H. Attacin—An Insect Immune Protein—Binds LPS and Triggers the Specific Inhibition of Bacterial Outer-Membrane Protein Synthesis. Microbiology 1998, 144, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Shiga, T.; Shibata, T.; Sako, M.; Maenaka, K.; Koshiba, T.; Mizumura, H.; Oda, T.; Kawabata, S. The N-Terminal Arg Residue Is Essential for Autocatalytic Activation of a Lipopolysaccharide-Responsive Protease Zymogen. J. Biol. Chem. 2014, 289, 25987–25995. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T.; Kamiya, M.; Aizawa, T.; Kumaki, Y.; Kikukawa, T.; Mizuguchi, M.; Demura, M.; Kawabata, S.; Kawano, K. Interaction between Tachyplesin I, an Antimicrobial Peptide Derived from Horseshoe Crab, and Lipopolysaccharide. BBA—Proteins Proteom. 2014, 1844, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wohland, T.; Ho, B.; Jeak, L.D. Perturbation of Lipopolysaccharide (LPS) Micelles by Sushi 3 (S3) Antimicrobial Peptide: The Importance of an Intermolecular Disulfide Bond in S3 Dimer for Binding, Disruption, and Neutralization of LPS. J. Biol. Chem. 2004, 279, 50150–50156. [Google Scholar] [CrossRef]
- Ren, J.-D.; Gu, J.-S.; Gao, H.-F.; Xia, P.-Y.; Xiao, G.-X. A Synthetic Cyclic Peptide Derived from Limulus Anti-Lipopolysaccharide Factor Neutralizes Endotoxin in Vitro and in Vivo. Int. Immunopharmacol. 2008, 8, 775–781. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, L.; Eckert, T.; Burg-Roderfeld, M.; Rojas-Macias, M.A.; Lütteke, T.; Krylov, V.B.; Argunov, D.A.; Datta, A.; Markart, P.; et al. Lysozyme’s Lectin-like Characteristics Facilitates Its Immune Defense Function. Q. Rev. Biophys. 2017, 50, e9. [Google Scholar] [CrossRef]
- Yu, H.; Dong, J.; Gu, Y.; Liu, H.; Xin, A.; Shi, H.; Sun, F.; Zhang, Y.; Lin, D.; Diao, H. The Novel Human β-Defensin 114 Regulates Lipopolysaccharide (LPS)-Mediated Inflammation and Protects Sperm from Motility Loss. J. Biol. Chem. 2013, 288, 12270–12282. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H.; Gu, Y.; Xin, A.; Zhang, Y.; Diao, H.; Lin, D. Human Beta-Defensin DEFB126 Is Capable of Inhibiting LPS-Mediated Inflammation. Appl. Microbiol. Biotechnol. 2013, 97, 3395–3408. [Google Scholar] [CrossRef] [PubMed]
- Tobias, P.S.; Soldau, K.; Gegner, J.A.; Mintz, D.; Ulevitch, R.J. Lipopolysaccharide Binding Protein-Mediated Complexation of Lipopolysaccharide with Soluble CD14. J. Biol. Chem. 1995, 270, 10482–10488. [Google Scholar] [CrossRef] [PubMed]
- Gazzano-Santoro, H.; Parent, J.B.; Grinna, L.; Horwitz, A.; Parsons, T.; Theofan, G.; Elsbach, P.; Weiss, J.; Conlon, P.J. High-Affinity Binding of the Bactericidal/Permeability-Increasing Protein and a Recombinant Amino-Terminal Fragment to the Lipid A Region of Lipopolysaccharide. Infect. Immun. 1992, 60, 4754–4761. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y. Binding of a Histidine-Rich Peptide to Porphyromonas gingivalis. FEMS Microbiol. Lett. 1991, 82, 253–256. [Google Scholar] [CrossRef]
- Appelmelk, B.J.; An, Y.Q.; Geerts, M.; Thijs, B.G.; de Boer, H.A.; MacLaren, D.M.; de Graaff, J.; Nuijens, J.H. Lactoferrin Is a Lipid A-Binding Protein. Infect. Immun. 1994, 62, 2628–2632. [Google Scholar] [CrossRef]
- De Haas, C.J.C.; Haas, P.J.; Van Kessel, K.P.M.; Van Strijp, J.A.G. Affinities of Different Proteins and Peptides for Lipopolysaccharide as Determined by Biosensor Technology. Biochem. Biophys. Res. Commun. 1998, 252, 492–496. [Google Scholar] [CrossRef]
- de Haas, C.J.C.; van der Tol, M.E.; Van Kessel, K.P.M.; Verhoef, J.; Van Strijp, J.A.G. A Synthetic Lipopolysaccharide-Binding Peptide Based on Amino Acids 27–39 of Serum Amyloid P Component Inhibits Lipopolysaccharide-Induced Responses in Human Blood. J. Immunol. 1998, 161, 3607–3615. [Google Scholar] [CrossRef]
- Larrick, J.W.; Hirata, M.; Balint, R.F.; Lee, J.; Zhong, J.; Wright, S.C. Human CAP18: A Novel Antimicrobial Lipopolysaccharide-Binding Protein. Infect. Immun. 1995, 63, 1291–1297. [Google Scholar] [CrossRef]
