The Metabolically Obese, Normal-Weight Phenotype in Young Rats Is Associated with Cognitive Impairment and Partially Preventable with Leptin Intake during Lactation
Abstract
:1. Introduction
2. Results
2.1. Body Weight, Adiposity, and Serum Parameters
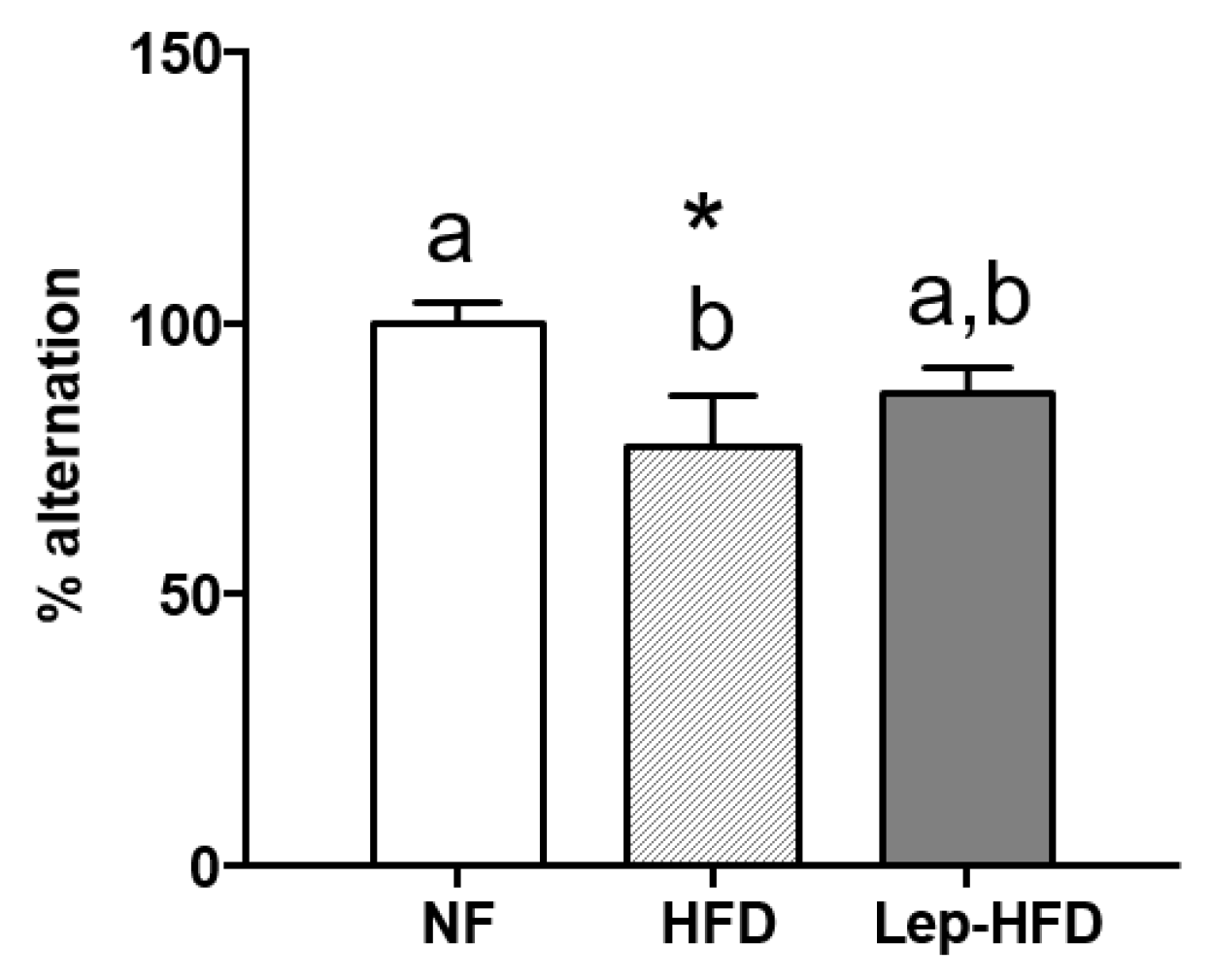
2.2. Effects on the Working Memory of a Young MONW Phenotype Induced with Isocaloric HFD Feeding in Animals That Received or Did Not Receive Leptin Supplementation during Lactation
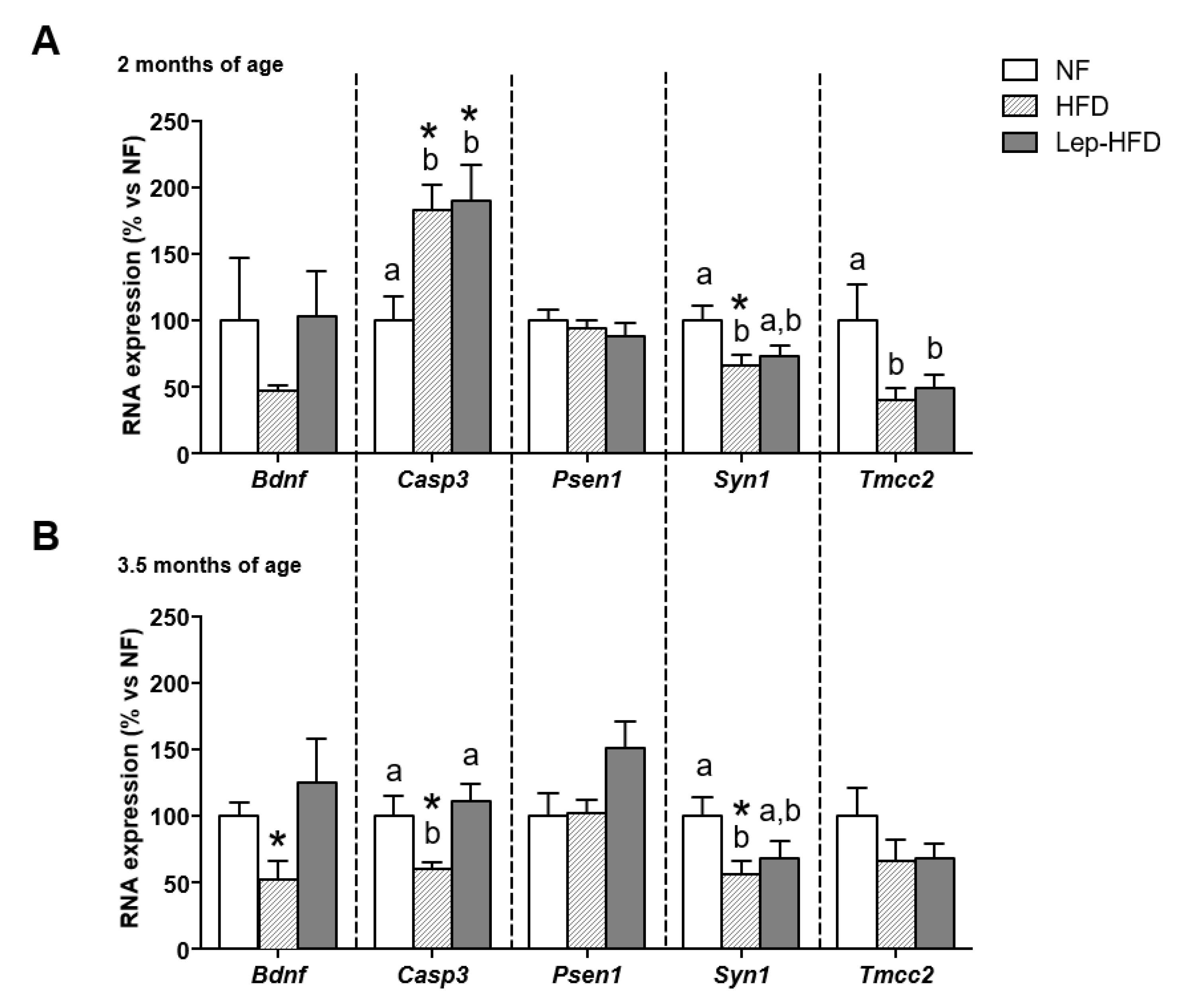
2.3. Effects on Hippocampal Gene Expression of a Young MONW Phenotype Induced with Isocaloric HFD Feeding in Animals That Received or Did Not Receive Leptin Supplementation during Lactation
2.4. Differential Expression of Genes Related to Cognitive Impairment in PBMCs of Rats with a Young MONW Phenotype Induced with Isocaloric HFD Feeding after Receiving or Not Receiving Leptin Supplementation during Lactation
2.5. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Adiposity
4.3. Blood Collection and PBMC Isolation
4.4. Quantification of Circulating Insulin, Leptin, and Glucose Levels
4.5. Total RNA Isolation
4.6. Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-qPCR) Analysis
4.7. Behavioural Testing: T-Maze Alternation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez-Miranda, J.; Perez-Martinez, P. It Is Time to Define Metabolically Obese but Normal-Weight (MONW) Individuals. Clin. Endocrinol. 2013, 79, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Pluta, W.; Dudzińska, W.; Lubkowska, A. Metabolic Obesity in People with Normal Body Weight (MONW)—Review of Diagnostic Criteria. Int. J. Environ. Res. Public Health 2022, 19, 624. [Google Scholar] [CrossRef]
- Bel-Serrat, S.; Mouratidou, T.; Huybrechts, I.; Labayen, I.; Cuenca-García, M.; Palacios, G.; Breidenassel, C.; Molnár, D.; Roccaldo, R.; Widhalm, K.; et al. Associations between Macronutrient Intake and Serum Lipid Profile Depend on Body Fat in European Adolescents: The Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) Study. Br. J. Nutr. 2014, 112, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Labayen, I.; Ruiz, J.R.; Ortega, F.B.; Huybrechts, I.; Rodríguez, G.; Jiménez-Pavón, D.; Roccaldo, R.; Nova, E.; Widhalm, K.; Kafatos, A.; et al. High