Control Theory and Systems Biology: Potential Applications in Neurodegeneration and Search for Therapeutic Targets
Abstract
1. Introduction
2. Results and Discussion
2.1. Systems Biology, Genome-Scale Metabolic Models (GEMs), and Omics
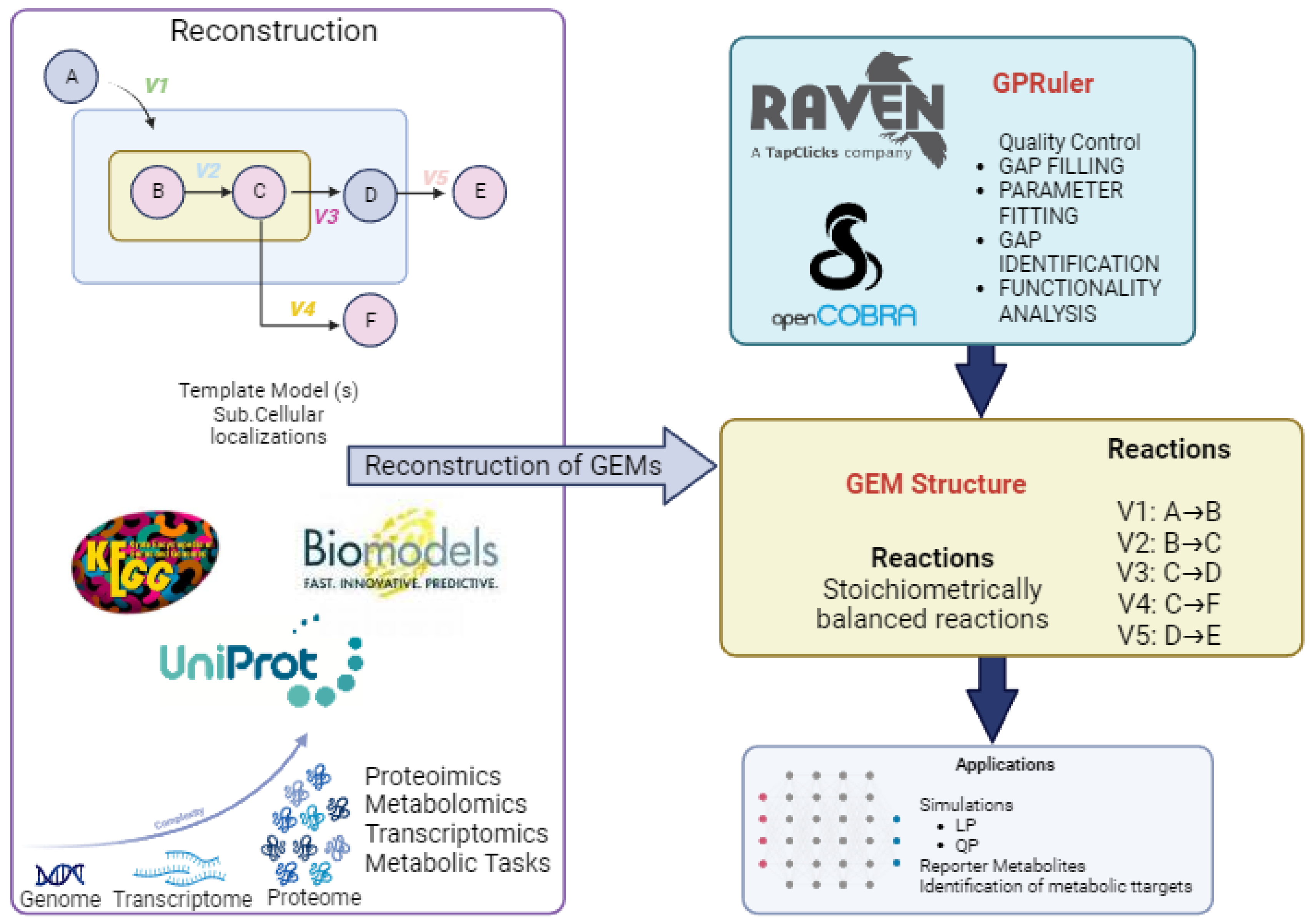
2.2. Key Steps for Performing a Reconstruction of Genomic-Scale Metabolic Models (GEMs)
2.2.1. Inference of Enzymes, Reaction Directionality, and Compartments
2.2.2. Tools for Genomic-Scale Metabolic Model Reconstruction
- RAVEN Toolbox (Version 2.8.6.0) (Reconstruction, Analysis, and Visualization of Metabolic Networks): RAVEN, a MATLAB-based tool, is critical in enabling constraint-based metabolic modeling. It facilitates the semi-automatic reconstruction of preliminary de novo models for specific organisms from the genomic sequence. RAVEN offers two distinct approaches to initiate GEM reconstruction: based on protein homology to an existing template model or de novo using reaction databases [88].
- GPRuler (Version 3.7): An open-source framework, GPRuler (Version 3.7) efficiently automates any living organism’s GPR rule reconstruction process. This framework has been validated in various case studies, demonstrating its ability to reproduce original GPR rules with high accuracy. The applicability and accuracy of GPRuler make it a valuable tool for generating accurate models [89].