- Al-Adwani, S.; Wallin, C.; Balhuizen, M.D.; Veldhuizen, E.J.A.; Coorens, M.; Landreh, M.; Végvári, Á.; Smith, M.E.; Qvarfordt, I.; Lindén, A.; et al. Studies on Citrullinated LL-37: Detection in Human Airways, Antibacterial Effects and Biophysical Properties. Sci. Rep. 2020, 10, 2376. [Google Scholar] [CrossRef]
- Youn, J.H.; Kwak, M.S.; Wu, J.; Kim, E.S.; Ji, Y.; Min, H.J.; Yoo, J.H.; Choi, J.E.; Cho, H.S.; Shin, J.S. Identification of Lipopolysaccharide-Binding Peptide Regions within HMGB1 and Their Effects on Subclinical Endotoxemia in a Mouse Model. Eur. J. Immunol. 2011, 41, 2753–2762. [Google Scholar] [CrossRef]
- Ghiselli, R.; Giacometti, A.; Cirioni, O.; Circo, R.; Mocchegiani, F.; Skerlavaj, B.; D’Amato, G.; Scalise, G.; Zanetti, M.; Saba, V. Neutralization of Endotoxin in Vitro and in Vivo by Bac7(1-35), a Proline-Rich Antibacterial Peptide. Shock 2003, 19, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Zughaier, S.; Svoboda, P.; Pohl, J. Structure-Dependent Immune Modulatory Activity of Protegrin-1 Analogs. Antibiotics 2014, 3, 694–713. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Dai, H.; Bommineni, Y.R.; Soulages, J.L.; Gong, Y.-X.; Prakash, O.; Zhang, G. Structure-Activity Relationships of Fowlicidin-1, a Cathelicidin Antimicrobial Peptide in Chicken. FEBS J. 2006, 273, 2581–2593. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Sugishita, K.; Miyajima, K. Interactions of an Antimicrobial Peptide, Magainin 2, with Lipopolysaccharide-Containing Liposomes as a Model for Outer Membranes of Gram-Negative Bacteria. FEBS Lett. 1999, 449, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, I.; Yomogida, S.; Tamura, H.; Hirata, M. Antibacterial Cathelicidin Peptide CAP11 Inhibits the Lipopolysaccharide (LPS)-Induced Suppression of Neutrophil Apoptosis by Blocking the Binding of LPS to Target Cells. Inflamm. Res. 2004, 53, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mu, L.; Zhuang, L.; Han, Y.; Liu, T.; Li, J.; Yang, Y.; Yang, H.; Wei, L. A Cecropin-like Antimicrobial Peptide with Anti-Inflammatory Activity from the Black Fly Salivary Glands. Parasit. Vectors 2015, 8, 561. [Google Scholar] [CrossRef]
- Meadows, C. Aseptic Sampling Best Practices Endotoxin Binding Affinity. Sartorius 2018, SP-4006-e, 1–6. [Google Scholar]
- Novitsky, T. The Problems with Plastics. Lal Update 1988, 6, 1–4. [Google Scholar]
- Péterfi, Z.; Kocsis, B. Comparison of Blocking Agents for an ELISA for LPS. J. Immunoass. 2000, 21, 341–354. [Google Scholar] [CrossRef]
- Kempler, G.; Ray, B. Nature of Freezing Damage on the Lipopolysaccharide Molecule of Escherichia coli B. Cryobiology 1978, 15, 578–584. [Google Scholar] [CrossRef]
- Douwes, J.; Versloot, P.; Hollander, A.; Heederik, D.; Doekes, G. Influence of Various Dust Sampling and Extraction Methods on the Measurement of Airborne Endotoxin. Appl. Environ. Microbiol. 1995, 61, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.M.; Seaver, A.; Calcott, P.H. Effect of Defined Lipopolysaccharide Core Defects on Resistance of Salmonella Typhimurium to Freezing and Thawing and Other Stresses. Appl. Environ. Microbiol. 1981, 42, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.P.; Tran, L.V.H.; Namgoong, H.; Kim, Y.H. Applications of Different Solvents and Conditions for Differential Extraction of Lipopolysaccharide in Gram-Negative Bacteria. J. Microbiol. 2019, 57, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Papadiamantis, A.G.; Klaessig, F.C.; Exner, T.E.; Hofer, S.; Hofstaetter, N.; Himly, M.; Williams, M.A.; Doganis, P.; Hoover, M.D.; Afantitis, A.; et al. Metadata Stewardship in Nanosafety Research: Community-Driven Organisation of Metadata Schemas to Support FAIR Nanoscience Data. Nanomaterials 2020, 10, 2033. [Google Scholar] [CrossRef] [PubMed]
- PubMed Keyword “Endotoxin” 5203 Events, Keyword “Lipopolysaccharide” 7853 Events. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 12 February 2023).