Fat Diets Are Associated with Higher Abdominal Adiposity Regardless of Physical Activity in Adolescents; the HELENA Study. Clin. Nutr. 2014, 33, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Se-Young, O.; Lee, D.; Tak, S.; Hong, M.; Park, S.M.; Cho, B.; Park, M. Characteristics of Diet Patterns in Metabolically Obese, Normal Weight Adults (Korean National Health and Nutrition Examination Survey III, 2005). Nutr. Metab. Cardiovasc. Dis. 2012, 22, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhuang, R.; Luo, X.; Yin, L.; Pang, C.; Feng, T.; You, H.; Zhai, Y.; Ren, Y.; Zhang, L.; et al. Prevalence of Metabolically Healthy Obese and Metabolically Obese but Normal Weight in Adults Worldwide: A Meta-Analysis. Horm. Metab. Res. 2015, 47, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Yates, K.F.; Sweat, V.; Yau, P.L.; Turchiano, M.M.; Convit, A. Impact of Metabolic Syndrome on Cognition and Brain. Arter. Thromb. Vasc. Biol. 2012, 32, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Flores-Cordero, J.A.; Pérez-Pérez, A.; Jiménez-Cortegana, C.; Alba, G.; Flores-Barragán, A.; Sánchez-Margalet, V. Obesity as a Risk Factor for Dementia and Alzheimer’s Disease: The Role of Leptin. Int. J. Mol. Sci. 2022, 23, 5202. [Google Scholar] [CrossRef]
- Cifre, M.; Palou, A.; Oliver, P. Cognitive Impairment in Metabolically-Obese, Normal-Weight Rats: Identification of Early Biomarkers in Peripheral Blood Mononuclear Cells. Mol. Neurodegener. 2018, 13, 14. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Su, F.; Li, C.; Chen, M. Immune Abnormalities and Differential Gene Expression in the Hippocampus and Peripheral Blood of Patients with Alzheimer’s Disease. Ann. Transl. Med. 2022, 10, 29. [Google Scholar] [CrossRef]
- Preston, J.D.; Reynolds, L.J.; Pearson, K.J. Developmental Origins of Health Span and Life Span: A Mini-Review. Gerontology 2018, 64, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Finn, S.; Culligan, E.P.; Snelling, W.J.; Sleator, R.D. Early Life Nutrition. Sci. Prog. 2018, 101, 332–359. [Google Scholar] [CrossRef]
- Palou, M.; Picó, C.; Palou, A. Leptin as a Breast Milk Component for the Prevention of Obesity. Nutr. Rev. 2018, 76, 875–892. [Google Scholar] [CrossRef]