- Methods for the automated reconstruction of genome-scale metabolic models: These methods, addressed in a review, discusses various tools and algorithms for the rapid reconstruction and analysis of metabolic models at the genomic scale. They highlight the importance of GEM reconstruction in supporting predictive analysis and the characterization of genomes based on sequence data. These reviewed and critical approaches provide a comprehensive view of the current state of available tools [90].
2.3. Classification of Genome-Scale Models: Steady-State and Dynamic Models
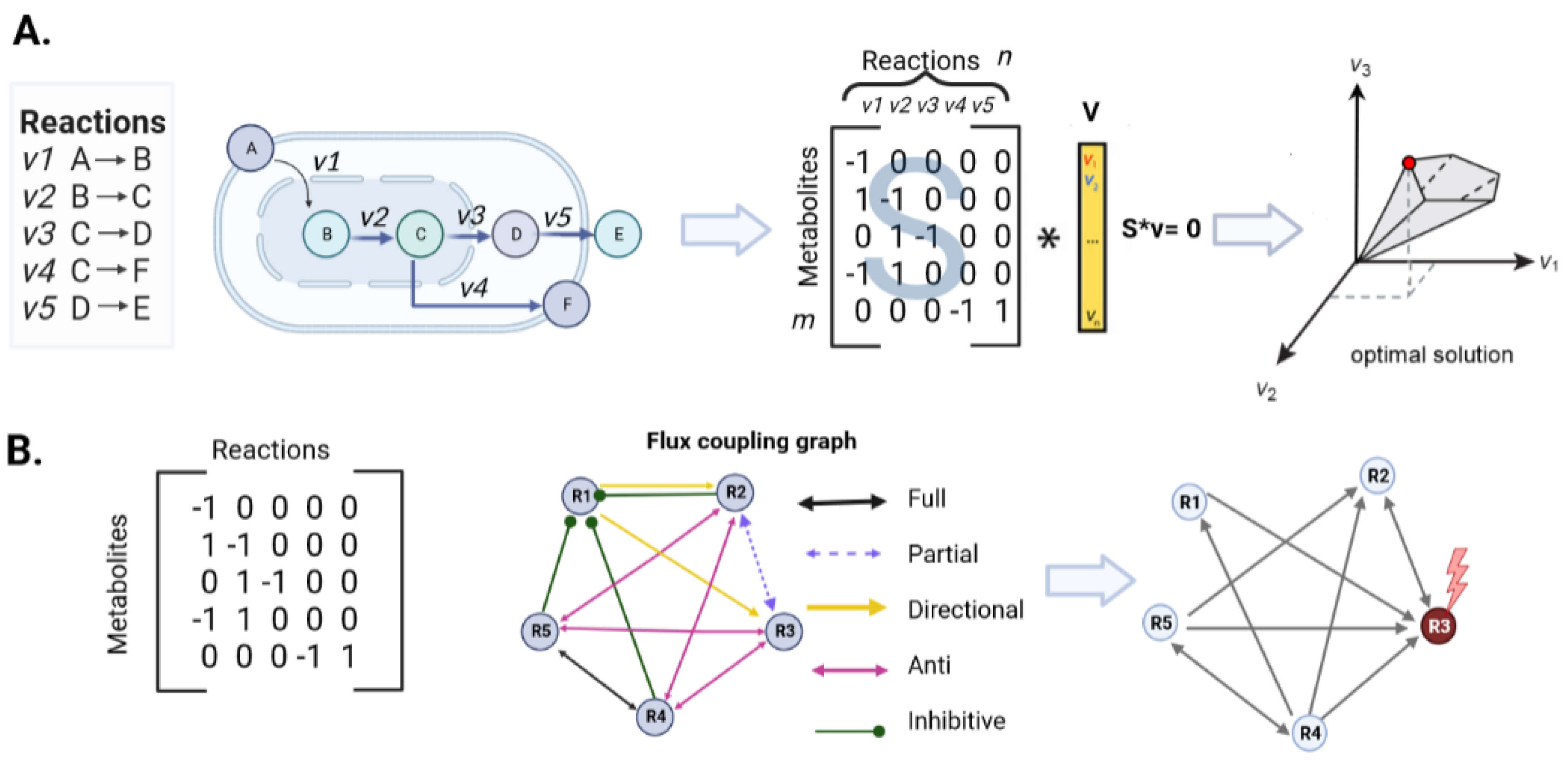
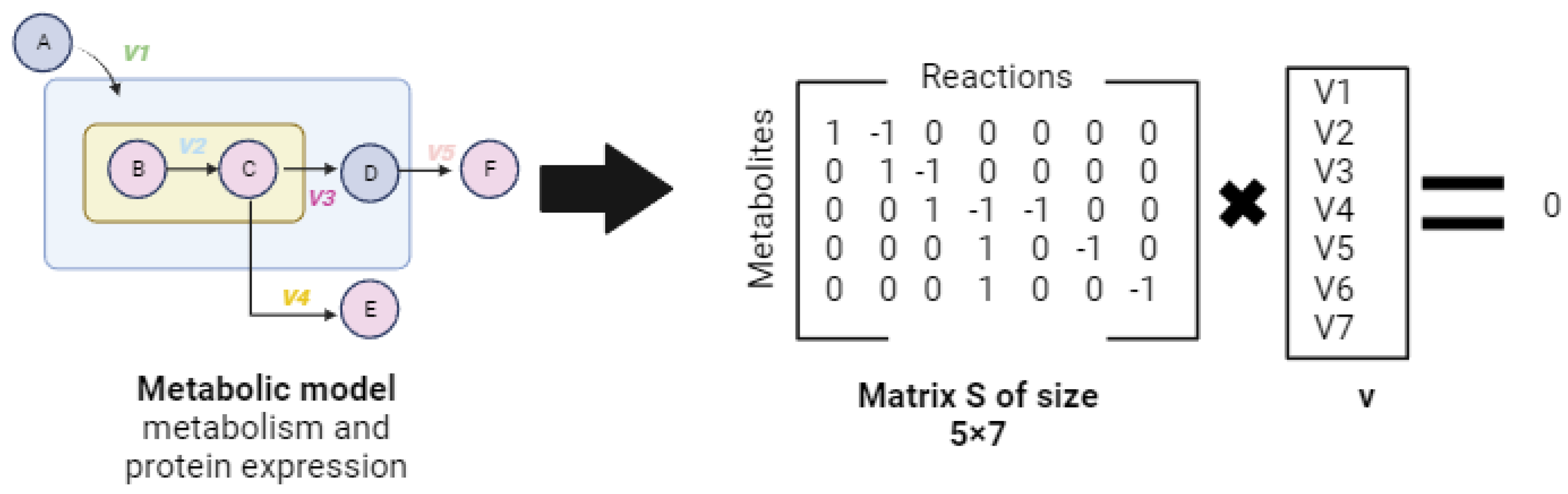
2.3.1. Steady-State Metabolic Models
2.3.2. Dynamic Models: A Comprehensive View
2.4. Topological Parameters for Identifying Drug Targets in an Enzyme-Centric Network
2.5. Genome-Scale Metabolic Models (GEMs) and System Controllability
2.6. MCA Can Be Applied to Both Dynamic and Steady-State Genome-Scale Metabolic Models
2.7. Control Theory Elements and Classification of Nodes in Complex Networks
2.7.1. Control Coefficients and the Power Decay Law
2.7.2. Feedback Vertex Set (FVS), a Dynamic Approach
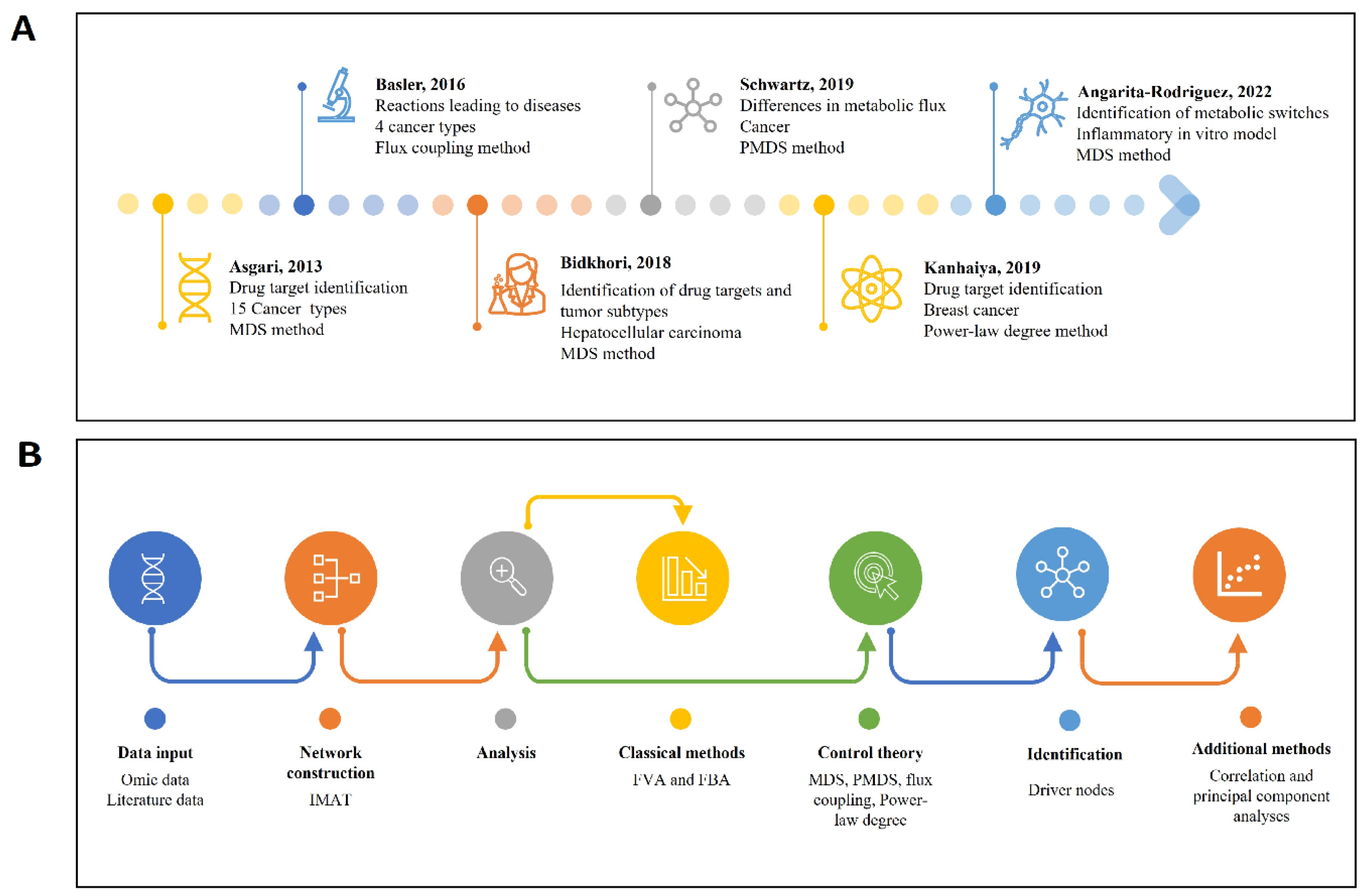
2.7.3. Minimal Dominant Set (MDS) and Probabilistic Blocking of Metabolic Fluxes Approach
- and , or
- and , or
- and , or
- and , or
- and ,and otherwise.