| Bacteria | Sugar Groups * | Phosphate Groups ** | Lipid A Acyl Chains | Length of Acyl Chains | Agonistic Activity *** | Citation |
|---|---|---|---|---|---|---|
| Acinetobacter baumannii | 2 | 6 (4 + 2) 7 (4 + 3) | 12–14 | Agonist | [46] | |
| Actinobacillus actinomycetemcomitans | 2 | 6 (4 + 2) | 14 | Agonist | [47] | |
| Alcaligenes faecalis | 2 | 6 (3 + 3) | 10–14 | Weak agonist | [48] | |
| Aquifex pyrophilus | 2 (1 + 1) | 0 | 5 (3 + 2) | 14–18 | Weak/No agonist | [23] |
| Bacteroides fragilis | 1 (0 + 1) | 5 (3 + 2) | 15–17 | Weak agonist | [49] | |
| Bacteroides vulgatus | 1 (0 + 1) | 5 (3 + 2) | 15–17 | Weak agonist | [50] | |
| Bartonella quintana | 2 | 5 (3 + 2) | 12–26 | Antagonist | [51] | |
| Bordetella parapertussis | 2 | 6 (4 + 2) | 10–16 | Weak/No agonist | [34] | |
| Bordetella pertussis | 2 | 5 (3 + 2) | 10–14 | Agonist | [34] | |
| Bradyrhizobium elkanii | 3 (2 + 1) | 0 | 6 (4 + 2) | 12–28 | Weak agonist/ antagonist | [24] |
| Brucella spp. | 1 (1 + 0) | 7 (4 + 3) | 12–16 | - | [30] | |
| Burkholderia multivorans | 2 (1 + 1) | 2 | 5 (3 + 2) | 14–16 | Agonist | [52] |
| Campylobacter jejuni | 2 (2 + 0) | 6 (4 + 2) | 14–16 | Agonist | [53] | |
| Chlamydia trachomatis | 2 | 5 (3 + 2) | 14–20 | Antagonist | [54] | |
| Chromobacterium violaceum | 2 | 6 (3 + 3) | 10–12 | Antagonist | [55] | |
| Colwellia hornerae | 2 | 5 (3 + 2) | 9–14 | - | [56] | |
| Colwellia piezophila | 2 | 5 (3 + 2) | 9–14 | - | [56] | |
| Echinicola pacifica | 1 (1 + 0) | 1 (0 + 1) | 4 (2 + 2) | 15–17 | Antagonist | [26] |
| Echinicola vietnamensis | 1 (1 + 0) | 1 (0 + 1) | 4 (2 + 2) | 15–16 | Antagonist | [26] |
| Escherichia coli | 2 | 6 (4 + 2) | 12–14 | Agonist | [57] | |
| Escherichia coli (12 °C) | 2 | 6 (3 + 3) | 12–14 | - | [40] | |
| Francisella tularensis | 1 (0 + 1) | 1 (0 + 1) | 4 (2 + 2) | 16–18 | No agonist and no antagonist | [58,59] |
| Fusobacterium nucleatum | 2 | 6 (4 + 2) | 14–16 | Agonist | [60] | |
| Haemophilus influenzae | 2 | 6 (4 + 2) | 14 | Agonist | [61] | |
| Halobacteroides lacunaris | 2 | 6 (3 + 3) | 10–12 | Antagonist | [62] | |
| Helicobacter pylori | Minor 2 Major 1 (0 + 1) | Minor 6 (4 + 2) Major 4 (2 + 2) | Minor 12–18 Major 16–18 | Major antagonist | [63] | |
| Klebsiella pneumoniae | 2 | 6 (4 + 2) | 12–14 | Agonist | [64] | |
| Legionella pneumophila | 2 | 6 (4 + 2) | 14–27 | No agonist | [27] | |
| Liberibacter crescens | 1 (0 + 1) | 5 (3 + 2) | 14–28 | - | [65] | |
| Marinomonas vaga | 1 (0 + 1) | 5 (2 + 3) | 10–12 | Weak agonist | [66] | |
| Moraxella catarrhalis | 3 (1 + 2) | 7 (3 + 4) | 10–12 | Agonist | [21,67] | |
| Neisseria meningitidis | 2 | 6 (3 + 3) | 12–14 | Agonist | [68] | |