- Picó, C.; Reis, F.; Egas, C.; Mathias, P.; Matafome, P. Lactation as a Programming Window for Metabolic Syndrome. Eur. J. Clin. Investig. 2021, 51, e13482. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, P.J.; Naninck, E.F.G.; van Goudoever, J.B.; Fitzsimons, C.; Joels, M.; Korosi, A. Perinatal Programming of Adult Hippocampal Structure and Function; Emerging Roles of Stress, Nutrition and Epigenetics. Trends Neurosci. 2013, 36, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, E.B.; Fischl, B.R.; Quinn, B.T.; Chong, W.K.; Gadian, D.G.; Lucas, A. Impact of Breast Milk on Intelligence Quotient, Brain Size, and White Matter Development. Pediatr. Res. 2010, 67, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Gillman, M.W. Risk of Overweight Among Adolescents Who Were Breastfed as Infants. JAMA 2001, 285, 2461. [Google Scholar] [CrossRef]
- Harder, T.; Bergmann, R.; Kallischnigg, G.; Plagemann, A. Duration of Breastfeeding and Risk of Overweight: A Meta-Analysis. Am. J. Epidemiol. 2005, 162, 397–403. [Google Scholar] [CrossRef]
- Granado, M.; Fuente-Martín, E.; García-Cáceres, C.; Argente, J.; Chowen, J.A. Leptin in Early Life: A Key Factor for the Development of the Adult Metabolic Profile. Obes. Facts 2012, 5, 138–150. [Google Scholar] [CrossRef]
- Jéquier, E. Leptin Signaling, Adiposity, and Energy Balance. Ann. N. Y. Acad. Sci. 2002, 967, 379–388. [Google Scholar] [CrossRef]
- Casabiell, X.; Piñeiro, V.; Tomé, M.A.; Peinó, R.; Dieguez, C.; Casanueva, F.F. Presence of Leptin in Colostrum and/or Breast Milk from Lactating Mothers: A Potential Role in the Regulation of Neonatal Food Intake. J. Clin. Endocrinol. Metab. 1997, 82, 4270–4273. [Google Scholar] [CrossRef]
- Sánchez, J.; Oliver, P.; Miralles, O.; Ceresi, E.; Picó, C.; Palou, A. Leptin Orally Supplied to Neonate Rats Is Directly Uptaken by the Immature Stomach and May Regulate Short-Term Feeding. Endocrinology 2005, 146, 2575–2582. [Google Scholar] [CrossRef]
- Reynés, B.; Cifre, M.; Palou, A.; Oliver, P. Perinatal Treatment with Leptin, but Not Celastrol, Protects from Metabolically Obese, Normal-Weight Phenotype in Rats. Nutrients 2022, 14, 2277. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Y.; Yang, Y.; Li, Z. Leptin Withdrawal after Birth: A Neglected Factor Account for Cognitive Deficit in Offspring of GDM Mother. Med. Hypotheses 2011, 77, 125–127. [Google Scholar] [CrossRef]
