Abstract
Cognitive impairment (CI) is a characteristic non-motor feature of Parkinson disease (PD) that poses a severe burden on the patients and caregivers, yet relatively little is known about its pathobiology. Cognitive deficits are evident throughout the course of PD, with around 25% of subtle cognitive decline and mild CI (MCI) at the time of diagnosis and up to 83% of patients developing dementia after 20 years. The heterogeneity of cognitive phenotypes suggests that a common neuropathological process, characterized by progressive degeneration of the dopaminergic striatonigral system and of many other neuronal systems, results not only in structural deficits but also extensive changes of functional neuronal network activities and neurotransmitter dysfunctions. Modern neuroimaging studies revealed multilocular cortical and subcortical atrophies and alterations in intrinsic neuronal connectivities. The decreased functional connectivity (FC) of the default mode network (DMN) in the bilateral prefrontal cortex is affected already before the development of clinical CI and in the absence of structural changes. Longitudinal cognitive decline is associated with frontostriatal and limbic affections, white matter microlesions and changes between multiple functional neuronal networks, including thalamo-insular, frontoparietal and attention networks, the cholinergic forebrain and the noradrenergic system. Superimposed Alzheimer-related (and other concomitant) pathologies due to interactions between α-synuclein, tau-protein and β-amyloid contribute to dementia pathogenesis in both PD and dementia with Lewy bodies (DLB). To further elucidate the interaction of the pathomechanisms responsible for CI in PD, well-designed longitudinal clinico-pathological studies are warranted that are supported by fluid and sophisticated imaging biomarkers as a basis for better early diagnosis and future disease-modifying therapies.
1. Introduction
Parkinson disease (PD), the most common movement disorder and the second most common neurodegenerative disease after Alzheimer disease (AD), is characterized by progressive degeneration not only of the dopaminergic striatonigral system, responsible for the core motor symptoms, but also of many other neurological systems and organs. These lesions are due to the widespread intraneuronal and neuritic deposition of phosphorylated α-synuclein (αSyn), forming Lewy bodies (LBs) and Lewy neurites, which are the morphological hallmarks of PD and related synucleinopathies. They are part of multiple pathways in the pathogenesis of PD which include oxidative stress, abnormal proteasome, mitochondrial, synaptic and lysosomal dysfunction, and neuroinflammation, which is related to the spreading of abnormal proteins in cortical and subcortical regions with the subsequent dysregulation of multiple neurotransmitter systems [1,2,3,4,5,6]. The resulting biochemical deficits induce a heterogenous clinical spectrum of motor and non-motor symptoms that contribute to the overall disease burden of this multisystem/organ disorder [7,8,9]. Cognitive impairment (CI) that has been increasingly recognized as an integral part of PD since its historical description by Charcot (1877) [10] shows a complex spectrum ranging from subtle cognitive decline (SCD) and mild cognitive impairment (MCI) to full-blown dementia (PDD), and it can be present already during the prodromal phase of PD [11,12]. SCD is a self-perceived decline in cognitive abilities with normal age-, sex- and education-adjusted performance on standardized cognitive tests indicating adequate cognitive functions [13], although there is currently no consensus on clinical criteria for SCD in PD [14]. Since it is already associated with brain metabolic changes in multiple cortical regions indicating early aberrant pathological changes [15], there is an increasing risk of developing dementia [16]. Parkinson disease with mild cognitive impairment (PD-MCI), a gradual decline in single or multiple cognitive domains [17,18] and a risk factor for PDD [19], is characterized by deficits in at least four cognitive domains (executive and visuospatial abilities, attention and memory), being severe enough to significantly affect routine functions of life [20,21,22,23]. Cognitive decline may occur in presymptomatic stages of PD [24] preceding the onset of dementia by up to 20 years as well as at the time of diagnosis or during the disease process. It has a high variability in severity, rate of progression and type of affected cognitive domains [3,25,26], but its structural and molecular backgrounds are incompletely understood [27,28]. CI has severe consequences even at early disease stages, poses a heavy burden on both patients and caregivers, affects the quality of life and is a risk factor for early mortality [14,29,30,31]. Novel genetic, fluid or imaging markers appear promising in facilitating the diagnosis and staging of CI in PD, and the stabilization or amelioration of cognitive outcomes is a goal of many ongoing clinical trials for disease-modifying treatment options. This article, based on literature research in PubMed and Google Scholar until November 2023, will critically review the currently available data about the epidemiology, essential clinical, neuroimaging, connectivity, morphological substrates, and biomarkers of CI in PD. The relations between PDD and dementia with Lewy bodies (DLB), considered as two heterogenic phenotypes of the LB continuum, will not be discussed in this review, since they have been reviewed recently [32,33,34,35,36,37], including connectivity studies in DLB [38].