3. Machine Learning (ML) and GEM Approaches to Determine Metabolic Markers
4. Overview of Systems Biology Applications with Control Theory in Chronic Diseases
5. Potential Applications in Neurodegenerative Disorders: A Focus on Alzheimer’s Disease
6. Future Directions
7. Materials and Methods
- Control theory OR Systems biology;
- Metabolic models on a genomic scale OR GEMS AND Control theory;
- Minimal dominant set (MDS) approach OR MDS OR Control theory AND Systems biology;
- Probabilistic blocking of metabolic fluxes approach AND Systems biology AND Control theory;
- Metabolic models on a genomic scale AND Control theory AND applications OR Neurodegeneration;
- GEMs OR genomic-scale metabolic models OR systems biology AND control theory AND applications OR neurodegeneration OR chronic diseases OR complex systems OR applications in neurodegeneration AND date limit 2000/01/01–2023/08/01.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hood, L.; Friend, S.H. Predictive, personalized, preventive, participatory (P4) cancer medicine. Nat. Rev. Clin. Oncol. 2011, 8, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Yousofshahi, M.; Ullah, E.; Stern, R.; Hassoun, S. MC3: A steady-state model and constraint consistency checker for biochemical networks. BMC Syst. Biol. 2013, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Hood, L.; Heath, J.R.; Phelps, M.E.; Lin, B. Systems biology and new technologies enable predictive and preventative medicine. Science 2004, 306, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J. Systems Biology of Metabolism: A Driver for Developing Personalized and Precision Medicine. Cell Metab. 2017, 25, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Rosario, D.; Boren, J.; Uhlen, M.; Proctor, G.; Aarsland, D.; Mardinoglu, A.; Shoaie, S. Systems Biology Approaches to Understand the Host–Microbiome Interactions in Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 716. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.B.; Winslow, A.R.; Strasser, S.D. Systems biology of neurodegenerative diseases. Integr. Biol. 2015, 7, 758–775. [Google Scholar] [CrossRef] [PubMed]
- Friedland, R.P.; Chapman, M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, P.; Cunha, R.A.; Oliveira, C. Neuroinflammation, Oxidative Stress and the Pathogenesis of Alzheimer’s Disease. Curr. Pharmacutical Des. 2010, 16, 2766–2778. [Google Scholar] [CrossRef]
- Coppedè, F.; Mancuso, M.; Siciliano, G.; Migliore, L.; Murri, L. Genes and the environment in neurodegeneration. Biosci. Rep. 2006, 26, 341–367. [Google Scholar] [CrossRef]
- Noorbakhsh, F.; Overall, C.M.; Power, C. Deciphering complex mechanisms in neurodegenerative diseases: The advent of systems biology. Trends Neurosci. 2009, 32, 88–100. [Google Scholar] [CrossRef]
- Li, C.; Dubbelaar, M.L.; Zhang, X.; Zheng, J.C. Editorial: Understanding the heterogeneity and spatial brain environment of neurodegenerative diseases through conventional and future methods. Front. Cell. Neurosci. 2023, 17, 1211273. [Google Scholar] [CrossRef] [PubMed]
- Young, A.L.; Marinescu, R.V.; Oxtoby, N.P.; Bocchetta, M.; Yong, K.; Firth, N.C.; Cash, D.M.; Thomas, D.L.; Dick, K.M.; Cardoso, J.; et al. Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat. Commun. 2018, 9, 4273. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Xie, S.X.; Baer, D.R.; Suh, E.; Van Deerlin, V.M.; Loh, N.J.; Irwin, D.J.; McMillan, C.T.; A Wolk, D.; Chen-Plotkin, A.; et al. Pathological combinations in neurodegenerative disease are heterogeneous and disease-associated. Brain 2023, 146, 2557–2569. [Google Scholar] [CrossRef] [PubMed]
- Erkkinen, M.G.; Kim, M.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed]
- Currais, A.; Goldberg, J.; Farrokhi, C.; Chang, M.; Prior, M.; Dargusch, R.; Daugherty, D.; Armando, A.; Quehenberger, O.; Maher, P.; et al. A comprehensive multiomics approach toward understanding the relationship between aging and dementia. Aging 2015, 7, 937–955. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network; Levine, D.A. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qiu, P.; Ji, Y. TCGA-Assembler: Open-source software for retrieving and processing TCGA data. Nat. Methods 2014, 11, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, A.; Lam, S.; Altay, O.; Li, X.; Yuan, M.; Zhang, C.; Arif, M.; Turkez, H.; Uhlén, M.; Shoaie, S.; et al. Revealing the molecular mechanisms of Alzheimer’s disease based on network analysis. Int. J. Mol. Sci. 2021, 22, 11556. [Google Scholar] [CrossRef]
- Basler, G.; Nikoloski, Z.; Larhlimi, A.; Barabási, A.-L.; Liu, Y.-Y. Control principles of metabolic networks. arXiv 2015. [Google Scholar] [CrossRef]
- Nielsen, J. Systems biology of metabolism. Annu. Rev. Biochem. 2017, 86, 245–275. [Google Scholar] [CrossRef]
- Orth, J.D.; Thiele, I.; Palsson, B.O. What is flux balance analysis? Nat. Biotechnol. 2010, 28, 245–248. [Google Scholar] [CrossRef]
- Thiele, I.; Palsson, B.O. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat. Protoc. 2010, 5, 93–121. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Kim, G.B.; Kim, W.J.; Kim, H.U.; Lee, S.Y. Current status and applications of genome-scale metabolic models. Genome Biol. 2019, 20, 121. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U.; Kim, T.Y.; Lee, S.Y. Metabolic flux analysis and metabolic engineering of microorganisms. Mol. Biosyst. 2007, 4, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hyduke, D.; Schellenberger, J.; Que, R.; Fleming, R.; Thiele, I.; Orth, J.; Feist, A.M.; Zielinski, D.; Bordbar, A.; Lewis, N.; et al. COBRA Toolbox 2.0. Res. Sq. Prepr. 2011. [Google Scholar] [CrossRef]
- Yan, J.; Hu, Z.; Li, Z.; Sun, S.; Guo, W. Network Control Models with Personalized Genomics Data for Understanding Tumor Heterogeneity in Cancer. Front. Oncol. 2022, 12, 891676. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Ratcliffe, R.G. Flux-balance modeling of plant metabolism. Front. Plant Sci. 2011, 2, 38. [Google Scholar] [CrossRef]
- Lewis, N.E.; Nagarajan, H.; Palsson, B.O. Constraining the metabolic genotype–phenotype relationship using a phylogeny of in silico methods. Nat. Rev. Microbiol. 2012, 10, 291–305. [Google Scholar] [CrossRef]
- Basler, G.; Nikoloski, Z.; Larhlimi, A.; Barabási, A.L.; Liu, Y.Y. Control of fluxes in metabolic networks. Genome Res. 2016, 26, 956–968. [Google Scholar] [CrossRef]
- Sauer, U. Metabolic networks in motion: 13 C-based flux analysis. Mol. Syst. Biol. 2006, 2, 62. [Google Scholar] [CrossRef]
- Terzer, M.; Stelling, J. Large-scale computation of elementary flux modes with bit pattern trees. Bioinformatics 2008, 24, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.M.; Otokuni, H.; Akutsu, T.; Nacher, J.C. Probabilistic controllability approach to metabolic fluxes in normal and cancer tissues. Nat. Commun. 2019, 10, 2725. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, V.; Nacher, J.C.; Akutsu, T.; Ishitsuka, M.; Osadcenco, A.; Sunitha, V.; Bagler, G.; Schwartz, J.-M.; Robertson, D.L. Network controllability analysis of intracellular signalling reveals viruses are actively controlling molecular systems. Sci. Rep. 2019, 9, 2066. [Google Scholar] [CrossRef] [PubMed]