- Bischof, G.N.; Park, D.C. Obesity and Aging. Psychosom. Med. 2015, 77, 697–709. [Google Scholar] [CrossRef]
- Melloni, R.H.; Apostolides, P.J.; Hamos, J.E.; DeGennaro, L.J. Dynamics of Synapsin I Gene Expression during the Establishment and Restoration of Functional Synapses in the Rat Hippocampus. Neuroscience 1994, 58, 683–703. [Google Scholar] [CrossRef]
- Jovanovic, J.N.; Czernik, A.J.; Fienberg, A.A.; Greengard, P.; Sihra, T.S. Synapsins as Mediators of BDNF-Enhanced Neurotransmitter Release. Nat. Neurosci. 2000, 3, 323–329. [Google Scholar] [CrossRef]
- Molteni, R.; Barnard, R.J.; Ying, Z.; Roberts, C.K.; Gómez-Pinilla, F. A High-Fat, Refined Sugar Diet Reduces Hippocampal Brain-Derived Neurotrophic Factor, Neuronal Plasticity, and Learning. Neuroscience 2002, 112, 803–814. [Google Scholar] [CrossRef]
- Hopkins, P.C.R. Neurodegeneration in a Drosophila Model for the Function of TMCC2, an Amyloid Protein Precursor-Interacting and Apolipoprotein E-Binding Protein. PLoS ONE 2013, 8, e55810. [Google Scholar] [CrossRef]
- Puig, K.L.; Floden, A.M.; Adhikari, R.; Golovko, M.Y.; Combs, C.K. Amyloid Precursor Protein and Proinflammatory Changes Are Regulated in Brain and Adipose Tissue in a Murine Model of High Fat Diet-Induced Obesity. PLoS ONE 2012, 7, e30378. [Google Scholar] [CrossRef]
- Mayeux, R.; Hyslop, P.S.G. Alzheimer’s Disease: Advances in Trafficking. Lancet Neurol. 2008, 7, 2–3. [Google Scholar] [CrossRef]
- Asaumi, M. Interaction of N-Terminal Acetyltransferase with the Cytoplasmic Domain of β-Amyloid Precursor Protein and Its Effect on Aβ Secretion. J. Biochem. 2005, 137, 147–155. [Google Scholar] [CrossRef]
- Snigdha, S.; Smith, E.D.; Prieto, G.A.; Cotman, C.W. Caspase-3 Activation as a Bifurcation Point between Plasticity and Cell Death. Neurosci. Bull. 2012, 28, 14–24. [Google Scholar] [CrossRef]
- Liew, C.-C.; Ma, J.; Tang, H.-C.; Zheng, R.; Dempsey, A.A. The Peripheral Blood Transcriptome Dynamically Reflects System Wide Biology: A Potential Diagnostic Tool. J. Lab. Clin. Med. 2006, 147, 126–132. [Google Scholar] [CrossRef]