2. Epidemiology of CI in PD
2.1. PDD—General Items
PD patients have a three to five times higher risk of developing CI than people without PD of similar age [25]. PDD has a four to six times increased lifetime incidence rate compared with age-matched controls [39,40]. The current prevalence of dementia is 31.3% (95% CI 20.1–40.1), with incidence rates from 42.6 to 112.5/100,000 person-years [41], indicating that around 10% of a PD population develop dementia per year [42]. The incidence of PDD increases with the duration of the disease from 3% to 30% of individuals followed for 5 years. Its prevalence is between 48% and 78% with a mean of 75% after survival for more than 10 years [43] and 83% after 20 years [40]. The relative risk for developing dementia in incident PD subjects compared to non-PD subjects was 2.47 (1.55–2.39) [39]. Systemic reviews suggest that 3–4% of dementia in the general population would be due to PDD; its estimated prevalence in the population older than 65 years being 0.2–0.5% [44]. The global pooled frequency of PDD is 26.3% with variations according to methodologies (14–55%) [45]. Its cumulated prevalence in PD patients with a mean age of 54–70 years is 17% at 5 years and 83% at 20 years after diagnosis and up to 95% by age 90 years [46]. Of note, there are several challenges in estimating the frequency of cognitive change in PD due to recently standardized diagnostic criteria variations in neuropsychological evaluation and differences in population sampling [47].
2.2. PD—Subjective Cognitive Impairment and MCI
Since there are no guidelines for PD-SCD, researchers have classified it differently, and some defined it as subjective CI without objective cognitive decline but poor performance in action naming [48] and an indicator of subsequent CI [49,50]. Its incidence, therefore, varied considerably between 25% and 85% [14,51,52,53,54,55,56].
MCI, a transitional stage between normal cognition and dementia, can occur in the presymptomatic phase of PD; it affects 19–30% of newly diagnosed, untreated (de novo) PD patients and has a frequency ranging from 21% to 70% with a mean of 25.8% [25,57,58,59,60,61]. About 30% of de novo PD patients complain of memory issues and are more likely to develop MCI after 2 years follow-up [50], but other factors, such as anxiety and affective symptoms, may contribute to the progression of cognitive deficits [62,63]. Although the reported estimates of cognitive dysfunction in non-demented PD patients vary between 19 and 55%, CI in non-demented PD is underestimated in practice [64]. At least 20–30% have at least mild cognitive changes even at the time of PD diagnosis [65], increasing to 40–50% after 5 years follow-up [66] and to 75% after survival of more than 10 years [40]. A recent meta-analysis reported a pooled MCI prevalence of 40% [67], while the estimated prevalence of MCI in the general population (age 60–90 years) ranges between 16 and 20% [68].