- Angarita-Rodríguez, A.; Mendoza-Mejía, N.; Gonzalez, J.; Aristizabal, A.F.; Hidalgo-Lanussa, O.; Rubio-Mesa, J.J.; Barreto, G.E.; Pinzon, A. Multi-Omics Integrative Analysis Coupled to Control Theory and Computational Simulation of a Genome-Scale metabolic Model Reveal Controlling Biological Switches in Human Astrocytes Under Palmitic Acid-Induced Lipotoxicity. Front. Syst. Biol. 2022, 2, 896265. [Google Scholar] [CrossRef]
- Liu, Y.; Barabási, A.-L. Control principles of complex systems. Rev. Mod. Phys. 2016, 88, 035006. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, S.; Feng, Y.; Liang, J.; Zeng, T.; Chen, L. Network controllability-based algorithm to target personalized driver genes for discovering combinatorial drugs of individual patients. Nucleic Acids Res. 2021, 49, e37. [Google Scholar] [CrossRef]
- Ideker, T.; Thorsson, V.; Ranish, J.A.; Christmas, R.; Buhler, J.; Eng, J.K.; Bumgarner, R.; Goodlett, D.R.; Aebersold, R.; Hood, L. Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science 2001, 292, 929–934. [Google Scholar] [CrossRef]
- Price, N.D.; Reed, J.L.; Palsson, B. Genome-scale models of microbial cells: Evaluating the consequences of constraints. Nat. Rev. Microbiol. 2004, 2, 886–897. [Google Scholar] [CrossRef]
- Otero, J.M.; Nielsen, J. Industrial systems biology. Biotechnol. Bioeng. 2010, 105, 439–460. [Google Scholar] [CrossRef]
- Pinu, F.R.; Beale, D.J.; Paten, A.M.; Kouremenos, K.; Swarup, S.; Schirra, H.J.; Wishart, D. Systems biology and multi-omics integration: Viewpoints from the metabolomics research community. Metabolites 2019, 9, 76. [Google Scholar] [CrossRef]
- Cisek, K.; Krochmal, M.; Klein, J.; Mischak, H. Full Reviews the application of multi-omics and systems biology to identify therapeutic targets in chronic kidney disease. Nephrol. Dial. Transplant. 2015, 31, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Redondo, J.; Piñero, D.; Eguiarte, L.E. Genomic, Transcriptomic and Epigenomic Tools to Study the Domestication of Plants and Animals: A Field Guide for Beginners. Front. Genet. 2020, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- LaFramboise, T. Single nucleotide polymorphism arrays: A decade of biological, computational and technological advances. Nucleic Acids Res. 2009, 37, 4181–4193. [Google Scholar] [CrossRef]
- Ansorge, W.J. Next generation DNA sequencing techniques and applications. New Biotechnol. 2010, 27, S3. [Google Scholar] [CrossRef]
- Mardis, E.R. Next-generation DNA sequencing methods. Annu. Rev. Genom. Hum. Genet. 2008, 9, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Smallbone, K.; Mendes, P.; Tech, V. Large-Scale Metabolic Models: From Reconstruction to Differential Equations. Ind. Biotechnol. 2013, 9, 179–184. [Google Scholar] [CrossRef]
- Machado, D.; Zhuang, K.H.; Sonnenschein, N.; Herrgård, M.J. Editorial: Current Challenges in Modeling Cellular Metabolism. Front. Bioeng. Biotechnol. 2015, 3, 193. [Google Scholar] [CrossRef]
- Kesić, S. Systems biology, emergence and antireductionism. Saudi J. Biol. Sci. 2015, 23, 584–591. [Google Scholar] [CrossRef]
- Lewis, N.E.; Abdel-Haleem, A.M. The evolution of genome-scale models of cancer metabolism. Front. Physiol. 2013, 4, 237. [Google Scholar] [CrossRef]
- Lee, D.-S.; Burd, H.; Liu, J.; Almaas, E.; Wiest, O.; Barabási, A.-L.; Oltvai, Z.N.; Kapatral, V. Comparative Genome-Scale Metabolic Reconstruction and Flux Balance Analysis of Multiple Staphylococcus aureus Genomes Identify Novel Antimicrobial Drug Targets. J. Bacteriol. 2009, 191, 4015–4024. [Google Scholar] [CrossRef]
- Suthers, P.F.; Zomorrodi, A.; Maranas, C.D. Genome-scale gene/reaction essentiality and synthetic lethality analysis. Mol. Syst. Biol. 2009, 5, 301. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, I.; Verma, S.; Kumar, S.; Jere, A.; Anamika, K. Multi-omics Data Integration, Interpretation, and Its Application. Bioinform. Biol. Insights 2020, 14, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Wanders, R.J.A.; Vaz, F.M.; Ferdinandusse, S.; van Kuilenburg, A.B.P.; Kemp, S.; van Karnebeek, C.D.; Waterham, H.R.; Houtkooper, R.H. Translational Metabolism: A multidisciplinary approach towards precision diagnosis of inborn errors of metabolism in the omics era. J. Inherit. Metab. Dis. 2019, 42, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Moolamalla, S.; Vinod, P. Genome-scale metabolic modelling predicts biomarkers and therapeutic targets for neuropsychiatric disorders. Comput. Biol. Med. 2020, 125, 103994. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Iwahashi, K.; Furukawa, A.; Ameno, K.; Kinoshita, H.; Ijiri, I.; Sekine, Y.; Suzuki, K.; Iwata, Y.; Minabe, Y.; et al. Acetaldehyde adducts in the brain of alcoholics. Arch. Toxicol. 2003, 77, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Yachie-Kinoshita, A.; Nishino, T.; Shimo, H.; Suematsu, M.; Tomita, M. A Metabolic Model of Human Erythrocytes: Practical Application of the E-Cell Simulation Environment. J. Biomed. Biotechnol. 2010, 2010, 642420. [Google Scholar] [CrossRef]
- Manninen, T.; Havela, R.; Linne, M.-L. Computational Models of Astrocytes and Astrocyte–Neuron Interactions: Characterization, Reproducibility, and Future Perspectives. In Computational Glioscience; Springer: Cham, Switzerland, 2019; pp. 423–454. [Google Scholar] [CrossRef]
- González, J.; Pinzón, A.; Angarita-Rodríguez, A.; Aristizabal, A.F.; Barreto, G.E.; Martín-Jiménez, C. Advances in Astrocyte Computational Models: From Metabolic Reconstructions to Multi-Omic Approaches. Front. Neurosci. 2020, 14, 35. [Google Scholar] [CrossRef]
- Pinzon, W.; Vega, H.; Gonzalez, J.; Pinzon, A. Mathematical Framework behind the Reconstruction and Analysis of Genome Scale Metabolic Models. Arch. Comput. Methods Eng. 2019, 26, 1593–1606. [Google Scholar] [CrossRef]
- Hastings, J.; Mains, A.; Virk, B.; Rodriguez, N.; Murdoch, S.; Pearce, J.; Bergmann, S.; Le Novère, N.; Casanueva, O. Multi-Omics and Genome-Scale Modeling Reveal a Metabolic Shift During C. Elegans Aging. Front. Mol. Biosci. 2019, 6, 2. [Google Scholar] [CrossRef]
- Ibarra, R.U.; Edwards, J.S.; Palsson, B.O. Escherichia coli K-12 undergoes adaptive evolution to achieve in silico predicted optimal growth. Nature 2002, 420, 186–189. [Google Scholar] [CrossRef]
- Orth, J.D.; Conrad, T.M.; Na, J.A.; Lerman, J.; Nam, H.; Feist, A.M.; Palsson, B. A comprehensive genome-scale reconstruction of Escherichia coli metabolism—2011. Mol. Syst. Biol. 2011, 7, 535. [Google Scholar] [CrossRef] [PubMed]
- Orth, J.D.; Fleming, R.M.T.; Palsson, B. Reconstruction and Use of Microbial Metabolic Networks: The Core Escherichia coli Metabolic Model as an Educational Guide. EcoSal Plus 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- Thiele, I.; Swainston, N.; Fleming, R.M.T.; Hoppe, A.; Sahoo, S.; Aurich, M.K.; Haraldsdottir, H.; Mo, M.L.; Rolfsson, O.; Stobbe, M.D.; et al. A community-driven global reconstruction of human metabolism. Nat. Biotechnol. 2013, 31, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. G:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Farooq, Q.U.A.; Shaukat, Z.; Aiman, S.; Li, C. Protein-protein interactions: Methods, databases, and applications in virus-host study. World J. Virol. 2021, 10, 288–300. [Google Scholar] [CrossRef]