- Reynés, B.; Priego, T.; Cifre, M.; Oliver, P.; Palou, A. Peripheral Blood Cells, a Transcriptomic Tool in Nutrigenomic and Obesity Studies: Current State of the Art. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1006–1020. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, M.; Wang, F.; Liu, J.; Zheng, H.; Lei, P. Relationship between Normal Weight Obesity and Mild Cognitive Impairment Is Reflected in Cognitive-related Genes in Human Peripheral Blood Mononuclear Cells. Psychogeriatrics 2020, 20, 35–43. [Google Scholar] [CrossRef]
- Altman, J.; Bayer, S.A. Migration and Distribution of Two Populations of Hippocampal Granule Cell Precursors during the Perinatal and Postnatal Periods. J. Comp. Neurol. 1990, 301, 365–381. [Google Scholar] [CrossRef]
- Couce, M.E.; Burguera, B.; Parisi, J.E.; Jensen, M.D.; Lloyd, R.V. Localization of Leptin Receptor in the Human Brain. Neuroendocrinology 1997, 66, 145–150. [Google Scholar] [CrossRef]
- Shioda, S.; Funahashi, H.; Nakajo, S.; Yada, T.; Maruta, O.; Nakai, Y. Immunohistochemical Localization of Leptin Receptor in the Rat Brain. Neurosci. Lett. 1998, 243, 41–44. [Google Scholar] [CrossRef]
- Morrison, C.D. Leptin Signaling in Brain: A Link between Nutrition and Cognition? Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2009, 1792, 401–408. [Google Scholar] [CrossRef]
- Harvey, J. Food for Thought: Leptin and Hippocampal Synaptic Function. Front. Pharmacol. 2022, 13, 882158. [Google Scholar] [CrossRef]
- Yan, B.C.; Choi, J.H.; Yoo, K.-Y.; Lee, C.H.; Hwang, I.K.; You, S.G.; Kang, I.-J.; Kim, J.-D.; Kim, D.; Kim, Y.-M.; et al. Leptin’s Neuroprotective Action in Experimental Transient Ischemic Damage of the Gerbil Hippocampus Is Linked to Altered Leptin Receptor Immunoreactivity. J. Neurol. Sci. 2011, 303, 100–108. [Google Scholar] [CrossRef]
- Irving, A.; Harvey, J. Regulation of Hippocampal Synaptic Function by the Metabolic Hormone Leptin: Implications for Health and Disease. Prog. Lipid Res. 2021, 82, 101098. [Google Scholar] [CrossRef]
- De Strooper, B.; Saftig, P.; Craessaerts, K.; Vanderstichele, H.; Guhde, G.; Annaert, W.; Von Figura, K.; Van Leuven, F. Deficiency of Presenilin-1 Inhibits the Normal Cleavage of Amyloid Precursor Protein. Nature 1998, 391, 387–390. [Google Scholar] [CrossRef]