About 60.5% of PD patients were diagnosed with MCI when comprehensive assessment was performed (MDS criteria level II), while this number was 23.3% when only brief assessment was available (Movement Disorder Society/MDS criteria level I); multiple domain impairment was most frequent (96.2%). In contrast, 42.3% of MCI cases developed dementia at follow-up [69]. Between 20 and 57% of PD patients were affected by MCI within the first 3–5 years after diagnosis [27,70] with a predominance of non-amnestic MCI (naMCI) [71]. MCI is found in 50% of patients with idiopathic REM sleep behavior disorder (RBD), a frequent precursor of PD, and in 73% of PD patients with RBD, but in only 11% of those without RBD [72]. MCI was almost threefold higher in PD patients with RBD compared to those without it (66% vs. 23%; p < 0.001), and subjective cognitive decline was reported in 89% of PD + RBD compared to 58% of those without RBD [73]. More recent studies reported a prevalence of MCI level I of 12.8% in the lower risk, 21.9% in the higher risk, and 64% in RBD patients, 66% of which had multidomain level II MCI, particularly attention and memory deficits [74].
The classification of MCI for 12 neuropsychological criteria found 14% PD-MCI using two scores in one domain at two standard deviations below normal scores and 89% PD-MCI with one score in one domain at one standard deviation below normative scores [75]. A multicenter analysis revealed an average prevalence of MCI of 25.8%, 18.9% in an incidental, untreated cohort, and 39.4% in advanced PD. The cognitive phenotype was heterogenous; the most common subtypes were amnestic and non-amnestic MCI (aMCI, naMCI) single domain (11.3% and 8.9%, respectively) [76]. More recent studies reported 69.3% PD-MCI, 16% PD with normal cognition (PD-NC) and 14.66% PDD [77] or 34% PD-MCI with 77.8% impaired in multiple cognitive domains [59].
Among patients with PD-NC, within 3 years, 25% (95% CI 20–30%) converted to PD-MCI and 2% (95% CI 1–7%) converted to PDD, whereas 28% (95% CI 20–37%) reverted back to normal cognitive functions [78]. Meanwhile, 59% of PD patients with persistent MCI within one year developed PDD [18]. The incidence of progression from PD-MCI to PDD was 98.0/1000 person-years with an annual conversion rate of 11% [79]. The PDD converters had higher frequencies of multidomain MCI and amnestic MCI with poorer performance of frontal/executive, memory and language function domains [80]. Cognitive domains with higher frequencies of impairment were found in verbal memory and attention/processing speed, but no significant differences in the prevalence of CI were found during a 5 years follow-up [81].
In conclusion, the incidence and prevalence of CI/dementia is higher in PD, and the development of CI (initially MCI) is accelerated in PD in comparison to the age-matched control population.
3. Risk Factors of CI in PD
Risk factors for CI/dementia in PD are old age, lower education, duration of disease and motor symptom severity, in particular predominant rigidity, postural imbalance, and various non-motor symptoms, such as depression, psychosis or hallucinations [39,82,83,84]. Cognitive dysfunction progresses more rapidly in patients with older disease onset, the frequency of MCI and dementia in young-onset patients (50 ± 8 years) being 5% and 10% compared to 25% and 22.3% in older ones (61 ± 8.3 years) (p < 0.0001) [85]. Women experience a worse global cognitive decline during the prodromal phase of PD [11]. RBD is a cognitive risk factor across individuals of all ages with recently diagnosed PD [74,86]. Increased homocystein levels in peripheral blood are another risk factor for cognitive decline in PD, although no association was found between the polymorphism of genes involved in homocystein metabolism [87]. Both diabetes and prediabetes also may negatively affect cognitive function in PD, with a significant interaction of diabetes status and age, but not with duration of PD [88]. Gut microbiome composition (reduced short-chain fatty acid-producing bacteria) has been associated with PD-CI [89]. Both increased uric acid levels and altered glomerular filtration rate with lower cerebrospinal fluid (CSF) amyloid-β (Aβ) and αSyn, and higher neurofilament light (NfL) in serum are associated with CI in PD [90,91]. Orthostatic hypotension prevalence is correlated to severity of CI, particularly with a lower performance in executive functions (EFs) [92]. It is a significant risk factor for CI in PD, especially in women and persons not suffering from hypotension [93]. Early hypotension, but not its symptom severity, increased dementia risk in PD patients by 14% while not being associated with other clinical or neuropathological variables [94]. PD patients with freezing of gait (FOG), an important risk factor for CI, exhibit worse global cognition, EF/attention, language memory and visuospatial functions [95,96,97]. Another risk factor for PDD is the overuse of anticholinergic drugs, especially in older PD patients with multimorbidities [98]. The presence of cerebral small vessel disease, often related to diabetes mellitus, hyperhomocysteinemia and hypertension, is an independent risk factor for PD-CI [99]. A review of the wide spectrum of risk factors of PD using the ICD-10 was published recently [100]. On the other hand, there is an association between poor cognitive functioning and risk of increased parkinsonism, including probable PD [101].