- Karlebach, G.; Shamir, R. Modelling and analysis of gene regulatory networks. Nat. Rev. Mol. Cell Biol. 2008, 9, 770–780. [Google Scholar] [CrossRef]
- Bauer, E.; Thiele, I. From metagenomic data to personalized in silico microbiotas: Predicting dietary supplements for Crohn’s disease. NPJ Syst. Biol. Appl. 2018, 4, 27. [Google Scholar] [CrossRef]
- Jamshidi, N.; Nigam, S.K.; Dalton, D. Drug transporters OAT1 and OAT3 have specific effects on multiple organs and gut microbiome as revealed by contextualized metabolic network reconstructions. Sci. Rep. 2022, 12, 18308. [Google Scholar] [CrossRef]
- Larhlimi, A.; David, L.; Selbig, J.; Bockmayr, A. F2C2: A fast tool for the computation of flux coupling in genome-scale metabolic networks. BMC Bioinform. 2012, 13, 57. [Google Scholar] [CrossRef]
- Pitkänen, E.; Jouhten, P.; Hou, J.; Syed, M.F.; Blomberg, P.; Kludas, J.; Oja, M.; Holm, L.; Penttilä, M.; Rousu, J.; et al. Comparative Genome-Scale Reconstruction of Gapless Metabolic Networks for Present and Ancestral Species. PLoS Comput. Biol. 2014, 10, e1003465. [Google Scholar] [CrossRef]
- Khan, K.; Jalal, K.; Khan, A.; Al-Harrasi, A.; Uddin, R. Comparative Metabolic Pathways Analysis and Subtractive Genomics Profiling to Prioritize Potential Drug Targets Against Streptococcus pneumoniae. Front. Microbiol. 2022, 12, 796363. [Google Scholar] [CrossRef] [PubMed]
- Mano, A.; Tuller, T.; Béjà, O.; Pinter, R.Y. Comparative classification of species and the study of pathway evolution based on the alignment of metabolic pathways. BMC Bioinform. 2010, 11, S38. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Deng, S.; Jin, G.; Wang, X.; Yu, Z.G. Bayesian network model for identification of pathways by integrating protein interaction with genetic interaction data. BMC Syst. Biol. 2017, 11, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Green, M.L.; Karp, P.D. A Bayesian method for identifying missing enzymes in predicted metabolic pathway databases. BMC Bioinform. 2004, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Li, J.; Cheng, J. Reconstruction of metabolic pathways by combining probabilistic graphical model-based and knowledge-based methods. BMC Proc. 2014, 8, S5. [Google Scholar] [CrossRef]
- Shah, H.A.; Liu, J.; Yang, Z.; Feng, J. Review of Machine Learning Methods for the Prediction and Reconstruction of Metabolic Pathways. Front. Mol. Biosci. 2021, 8, 634141. [Google Scholar] [CrossRef] [PubMed]
- De Martino, D.; MC Andersson, A.; Bergmiller, T.; Guet, C.C.; Tkačik, G. Statistical mechanics for metabolic networks during steady state growth. Nat. Commun. 2018, 9, 2988. [Google Scholar] [CrossRef]
- Sridhara, V.; Meyer, A.G.; Rai, P.; Barrick, J.E.; Ravikumar, P.; Segrè, D.; Wilke, C.O. Predicting growth conditions from internal metabolic fluxes in an in-silico model of E. coli. PLoS ONE 2014, 9, e114608. [Google Scholar] [CrossRef]
- Emiola, A.; Oh, J. High throughput in situ metagenomic measurement of bacterial replication at ultra-low sequencing coverage. Nat. Commun. 2018, 9, 4956. [Google Scholar] [CrossRef]
- Emiola, A.; Zhou, W.; Oh, J. Metagenomic growth rate inferences of strains in situ. Sci. Adv. 2020, 6, eaaz2299. [Google Scholar] [CrossRef]
- Gönen, M. Statistical aspects of gene signatures and molecular targets. Gastrointest. Cancer Res. 2009, 3 (Suppl. 2), S19–S21. [Google Scholar] [PubMed]
- Theilhaber, J.; Chiron, M.; Dreymann, J.; Bergstrom, D.; Pollard, J. Construction and optimization of gene expression signatures for prediction of survival in two-arm clinical trials. BMC Bioinform. 2020, 21, 333. [Google Scholar] [CrossRef] [PubMed]
- Sauer, D.B.; Wang, D.N. Predicting the optimal growth temperatures of prokaryotes using only genome derived features. Bioinformatics 2019, 35, 3224–3231. [Google Scholar] [CrossRef] [PubMed]
- Brunk, E.; Sahoo, S.; Zielinski, D.C.; Altunkaya, A.; Dräger, A.; Mih, N.; Gatto, F.; Nilsson, A.; Preciat Gonzalez, G.A.; Aurich, M.K.; et al. Recon3D enables a three-dimensional view of gene variation in human metabolism. Nat. Biotechnol. 2018, 36, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Passi, A.; Tibocha-Bonilla, J.D.; Kumar, M.; Tec-Campos, D.; Zengler, K.; Zuniga, C. Genome-Scale Metabolic Modeling Enables In-Depth Understanding of Big Data. Metabolites 2021, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Seif, Y.; Palsson, B.Ø. Path to improving the life cycle and quality of genome-scale models of metabolism. Cell Syst. 2021, 12, 842–859. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Marcišauskas, S.; Sánchez, B.J.; Domenzain, I.; Hermansson, D.; Agren, R.; Nielsen, J.; Kerkhoven, E.J. RAVEN 2.0: A versatile toolbox for metabolic network reconstruction and a case study on Streptomyces coelicolor. PLoS Comput. Biol. 2018, 14, e1006541. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.; Damiani, C.; Pescini, D. GPRuler: Metabolic gene-protein-reaction rules automatic reconstruction. PLoS Comput. Biol. 2021, 17, e1009550. [Google Scholar] [CrossRef]
- Machado, D.; Andrejev, S.; Tramontano, M.; Patil, K.R. Fast automated reconstruction of genome-scale metabolic models for microbial species and communities. Nucleic Acids Res. 2018, 46, 7542–7553. [Google Scholar] [CrossRef]
- Loriaux, P.M.; Hoffmann, A. A Framework for Modeling the Relationship between Cellular Steady-State and Stimulus-Responsiveness. Methods Cell Biol. 2012, 110, 81–109. [Google Scholar] [CrossRef]
- Loriaux, P.M.; Tesler, G.; Hoffmann, A. Characterizing the Relationship between Steady State and Response Using Analytical Expressions for the Steady States of Mass Action Models. PLoS Comput. Biol. 2013, 9, e1002901. [Google Scholar] [CrossRef] [PubMed]
- Palsson, B. Metabolic systems biology. FEBS Lett. 2009, 583, 3900–3904. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Dutta, A. Computational systems biology in disease modeling and control, review and perspectives. NPJ Syst. Biol. Appl. 2022, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Feiglin, A.; Hacohen, A.; Sarusi, A.; Fisher, J.; Unger, R.; Ofran, Y. Static network structure can be used to model the phenotypic effects of perturbations in regulatory networks. Bioinformatics 2012, 28, 2811–2818. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gillespie, S.L.; Neal, J.L.; Christian, L.M.; Szalacha, L.A.; McCarthy, D.O.; Salsberry, P.J. Interleukin-1 Receptor Antagonist Polymorphism and Birth Timing. Nurs. Res. 2017, 66, 95–104. [Google Scholar] [CrossRef]
- Galindez, G.; Sadegh, S.; Baumbach, J.; Kacprowski, T.; List, M. Network-based approaches for modeling disease regulation and progression. Comput. Struct. Biotechnol. J. 2023, 21, 780–795. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Z.P. Analysis of topological parameters of complex disease genes reveals the importance of location in a biomolecular network. Genes 2019, 10, 143. [Google Scholar] [CrossRef]
- Assenov, Y.; Ramírez, F.; Schelhorn, S.-E.; Lengauer, T.; Albrecht, M. Computing topological parameters of biological networks. Bioinformatics 2008, 24, 282–284. [Google Scholar] [CrossRef]