- Pitsi, D.; Octave, J.-N. Presenilin 1 Stabilizes the C-Terminal Fragment of the Amyloid Precursor Protein Independently of γ-Secretase Activity. J. Biol. Chem. 2004, 279, 25333–25338. [Google Scholar] [CrossRef]
- Erol, A. An Integrated and Unifying Hypothesis for the Metabolic Basis of Sporadic Alzheimer’s Disease. J. Alzheimer’s Dis. 2008, 13, 241–253. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Norman, E.D.; Lee, K.; Cutler, R.G.; Telljohann, R.S.; Egan, J.M.; Mattson, M.P. Diet-Induced Insulin Resistance Impairs Hippocampal Synaptic Plasticity and Cognition in Middle-Aged Rats. Hippocampus 2008, 18, 1085–1088. [Google Scholar] [CrossRef]
- Harvey, J.; Solovyova, N.; Irving, A. Leptin and Its Role in Hippocampal Synaptic Plasticity. Prog. Lipid Res. 2006, 45, 369–378. [Google Scholar] [CrossRef]
- Sripetchwandee, J.; Chattipakorn, N.; Chattipakorn, S.C. Links Between Obesity-Induced Brain Insulin Resistance, Brain Mitochondrial Dysfunction, and Dementia. Front. Endocrinol. 2018, 9, 496. [Google Scholar] [CrossRef]
- Ding, Q.; Zhao, Y.; Yang, Y.; Chen, Z. Cognitive Impairment Due to Leptin Withdrawal in Rat Offspring of Dams with Maternal Diet-Induced Obesity. Med. Sci. Monit. 2018, 24, 6208–6217. [Google Scholar] [CrossRef]
- Beig, A.; Agbaria, R.; Dahan, A. The Use of Captisol (SBE7-β-CD) in Oral Solubility-Enabling Formulations: Comparison to HPβCD and the Solubility–Permeability Interplay. Eur. J. Pharm. Sci. 2015, 77, 73–78. [Google Scholar] [CrossRef]
- Díaz-Rúa, R.; Keijer, J.; Caimari, A.; van Schothorst, E.M.; Palou, A.; Oliver, P. Peripheral Blood Mononuclear Cells as a Source to Detect Markers of Homeostatic Alterations Caused by the Intake of Diets with an Unbalanced Macronutrient Composition. J. Nutr. Biochem. 2015, 26, 398–407. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and β-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Oliver, P.; Reynés, B.; Caimari, A.; Palou, A. Peripheral Blood Mononuclear Cells: A Potential Source of Homeostatic Imbalance Markers Associated with Obesity Development. Pflug. Arch. 2013, 465, 459–468. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Caimari, A.; Oliver, P.; Keijer, J.; Palou, A. Peripheral Blood Mononuclear Cells as a Model to Study the Response of Energy Homeostasis-Related Genes to Acute Changes in Feeding Conditions. OMICS 2010, 14, 129–141. [Google Scholar] [CrossRef]