4. Genetic Factors of PD-CI
Although most cases of PD are sporadic and only approximately 7–10% result from monogenic cause, either autosomal dominant or recessive [102,103], nearly all PD is genetically influenced. More than 100 genes or genetic loci have been identified, and most cases likely arise from interactions among many common or rare genetic variants [4]. CI has been reported in all monogenic PD forms with variable rates. Currently, there are seven well-described genes that increase the risk for PD with variable rates of penetrance: in addition to SNCA, LRRK2, VPS35, PRKN, PINK1, DJ1 and the glucocerebrosidase gene GBA [104], there are PD risk variants, including PITX3, TMEM106B, SNCA Rep1, APOE ε4, COMT and MAPT H1/H1 with respective relationships to cognition [105]. Furthermore, seven upregulated genes, namely SNAP25, GRIN2A, GABRG2, GABRA1, GRIA1, SLC17A6, and SYN1, have been identified, which are significantly correlated with PDD [106]. GBA mutations have a negative impact on cognition, whereas LRRK2-associated disease may have a milder cognitive phenotype, the others having a potential effect on cognitive outcome [107]. GBA-PD is associated with faster motor and cognitive decline, especially with impaired visuospatial/EFs [108,109], whereas the rs12411216 variant of GBA was significantly associated with PD-MCI [110]. PARK16 rs6679073 carriers were less likely to develop MCI compared to non-carriers in a 4-year follow-up study, suggesting that this variant may have a neuroprotective effect on cognitive functions [111]. Longitudinal cognitive decline in patients with GBA-PD was more severe than in those with LRRK2/GBA-PD, which more closely resembled LRRK2-PD. This supports the notion of a dominant association in individuals who carry both alleles and raises the possibility of an LRRK2 and GBA interaction, although its biological basis is not clear [112]. APOE ε4 has been identified as a major risk factor for cognitive progression in PD [113]. It has an age-dependent effect on decline in global cognition as well as in most cognitive domains [114,115]. Carriers of APOE ε4 and GBA mutations had faster cognitive decline and were at higher risk of progression to dementia [116], whereas no significant gene-related effects were observed for MAPT and SNCA rs356219 [117]. In PD patients, aquaporin-4 polymorphism (rs162009) was associated with slower dementia conversion and better performance in semantic fluency, while subtype rs68006382 was associated with faster progression to MCI, worse performance in semantic fluency and other cognitive domains. Genetic variations of aquaporin-4 may contribute to an altered rate of cognitive decline probably due to subsequent alterations of glymphatic efficacy, which is a sleep-enhanced brain waste clearance system [118]. Finally, a possible role of glial cell line-derived neurotrophic factor (GDNF) on CI in PD has been suggested, although α- and β-GDNF are not used for predicting CI in PD [119].
In conclusion, recent genetic studies have shown that at least seven well-described genes and a large number of variants are important risk factors for cognitive decline in PD; linking genetic profiles to cognitive outcomes may have an important clinical impact.
5. Development and Clinical Profile of CI in PD
Cognitive changes in PD manifest early and are more heterogenous than previously appreciated [64,65]. Their onset is insidious and the evolution is progressive with a mean annual Mini-Mental State Examination (MMSE) decline of approximately 2.3 points [120]. However, some PD patients may exhibit early cognitive deficits that will not evolve into PDD or persist over an extended period [121]. PD-MCI may begin before the motor symptoms [122] and even 5 years before clinical diagnosis of PDD [123]. Males usually show a more rapid progression of CI than females [124], in particular during the pre-diagnostic phase that exceeds rates associated with normal aging [125].