| Pasteurella multocida (major) | 1 (1 + 0) | 5 (4 + 1) | 14 | - | [35] | |
| Pasteurella multocida (minor) | 1 (1 + 0) | 2 | 6 (4 + 2) | 14 | Agonist | [35] |
| Porphyromonas gingivalis | 1 (0 + 1) | 5 (3 + 2) | 15–17 | Weak agonist | [69] | |
| Proteus mirabilis | 1 (1 + 0) | 2 | 7 (4 + 3) | 14–16 | - | [70] |
| Pseudomonas aeruginosa | 2 | 6 (3 + 3) | 10–12 | - | [71] | |
| Psychrobacter cryohalolentis | 2 | 6 (4 + 2) | 8–12 | - | [56] | |
| Psychromonas marina | 2 | 6 (4 + 2) | 12–14 | - | [72] | |
| Ralstonia eutropha | 1 (1 + 0) | 2 (1 + 1) | 6 (3 + 3) | 14 | Weak Agonist | [73] |
| Ralstonia mannitolilytica | 2 (1 + 1) | 2 (1 + 1) | 6 (3 + 3) | 14–16 | Agonist | [73] |
| Ralstonia pickettii | 2 (1 + 1) | 2 (1 + 1) | 5 (3 + 2) | 14 | No agonist | [73] |
| Rhizobium leguminosarum | 1 (1 + 0) | 0 | 5 (3 + 2) | 14–28 | - | [25] |
| Rhodobacter capsulatus | 3 (1 + 2) | 5 (3 + 2) | 10–14 | Antagonist | [20] | |
| Rhodobacter sphaeroides | 2 | 5 (3 + 2) | 10–14 | Antagonist | [33] | |
| Salmonella minnesota | 2 | 7 (4 + 3) | 12–16 | Agonist | [74] | |
| Salmonella typhimurium | 2 | 6 (4 + 2) | 12–14 | Agonist | [75] | |
| Serratia marcescens | 2 | 5 (4 + 1) | 14 | Agonist | [76] | |
| Sphaerotilus natans | 3 (2 + 1) | 6 (3 + 3) | 10–14 | Agonist | [22] | |
| Spiribacter salinus | 2 | 5 (2 + 3) | 10–14 | - | [77] | |
| Vibrio cholerae | 2 | 6 (4 + 2) | 12–14 | - | [78] | |
| Vibrio fischeri | 1 (1 + 0) | 5 (3 + 2) | 12–14 | - | [32] | |
| Yersinia pestis | 2 | 25–27 °C: 6 (4 + 2) 37 °C: 4 (2 + 2) | 12–16 14 | Agonist No agonist | [41,79] | |
| Zunongwangia profunda | 1 (0 + 1) | Minor 4 (2 + 2) Major 5 (3 + 2) | Minor 15–17 Major 15–17 | - | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fux, A.C.; Casonato Melo, C.; Michelini, S.; Swartzwelter, B.J.; Neusch, A.; Italiani, P.; Himly, M. Heterogeneity of Lipopolysaccharide as Source of Variability in Bioassays and LPS-Binding Proteins as Remedy. Int. J. Mol. Sci. 2023, 24, 8395. https://doi.org/10.3390/ijms24098395
Fux AC, Casonato Melo C, Michelini S, Swartzwelter BJ, Neusch A, Italiani P, Himly M. Heterogeneity of Lipopolysaccharide as Source of Variability in Bioassays and LPS-Binding Proteins as Remedy. International Journal of Molecular Sciences. 2023; 24(9):8395. https://doi.org/10.3390/ijms24098395
Chicago/Turabian StyleFux, Alexandra C., Cristiane Casonato Melo, Sara Michelini, Benjamin J. Swartzwelter, Andreas Neusch, Paola Italiani, and Martin Himly. 2023. "Heterogeneity of Lipopolysaccharide as Source of Variability in Bioassays and LPS-Binding Proteins as Remedy" International Journal of Molecular Sciences 24, no. 9: 8395. https://doi.org/10.3390/ijms24098395