- Deacon, R.M.J.; Rawlins, J.N.P. T-Maze Alternation in the Rodent. Nat. Protoc. 2006, 1, 7–12. [Google Scholar] [CrossRef]
- d’Isa, R.; Comi, G.; Leocani, L. Apparatus Design and Behavioural Testing Protocol for the Evaluation of Spatial Working Memory in Mice through the Spontaneous Alternation T-Maze. Sci. Rep. 2021, 11, 21177. [Google Scholar] [CrossRef]

| NF | HFD | Lep-HFD | |
|---|---|---|---|
| Body weight (g) | 409 ± 13 | 416 ± 6 | 410 ± 6 |
| Fat mass (%) | 19.4 ± 0.5 a | 23.9 ± 1.8 b,* | 22.9 ± 1.2 a,b,* |
| Adiposity index (%) | 8.36 ± 0.26 a | 11.2 ± 0.6 b,* | 10.4 ± 0.4 b,* |
| Weight of adipose tissues (g) | |||
| Epidiymal fat | 9.59 ± 0.66 a | 13.7 ± 0.5 b,* | 11.1 ± 0.5 a |
| Mesenteric fat | 4.04 ± 0.26 a | 5.46 ± 0.4 b,* | 4.83 ± 0.30 a |
| Retroperitoneal fat | 11.0 ± 0.9 a | 13.1 ± 0.4 b | 13.2 ± 0.7 b |
| Inguinal fat | 9.73 ± 0.86 a | 13.9 ± 1.2 b,* | 13.5 ± 0.9 b,* |
| Visceral fat content (g) | 24.6 ± 1.5 a | 32.3 ± 1.0 b,* | 29.1 ± 1.2 b,* |
| Leptin (ng·ml−1)—feeding | 12.6 ± 1 a | 18.1 ± 2.3 b,* | 12.5 ± 1.3 a |
| Glucose (mg·dl−1)—fasting | 101 ± 3 a | 120 ± 7 b,* | 114 ± 4 a,* |
| Insulin (µg·L−1)—fasting | 0.18 ± 0.02 a | 0.33 ± 0.05 b,* | 0.24 ± 0.03 a,b |
| HOMA-IR | 1.32 ± 0.30 a | 2.5 ± 0.5 b | 1.7 ± 0.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifre, M.; Palou, A.; Oliver, P. The Metabolically Obese, Normal-Weight Phenotype in Young Rats Is Associated with Cognitive Impairment and Partially Preventable with Leptin Intake during Lactation. Int. J. Mol. Sci. 2024, 25, 228. https://doi.org/10.3390/ijms25010228
Cifre M, Palou A, Oliver P. The Metabolically Obese, Normal-Weight Phenotype in Young Rats Is Associated with Cognitive Impairment and Partially Preventable with Leptin Intake during Lactation. International Journal of Molecular Sciences. 2024; 25(1):228. https://doi.org/10.3390/ijms25010228
Chicago/Turabian StyleCifre, Margalida, Andreu Palou, and Paula Oliver. 2024. "The Metabolically Obese, Normal-Weight Phenotype in Young Rats Is Associated with Cognitive Impairment and Partially Preventable with Leptin Intake during Lactation" International Journal of Molecular Sciences 25, no. 1: 228. https://doi.org/10.3390/ijms25010228