The mean time from onset of PD to severe CI/dementia is approximately 10 to 15 years, although there is wide individual variance [39], and even in the absence of full-blown dementia, 30–67% of patients with advanced PD show clinically moderate CI [126].
MCI, according to current clinical criteria, includes amnestic and non-amnestic types (aMCI and naMCI) [127], which may involve single or multiple cognitive domains. The latter was observed in 99.8% of the PD-MCI patients [59]. The heterogeneity of early de novo PD-MCI suggested the existence of three distinct memory phenotypes: cluster A (65.9%) included memory unimpaired patients, cluster B (23.2%) included those with mild episodic memory disorder (prefrontal executive-dependent phenotype), and cluster C (10.9%) included those with severe episodic disorder, which is related to the co-occurrence of hippocampal-dependent deficits with premotor executive memory dysfunctions. These phenotypes did not differ in terms of motor and other non-motor features, but the attention/executive deficits progressively increased from cluster A to cluster C, which had worse quality of life compared to others. Brain structural imaging correlates substantiated this cluster model [128].
The involvement of the cognitive domains of EF, which includes attention and working memory, is most prominent in early PD [25,129,130], while visuospatial function and global cognitive deficits occur in the mid-stage of the disease [131,132,133]. While deficits in EFs and visuospatial skills were apparent in PD patients compared to controls, many aspects of cognition remained intact [134]. Patients with poor baseline visual performance showed greater decreases in cognition over time and predicted a greater probability of dementia, whereas the retinal thickness of the ganglion cell-inner plexiform layer did not predict cognitive decline [135]. The cognitive scores of the frontal/EF domain contribute the most to predicting dementia [136].
A three-stage clinical sequence related to cognition has been proposed for PD patients, with SCD as the prodromal phase, which is followed by MCI and finally leading to dementia [15,137,138]. Follow-up studies showed a higher risk of developing PD-MCI and PDD for patients with PD-SCD compared to those without SCD at baseline [49,50,53,137]. Lower baseline attention and language scores were associated with progression to MCI, whereas higher baseline scores in all cognitive domains except EF were associated with clinical and psychometric reversion to “normal” cognition [139]. However, an increased risk of PDD conversion persists even in these reverters [78]. PD-MCI patients showed a specific pattern of language dysfunctions [48], while PD-MCI converters had more severe cognitive deficits in frontal EF, immediate verbal memory and visual recognition memory compared to non-converters [140,141]. A meta-analysis of cognitive outcome and prediction in PD-MCI conversion to PDD was made recently [142].
The Movement Disorder Society Task Force (MDSTF) has delineated the following diagnostic criteria for PD-MCI [17,143]: characterization of the clinical syndrome (inclusion criteria), gradual decline in the context of established PD, cognitive ability and deficits in neuropsychological testing or a scale of global cognitive deficits not sufficient to interfere with functional independence; exclusion criteria (PDD, other explanation for CI and PD-associated co-morbidities). The MDSTF has also suggested diagnostic criteria for PDD [20,144] with these most important cognitive features (see [33,37]).
The impairment of more than one cognitive domain, e.g., executive dysfunction, attention, verbal fluency impairment, both phonetic and semantic, is more pronounced in early and moderate stages of PD, executive dysfunction being the most pronounced. Memory complaints are the presenting cognitive problem in 67% of patients with PDD, which affect short-term memory, verbal, non-verbal and visual domains. Construction, praxis and visuospatial functions are more severely impaired than in AD, but language deficits and aphasia are less severe. Fluctuating cognition is present but less often than in DLB.