- Mitrea, C.; Taghavi, Z.; Bokanizad, B.; Hanoudi, S.; Tagett, R.; Donato, M.; Voichiţa, C.; Drăghici, S. Methods and approaches in the topology-based analysis of biological pathways. Front. Physiol. 2013, 4, 278. [Google Scholar] [CrossRef]
- Asgari, Y.; Salehzadeh-Yazdi, A.; Schreiber, F.; Masoudi-Nejad, A. Controllability in cancer metabolic networks according to drug targets as driver nodes. PLoS ONE 2013, 8, e79397. [Google Scholar] [CrossRef]
- Ravindran, V.; Sunitha, V.; Bagler, G. Identification of critical regulatory genes in cancer signaling network using controllability analysis. Phys. A Stat. Mech. Its Appl. 2017, 474, 134–143. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, C.; Di, Z.; Wang, W.X.; Lai, Y.C. Exact controllability of complex networks. Nat. Commun. 2013, 4, 2447. [Google Scholar] [CrossRef]
- Holzhütter, H. The principle of flux minimization and its application to estimate stationary fluxes in metabolic networks. Eur. J. Biochem. 2004, 271, 2905–2922. [Google Scholar] [CrossRef]
- Sarathy, C.; Breuer, M.; Kutmon, M.; Adriaens, M.E.; Evelo, C.T.; Arts, I.C.W. Comparison of metabolic states using genome-scale metabolic models. PLoS Comput. Biol. 2021, 17, e1009522. [Google Scholar] [CrossRef]
- Hsiao, V.; Swaminathan, A.; Murray, R.M. Control Theory for Synthetic Biology: Recent Advances in System Characterization, Control Design, and Controller Implementation for Synthetic Biology. IEEE Control Syst. 2018, 38, 32–62. [Google Scholar] [CrossRef]
- Schafer, J.R.A.; Fell, D.A.; Rothman, D.; Shulman, R.G. Protein phosphorylation can regulate metabolite concentrations rather than control flux: The example of glycogen synthase. Proc. Natl. Acad. Sci. USA 2004, 101, 1485–1490. [Google Scholar] [CrossRef]
- Schmidt, H.; Jacobsen, E.W. Linear systems approach to analysis of complex dynamic behaviours in biochemical networks. Syst. Biol. 2004, 1, 149–158. [Google Scholar] [CrossRef][Green Version]
- Rao, V.S.H.; Rao, P.R.S. Dynamic Models and Control of Biological Systems; Springer: New York, NY, USA, 2009. [Google Scholar][Green Version]
- Jakubowski, H.; Flatt, P. Fundamentals of Biochemistry II—Bioenergetics and Metabolism; LibreTexts: Davis, CA, USA, 2023. [Google Scholar][Green Version]
- Moreno-Sánchez, R.; Saavedra, E.; Rodríguez-Enríquez, S.; Olín-Sandoval, V. Metabolic Control Analysis: A Tool for Designing Strategies to Manipulate Metabolic Pathways. J. Biomed. Biotechnol. 2008, 2008, 597913. [Google Scholar] [CrossRef]
- Knüpfer, C.; Beckstein, C. Function of dynamic models in systems biology: Linking structure to behaviour. J. Biomed. Semant. 2013, 4, 24. [Google Scholar] [CrossRef]
- Lalwani, M.A.; Zhao, E.M.; Avalos, J.L. ScienceDirect Current and future modalities of dynamic control in metabolic engineering. Curr. Opin. Biotechnol. 2018, 52, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Hartline, C.J.; Schmitz, A.C.; Han, Y.; Zhang, F. Dynamic control in metabolic engineering: Theories, tools, and applications. Metab. Eng. 2020, 63, 126–140. [Google Scholar] [CrossRef]
- Pan, M.; Gawthrop, P.J.; Cursons, J.; Crampin, E.J. Modular assembly of dynamic models in systems biology. PLoS Comput. Biol. 2021, 17, e1009513. [Google Scholar] [CrossRef]
- Raue, A.; Schilling, M.; Bachmann, J.; Matteson, A.; Schelke, M.; Kaschek, D.; Hug, S.; Kreutz, C.; Harms, B.D.; Theis, F.J.; et al. Lessons Learned from Quantitative Dynamical Modeling in Systems Biology. PLoS ONE 2013, 8, e74335. [Google Scholar] [CrossRef]
- Ni, C.; Dinh, C.V.; Prather, K.L. Dynamic Control of Metabolism. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 519–541. [Google Scholar] [CrossRef]
- Azeloglu, E.U.; Iyengar, R. Good practices for building dynamical models in systems biology. Sci. Signal. 2015, 8, fs8. [Google Scholar] [CrossRef]
- Miskovic, L.; Tokic, M.; Savoglidis, G.; Hatzimanikatis, V. Control Theory Concepts for Modeling Uncertainty in Enzyme Kinetics of Biochemical Networks. Ind. Eng. Chem. Res. 2019, 58, 13544–13554. [Google Scholar] [CrossRef]
- Wildermuth, M.C. Minireview Metabolic control analysis: Biological applications and insights. Genome Biol. 2000, 1, reviews1031.1. [Google Scholar] [CrossRef]
- Braun, U.; Harneit, A.; Pergola, G.; Menara, T.; Schäfer, A.; Betzel, R.F.; Zang, Z.; Schweiger, J.I.; Zhang, X.; Schwarz, K.; et al. Brain network dynamics during working memory are modulated by dopamine and diminished in schizophrenia. Nat. Commun. 2021, 12, 3478. [Google Scholar] [CrossRef]
- Villoslada, P.; Steinman, L.; Baranzini, S.E. Systems biology and its application to the understanding of neurological diseases. Ann. Neurol. 2009, 65, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Lin, D.W.; Eames, A.; Chandrasekaran, S. Next-Generation Genome-Scale Metabolic Modeling through Integration of Regulatory Mechanisms. Metabolites 2021, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Moulin, C.; Tournier, L.; Peres, S. Combining Kinetic and Constraint-Based Modelling to Better Understand Metabolism Dynamics. Processes 2021, 9, 1701. [Google Scholar] [CrossRef]
- Vasilakou, E.; Machado, D.; Theorell, A.; Rocha, I.; Nöh, K.; Oldiges, M.; Wahl, S.A. Current state and challenges for dynamic metabolic modeling. Curr. Opin. Microbiol. 2016, 33, 97–104. [Google Scholar] [CrossRef]
- Reder, C. Metabolic control theory: A structural approach. J. Theor. Biol. 1988, 135, 175–201. [Google Scholar] [CrossRef]
- Hofmeyr, J.-H.S.; Kacser, H.; Merwe, K.J. Metabolic control analysis of moiety-conserved cycles. JBIC J. Biol. Inorg. Chem. 1986, 155, 631–640. [Google Scholar] [CrossRef]
- Shinzawa, Y.; Akutsu, T.; Nacher, J.C. Uncovering and classifying the role of driven nodes in control of complex networks. Sci. Rep. 2021, 11, 9627. [Google Scholar] [CrossRef]
- Sajitz-Hermstein, M.; Nikoloski, Z. Structural Control of Metabolic Flux. PLoS Comput. Biol. 2013, 9, e1003368. [Google Scholar] [CrossRef][Green Version]
- Annamalai, K.; Puri, I.K.; Jog, M.A. Thermodynamics and Biological Systems. In Advanced Thermodynamics Engineering; CRC Press: Boca Raton, FL, USA, 2020; pp. 755–850. [Google Scholar] [CrossRef]
- Brown, G.C.; Hafner, R.P.; Brand, M.D. A ‘top-down’ approach to the determination of control coefficients in metabolic control theory. JBIC J. Biol. Inorg. Chem. 1990, 188, 321–325. [Google Scholar] [CrossRef]
- Guo, W.-F.; Zhang, S.W.; Shi, Q.Q.; Zhang, C.-M.; Zeng, T.; Chen, L. A novel algorithm for finding optimal driver nodes to target control complex networks and its applications for drug targets identification. BMC Genom. 2018, 19, 924. [Google Scholar] [CrossRef]
- Guo, W.-F.; Zhang, S.-W.; Zeng, T.; Li, Y.; Gao, J.; Chen, L. A novel network control model for identifying personalized driver genes in cancer. PLoS Comput. Biol. 2019, 15, e1007520. [Google Scholar] [CrossRef]
- Nacher, J.C.; Akutsu, T. Dominating scale-free networks with variable scaling exponent: Heterogeneous networks are not difficult to control. New J. Phys. 2012, 14, 073005. [Google Scholar] [CrossRef]
- Fiedler, B.; Mochizuki, A.; Kurosawa, G.; Saito, D. Dynamics and Control at Feedback Vertex Sets. I: Informative and Determining Nodes in Regulatory Networks. J. Dyn. Differ. Equ. 2013, 25, 563–604. [Google Scholar] [CrossRef]