The nosological classification of PDD was based on the DSM-IV criteria [145], while the MDSTF defined two levels of diagnostic certainty: possible and probable [20]. Dementia was defined as a syndrome of insidious onset and the progressive decline of cognition and functional capacity from a premorbid level, which is not attributable to motor and autonomic symptoms. A study of diagnostic step I allowing the diagnosis of possible PDD showed a sensitivity and specificity of 78% and 95%, respectively, with a positive-predictive value of 70% and a negative predictive value of 97%, indicating an accuracy of 93.4% [146], while the DSM-IV criteria failed to identify 22% of patients fulfilling MDSTF criteria. After the MDS proposals, great advances have occurred in the field of cognitive assessment in PDD, although many studies concluded that the accuracy of these instruments was not entirely satisfactory [147,148,149,150]. A critical point was that these guidelines did not address limitations inherent to populations with low levels of formal education. Since education is a major factor underlying cognitive performance, a revision of the current guidelines appears necessary considering differences among populations with better scores in cognition [151].
6. Neuroimaging Findings in CI in PD (See Supplement Table S1 [152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215])
Although there is a continuum from PD-NC via SCD to PD-MCI to PDD, it appears of interest to describe the essential neuroimaging findings for these cognitive stages separately.
6.1. Gray Matter Changes in PD-MCI
The clinical heterogeneity of PD-MCI is reflected in the variability of structural imaging findings, but the identification of a structural signature of this cognitive deficit remains challenging [42]. Structural atrophy may not be associated with any cognitive domain with the exception of visuospatial measures in primary sensory and motor cortices [216]. In PD-NC patients, structural neuroimaging (MRI) may be unchanged or shows mild diffuse brain atrophy with slight changes of the medial temporal lobes [152,153]. Compared to a control group, PD-NC individuals suffered from gray matter (GM) atrophy mainly in prefrontal and limbic lobes and left temporal gyrus [156]. Voxel-based morphometry (VBM) in PD patients with SCD or subjective memory complaints revealed reduced GM intensities in anterior cingulate and right parietal lobe [55,217] and mild thinning of the midsagittal corpus callosum [218]. Mild atrophy affected the orbitofrontal regions, left superior parietal lobule, and more widespread limbic and fronto-parietal cortices [158]. PD-MCI patients showed baseline thalamus atrophy and progressive atrophy in the thalamus, caudate nucleus, presubiculum, and cornu ammonis, which was associated with executive and memory dysfunctions [173]. Cortical thinning in parietotemporal regions but also total GM volume reduction and ventricular enlargement were associated with CI in executive, visuospatial/perceptual and memory domains [159]. GM reduction in temporal, parietal and occipital regions was found in PD individuals with aMCI and to a lesser degree, naMCI, with executive MCI in frontal and striatal, and in those with non-executive MCI, in posterior regions [160]. PD patients with cognitive decline after 18 months, compared to those with stable condition at baseline, had lower cortical thickness within the prefrontal, medial and lateral temporal cortices [162]. PD-MCI showed a significant enlargement of bilateral temporal and left frontal lateral ventricles [154]. After 2 years of follow-up in PD-NC patients, those with a greater third ventricle width developed CI [155].
The reduction in GM density in superior frontal cortex and cerebellum was related to cognitive performance in early PD-MCI [163]. PD-NC patients converting to MCI showed cortical thinning in medial and superior frontal, inferior temporal, cingulate and supramarginal gyri, whereas those with stable normal cognition suffered from progressive cortical thinning mainly in parietal and occipital regions [167].
PD-MCI patients showed reduced gray matter volume (GMV) in the temporal and parietal cortex, putamen, amygdala, hippocampus and cerebellum [166]. Global cognitive dysfunction (mainly non-memory related) was associated with atrophy in the frontal lobe, left middle and inferior temporal gyrus or in the left superior temporal, right fusiform and bilateral lingual regions [219]. A meta-analysis reported higher GM atrophy in the bilateral prefrontal cortex, left insula and angula gyrus, right supramarginal gyrus and midcingulate cortex in the PD-MCI cohort [164], while another meta-analysis reported GM atrophy in the left anterior insula, left inferior and orbital frontal gyrus [168]. The atrophy of limbic lobes was associated with impaired memory, frontal lobe atrophy and frontostriatal metrics with attention/executive decline [156,161].
Early drug-naive PD-MCI showed atrophy of the right entorhinal cortex [220]. Morphometric studies in early PD-MCI patients showed reduced limbic subcortical structures [221]; the hippocampal subfields subiculum, presubiculum, CA1 and fimbria were strongly correlated with CI, subfields GC-DG and fimbria being sensitive in detecting early stage of CI, while the left parasubiculum and presubiculum predicted conversion from PD-NC to PD-MCI [171]. A smaller CA1 region and reduced hippocampal–amygdaloid transition area volume were observed [169]. A 4-year follow-up study reported that both PD-NC and MCI patients showed a more severe decline in anterior and posterior hippocampus volume with a significant reduction in the bilateral CA4 subregion, other hippocampal subfields and the right presubiculum [170]. A statistically significant difference was observed for the raw hippocampal volumes between PD-MCI and PD-NC [222]; a low baseline hippocampal volume and fornix fractional anisotrophy (FA) were associated with faster cognitive decline [161]. Thus, involvement of the temporo-parietal cortex, lateral ventricular enlargement and selective vulnerability of the hippocampus could be structural biomarkers for conversion to PD-MCI [154,157,223,224].
The volumetric distribution of the subcortical structures (caudate nucleus, putamen, thalamus and amygdala) in PD-MCI exhibited an overlap with the PD-NC group due to a lack of spatial specificity in their atrophy levels, but it was able to predict CI [225]. A high free water fraction in the dorsal caudate nucleus and bilateral nucleus basalis of Meynert (NBM) was associated with cognitive decline across several domains except memory [172]. Cortical microstructural changes (increased intracortical diffusivity) in the absence of cortical thinning were associated with cognitive and psychiatric symptoms [226].
6.2. White Matter Lesions in PD-MCI
White matter (WM) damage often precedes GM atrophy, the cumulative burden of WM lesions correlating with CI in PD cases without co-pathologies [174]. Prominent WM changes were observed in the earliest stages of PD with intact GMV [178,227,228], and the white matter hyperintensity (WMH) burden differed from that in PD-NC [229]. Specific brain regions, including periventricular and deep WM, had significantly higher WMH load in the PD-MCI than in the PD-NC group [175]. Total and periventricular WMHs at baseline predicted a decline in global cognition, while the total WMH burden predicted the decline of EFs (both p < 0.05). Occipital WMH volumes predicted declines in global cognition, visuomotor attention and visuospatial memory, while WMH volumes at baseline did not predict motor decline [176].
De novo PD subjects with high baseline WMH had significantly greater cognitive decline than those with low WMH load [230], and early episodic memory impairment was related to WMH, mainly in the prefrontal and temporal lobes [177]. WMH volume, changing over time, was associated with an impairment of global cognition, EF and language, whereas WM microstructural lesions did not change significantly [231]. However, a reduction in WM volume was not a consistent finding in PD-MCI compared with healthy controls (HCs) [232,233,234], and WMH often did not impact cognitive function in newly diagnosed PD [235], while in other studies, cognitive deficits were not associated with WMH but with neuronal deficits [236]. This suggests that for some phenotypes of MCI, WM lesions may not be prominent in early stages of PD [42]. On the other hand, microstructural damage in the major motor and associative WM tracts was present and progressed even in early phases of PD [237]. PD-MCI had similar but more severe and widespread WM degeneration in commissural fibers and association projections than PD-NC. The FA of the anterior part of right inferior fronto-occipital fasciculus was positively correlated with the Montreal Cognitive Assessment scale, EF and visuospatial function, while the mean diffusivity of the left superior longitudinal fasciculus was negatively correlated. Thus, regional tract-specific microstructural degeneration, especially in association fibers, is a reliable indicator of PD-MCI [185]. Tract-based FA values decreased in the bilateral corticospinal tract, anterior and posterior cingulum, fornix, bilateral superior thalamic radiation, corpus callosum, bilateral superior and inferior longitudinal fasciculus, superior and inferior fronto-occipital fascicles, and bilateral parieto-occipital tract [181]. Widespread frontostriatal WM tract FA reduction was seen in PD-CI [184]. WM abnormalities, associated with significantly lower neurite density and orientation dispersion in the prefrontal region, the cingulum bundle and in thalamo-frontal tracts, were related to cognitive decline [58,182].