- Mochizuki, A.; Fiedler, B.; Kurosawa, G.; Saito, D. Dynamics and control at feedback vertex sets. II: A faithful monitor to determine the diversity of molecular activities in regulatory networks. J. Theor. Biol. 2013, 335, 130–146. [Google Scholar] [CrossRef]
- Vinayagam, A.; Gibson, T.E.; Lee, H.-J.; Yilmazel, B.; Roesel, C.; Hu, Y.; Kwon, Y.; Sharma, A.; Liu, Y.-Y.; Perrimon, N.; et al. Controllability analysis of the directed human protein interaction network identifies disease genes and drug targets. Proc. Natl. Acad. Sci. USA 2016, 113, 4976–4981. [Google Scholar] [CrossRef]
- Kenett, Y.N.; Medaglia, J.D.; Beaty, R.E.; Chen, Q.; Betzel, R.F.; Thompson-Schill, S.L.; Qiu, J. Driving the brain towards creativity and intelligence: A network control theory analysis. Neuropsychologia 2018, 118, 79–90. [Google Scholar] [CrossRef]
- Liu, X.; Pan, L. Detection of driver metabolites in the human liver metabolic network using structural controllability analysis. BMC Syst. Biol. 2014, 8, 51. [Google Scholar] [CrossRef]
- Liu, X.; Pan, L. Identifying Driver Nodes in the Human Signaling Network Using Structural Controllability Analysis. IEEE/ACM Trans. Comput. Biol. Bioinform. 2014, 12, 467–472. [Google Scholar] [CrossRef]
- Wang, Y. Identifying neuron subtype-specific metabolic network changes in single cell transcriptomics of Alzheimer’s Disease using perturb-Met. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bassett, D.S.; Khambhati, A.N.; Grafton, S.T. Emerging Frontiers of Neuroengineering: A Network Science of Brain Connectivity. Annu. Rev. Biomed. Eng. 2017, 19, 327–352. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Fromion, V.; Westerhoff, H.V. (Im)Perfect robustness and adaptation of metabolic networks subject to metabolic and gene-expression regulation: Marrying control engineering with metabolic control analysis (Im) Perfect robustness and adaptation of metabolic networks subject to met. BMC Syst. Biol. 2013, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Imielinski, M.; Klitgord, N.; Belta, C. Investigating the genomic basis of metabolic robustness through in silico flux analysis. In Proceedings of the 2008 47th IEEE Conference on Decision and Control, Cancun, Mexico, 9–11 December 2008; pp. 793–798. [Google Scholar]
- Saavedra, E.; Moreno-Sánchez, R. Metabolic Control Theory. In Encyclopedia of Systems Biology; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Westerhoff, H.V.; Kolodkin, A.; Conradie, R.; Wilkinson, S.J.; Bruggeman, F.J.; Krab, K.; van Schuppen, J.H.; Hardin, H.; Bakker, B.M.; Moné, M.J.; et al. Systems biology towards life in silico: Mathematics of the control of living cells. J. Math. Biol. 2009, 58, 7–34. [Google Scholar] [CrossRef]
- Iglesias, P.A.; Ingalls, B.P. Control Theory and Systems Biology, 1st ed.; MIT Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Moreno-Sánchez, R.; Saavedra, E.; Rodríguez-Enríquez, S.; Gallardo-Pérez, J.C.; Quezada, H.; Westerhoff, H.V. Metabolic control analysis indicates a change of strategy in the treatment of cancer. Mitochondrion 2010, 10, 626–639. [Google Scholar] [CrossRef]
- Marashi, S.; Bockmayr, A. BioSystems Flux coupling analysis of metabolic networks is sensitive to missing reactions. Biosystems 2011, 103, 57–66. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Lu, S.; O’Sullivan, B.; Razgon, I. A fixed-parameter algorithm for the directed feedback vertex set problem. J. ACM 2008, 55, 1–19. [Google Scholar] [CrossRef]
- Belykh, V.N.; Belykh, I.V.; Hasler, M. Connection graph stability method for synchronized coupled chaotic systems. Phys. D Nonlinear Phenom. 2004, 195, 159–187. [Google Scholar] [CrossRef]
- Maarleveld, T.R.; Khandelwal, R.A.; Olivier, B.G.; Teusink, B.; Bruggeman, F.J. Basic concepts and principles of stoichiometric modeling of metabolic networks. Biotechnol. J. 2013, 8, 997–1008. [Google Scholar] [CrossRef]
- Burgard, A.P.; Nikolaev, E.V.; Schilling, C.H.; Maranas, C.D. Flux Coupling Analysis of Genome-Scale Metabolic Network Reconstructions. Genome Res. 2004, 14, 301–312. [Google Scholar] [CrossRef]
- David, L.; Marashi, S.A.; Larhlimi, A.; Mieth, B.; Bockmayr, A. FFCA: A feasibility-based method for flux coupling analysis of metabolic networks. BMC Bioinform. 2011, 12, 236. [Google Scholar] [CrossRef]
- Larhlimi, A.; Bockmayr, A. A New Approach to Flux Coupling Analysis of Metabolic Networks. In International Symposium on Computational Life Science; Springer: Berlin/Heidelberg, Germany, 2006; pp. 205–215. [Google Scholar]
- Poolman, M.G.; Sebu, C.; Pidcock, M.K.; Fell, D.A. Modular decomposition of metabolic systems via null-space analysis. J. Theor. Biol. 2007, 249, 691–705. [Google Scholar] [CrossRef]
- Tomar, N.; De, R.K. A Comprehensive View on Metabolic Pathway Analysis Methodologies. Curr. Bioinform. 2014, 9, 295–305. [Google Scholar] [CrossRef]
- Kim, D.H.E.; Motter, A. Slave nodes and the controllability of metabolic networks. New J. Phys. 2009, 11, 113047. [Google Scholar] [CrossRef][Green Version]
- Furtado, E.L. FireScholars Artificial Intelligence: An Analysis of Alan Turing’s Role in the Conception and Development of Intelligent Machinery; Southeastern University: Lakeland, FL, USA, 2018. [Google Scholar][Green Version]
- Sidak, D.; Schwarzerová, J.; Weckwerth, W.; Waldherr, S. Interpretable machine learning methods for predictions in systems biology from omics data. Front. Mol. Biosci. 2022, 9, 926623. [Google Scholar] [CrossRef]
- Thiyagalingam, J.; Shankar, M.; Fox, G.; Hey, T. Scientific machine learning benchmarks. Nat. Rev. Phys. 2022, 4, 413–420. [Google Scholar] [CrossRef]
- Zampieri, G.; Vijayakumar, S.; Yaneske, E.; Angione, C. Machine and deep learning meet genome-scale metabolic modeling. PLoS Comput. Biol. 2019, 15, e1007084. [Google Scholar] [CrossRef]
- Janiesch, C.; Zschech, P.; Heinrich, K. Machine learning and deep learning. Electron. Mark. 2021, 31, 685–695. [Google Scholar] [CrossRef]
- Oliveira, A.; Cunha, E.; Cruz, F.; Capela, J.; Sequeira, J.C.; Sampaio, M.; Sampaio, C.; Dias, O. Systematic assessment of template-based genome-scale metabolic models created with the BiGG Integration Tool. J. Integr. Bioinform. 2022, 19, 20220014. [Google Scholar] [CrossRef]
- Cheng, Y.; Bi, X.; Xu, Y.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Machine learning for metabolic pathway optimization: A review. Comput. Struct. Biotechnol. J. 2023, 21, 2381–2393. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Angione, C. Protocol for hybrid flux balance, statistical, and machine learning analysis of multi-omic data from the cyanobacterium Synechococcus sp. PCC 7002. STAR Protoc. 2021, 2, 100837. [Google Scholar] [CrossRef]
- Osorio, D.; Gonzalez, J.; Pinzon, A. ‘exp2flux’ Convert Gene EXPression Data to FBA FLUXes; The R Project for Statistical Computing: Vienna, Austria, 2016. [Google Scholar] [CrossRef]
- Xu, C.; Jackson, S.A. Machine learning and complex biological data. Genome Biol. 2019, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Cortassa, S.; Caceres, V.; Bell, L.N.; O’rourke, B.; Paolocci, N.; Aon, M.A. From Metabolomics to Fluxomics: A Computational Procedure to Translate Metabolite Profiles into Metabolic Fluxes. Biophys. J. 2015, 108, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Hoffmann, S.; Gerasch, A.; Gille, C.; Holzhütter, H.G. FASIMU: Flexible software for flux-balance computation series in large metabolic networks. BMC Bioinform. 2011, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.H.; Szczepski, K.; Al-Younis, I.; Lachowicz, J.I.; Jaremko, M. Fluxomics—New Metabolomics Approaches to Monitor Metabolic Pathways. Front. Pharmacol. 2022, 13, 805782. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Poulos, R.C.; Liu, J.; Zhong, Q. Machine learning for multi-omics data integration in cancer. iScience 2022, 25, 103798. [Google Scholar] [CrossRef] [PubMed]
- Strain, B.; Morrissey, J.; Antonakoudis, A.; Kontoravdi, C. Genome-scale models as a vehicle for knowledge transfer from microbial to mammalian cell systems. Comput. Struct. Biotechnol. J. 2023, 21, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Kanhaiya, K.; Tyagi-Tiwari, D. Identification of Drug Targets in Breast Cancer Metabolic Network. J. Comput. Biol. 2020, 27, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Gatto, F.; Nookaew, I.; Nielsen, J. Chromosome 3p loss of heterozygosity is associated with a unique metabolic network in clear cell renal carcinoma. Proc. Natl. Acad. Sci. USA 2014, 111, E866–E875. [Google Scholar] [CrossRef] [PubMed]
- Bidkhori, G.; Benfeitas, R.; Klevstig, M.; Zhang, C.; Nielsen, J.; Uhlen, M.; Boren, J.; Mardinoglu, A. Metabolic network-based stratification of hepatocellular carcinoma reveals three distinct tumor subtypes. Proc. Natl. Acad. Sci. USA 2018, 115, E11874–E11883. [Google Scholar] [CrossRef] [PubMed]
- Bidkhori, G.; Benfeitas, R.; Elmas, E.; Kararoudi, M.N.; Arif, M.; Uhlen, M.; Nielsen, J.; Mardinoglu, A. Metabolic network-based identification and prioritization of anticancer targets based on expression data in hepatocellular carcinoma. Front. Physiol. 2018, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: Common mechanisms and strategies for new treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef]
- Koníčková, D.; Menšíková, K.; Tučková, L.; Hényková, E.; Strnad, M.; Friedecký, D.; Stejskal, D.; Matěj, R.; Kaňovský, P. Biomarkers of Neurodegenerative Diseases: Biology, Taxonomy, Clinical Relevance, and Current Research Status. Biomedicines 2022, 10, 1760. [Google Scholar] [CrossRef]
- Kishk, A.; Pacheco, M.P.; Heurtaux, T.; Sinkkonen, L.; Pang, J.; Fritah, S.; Niclou, S.P.; Sauter, T. Review of Current Human Genome-Scale Metabolic Models for Brain Cancer and Neurodegenerative Diseases. Cells 2022, 11, 2486. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Han, Y.; Jiang, J. Different glucose metabolic brain networks between Subjective Cognitive Decline and Health Control based on graph theory. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Montreal, QC, Canada, 27 August 2020; pp. 1084–1087. [Google Scholar]
- Baloni, P.; Arnold, M.; Buitrago, L.; Nho, K.; Moreno, H.; Huynh, K.; Brauner, B.; Louie, G.; Kueider-Paisley, A.; Suhre, K.; et al. Multi-Omic analyses characterize the ceramide/sphingomyelin pathway as a therapeutic target in Alzheimer’s disease. Commun. Biol. 2022, 5, 1074. [Google Scholar] [CrossRef]
- Campbell, N.L.; Unverzagt, F.; LaMantia, M.A.; Khan, B.A.; Boustani, M.A. Risk factors for the progression of mild cognitive impairment to dementia. Clin. Geriatr. Med. 2013, 29, 873–893. [Google Scholar] [CrossRef]
- Castrillo, J.I.; Lista, S.; Hampel, H.; Ritchie, C.W. Systems Biology Methods for Alzheimer’s Disease Research toward Molecular Signatures, Subtypes, and Stages and Precision Medicine: Application in Cohort Studies and Trials. In Methods in Molecular Biology; Humana Press Inc.: New York, NY, USA, 2018; Volume 1750, pp. 31–66. [Google Scholar] [CrossRef]

| Methods | Authors | Network Styles | Dynamics | Input | Principle | Optimization |
|---|---|---|---|---|---|---|
| MDS | [29,134] | Undirected networks | Nonlinear | Adjacency matrix/ stoichiometric matrix | Optimization based on KNN and K-means using Euclidean distances and Bootstrap. | Linear discrete |
| Probabilistic controllability approach | [32] | Undirected networks | Nonlinear | Adjacency matrix/ stoichiometric matrix | Optimization based on the shortest path between nodes based on an index built using the correlation between reactions. | Linear continuous |
| DFVS | [135,136] | Directed networks | Nonlinear | Adjacency matrix/ stoichiometric matrix | Optimization based on nonlinear functions in a system of ODE that define the structure of the graph. | Nonlinear continuous/ discrete |
| Author | Outcome | Sample Type | Omics Data Type | Main Outcome or Application | Method |
|---|---|---|---|---|---|
| [34] | Human astrocytes stimulated with palmitic acid | Cultured cells | Transcriptomic and proteomic | Identification of metabolic switches | FBA, FVA, MDS |
| [174] | Breast cancer | Cancer and normal samples | Based on previous GEMs [175] Transcriptomic and proteomic | Identification of drug targets | Topological properties and power-law degree |
| [32] | Healthy and cancer states | Tissues | Based on previous GEMs (Basler et al. [29] and Gatto et al. [175]) | Differences in metabolic flux | PMDS |
| [176] | Hepatocellular carcinoma patients | Cancerous liver samples and non-affected tissue | Transcriptomic | Detection of tumor subtypes | FBA, minimum driver node sets—MDS |
| [29] | Breast, lung, renal, and urothelial cancers | 4 cancer and healthy sample networks | Based on previous GEMs [175] Transcriptomic and proteomic | Reactions leading to cancer | Flux coupling |
| [101] | 15 cancer types | Normal and corresponding cancer cells | Transcriptomic | Identification of drug targets as driver nodes | MDS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angarita-Rodríguez, A.; González-Giraldo, Y.; Rubio-Mesa, J.J.; Aristizábal, A.F.; Pinzón, A.; González, J. Control Theory and Systems Biology: Potential Applications in Neurodegeneration and Search for Therapeutic Targets. Int. J. Mol. Sci. 2024, 25, 365. https://doi.org/10.3390/ijms25010365
Angarita-Rodríguez A, González-Giraldo Y, Rubio-Mesa JJ, Aristizábal AF, Pinzón A, González J. Control Theory and Systems Biology: Potential Applications in Neurodegeneration and Search for Therapeutic Targets. International Journal of Molecular Sciences. 2024; 25(1):365. https://doi.org/10.3390/ijms25010365
Chicago/Turabian StyleAngarita-Rodríguez, Andrea, Yeimy González-Giraldo, Juan J. Rubio-Mesa, Andrés Felipe Aristizábal, Andrés Pinzón, and Janneth González. 2024. "Control Theory and Systems Biology: Potential Applications in Neurodegeneration and Search for Therapeutic Targets" International Journal of Molecular Sciences 25, no. 1: 365. https://doi.org/10.3390/ijms25010365
APA StyleAngarita-Rodríguez, A., González-Giraldo, Y., Rubio-Mesa, J. J., Aristizábal, A. F., Pinzón, A., & González, J. (2024). Control Theory and Systems Biology: Potential Applications in Neurodegeneration and Search for Therapeutic Targets. International Journal of Molecular Sciences, 25(1), 365. https://doi.org/10.3390/ijms25010365