WM abnormalities appeared to be widespread [42], involving multiple brain regions with heterogenous patterns and abnormal diffusivity variables in WM adjacent to cortices and limbic substructures [238]. More comprehensive information on WM changes was obtained by combining diffusion tensor imaging (DTI) indices, with an accuracy of 91.67% and sensitivity of 92.86%, thus achieving the best classification in this data set [217].
The involvement of the corpus callosum, cingulum and major association tracts in PD-MCI but not in PD-NC [157,178,179] as well as increased hyperintensity in frontal and interhemispheric WM (genu and body of corpus callosum) were seen [178,180,239]. Thinning of the corpus callosum was correlated with the thickness of the left orbitofrontal cortex in PD-MCI [218]. The corpus callosum and cingulum bundle showed the same trend to decline with cognitive dysfunction [240]. MCI in early PD stages was associated with poorer olfactory function and disordered WM integrity in the anterior olfactory structures [183]. The bilateral cortico-amygdaloid transition area (CAT) and sectors superficial cortex-like region (sCLR) volumes of PD-MCI and PDD patients were less than those of PD-NC and HC ones. The left CAT and sCLR volumes discriminated CI in PD and were associated with hyposmia [241].
In conclusion, GM changes in PD-MCI involve prefrontal areas and hippocampal subregions, WM lesions being more typically observed in the corpus callosum and limbic structures, while WMH burdens in periventricular and deep areas are widespread in early stages of CI in PD, indicating that the impairment of multiple GM and WM may be important causes of cognitive dysfunction in PD-MCI.
6.3. Neuroimaging Findings in Converters to PDD (see Supplement Table S2)
In comparison to PD-MCI patients who did not progress to PDD, the converters showed lower GM densities in prefrontal areas, the left insular cortex, the insula, and the bilateral caudate nucleus and lesser cortical thickness extending from frontotemporal cortices to posterior cortical areas [140,167,242]. They suffered faster rate of GM thinning in temporal and medial occipital lobes, limbic subcortical structures [221] and progressive atrophy in frontal lobes [243]. Their cortex was thinner than that of non-converters, bilaterally in the frontal, insula and left middle temporal areas, displaying a more widespread pattern [244]. While PD-MCI patients showed decreased cortical thickness in the left superior temporal, insula, right fusiform and bilateral lingual regions, in those converting to PDD, widespread cortical thinning was detected, including in the left superior temporal gyrus, insula and bilateral fusiform areas [219].
A progressive atrophy of basal ganglia after one year follow-up and widespread cortical thinning over 3–6 years were observed [237]. The PD-MCI converters had lower cortical thickness within the prefrontal, medial and lateral temporal regions compared with the stable group [162], as well as larger WMH volume and higher hyperintensity scales than non-converters, demonstrating that the WMH burden is related to ongoing decline in frontal-lobe-based cognitive performance [245].
6.4. Gray Matter Lesions in PDD (See Supplement Table S3)
GM atrophy in PDD was more severe and extensive than in PD-MCI [166]. Whole brain atrophy showed an increase of 1.12% in PDD patients compared to 0.31% in the non-demented group and 0.34% in healthy age-matched controls. Decreased GM volume in PDD patients in the temporo-parietal region was confirmed by subsequent studies [42,224]. Atrophy affected the bilateral temporal gyrus, posterior cingulate and left cingulate gyrus, right parahippocampal lobe, hippocampus, right cuneus and precuneus, left inferior frontal gyrus and left insular lobe [246,247], with unilateral insular involvement in PD-MCI extending to bilateral insular affection in PDD [164]. Bilateral frontal atrophy [248], basal forebrain atrophy [249,250], and decreased volume of the claustrum were associated with cognitive decline [251,252]. Meta-analysis showed consistent GM loss in bilateral medial temporal lobes and striatum [253]. PDD had lower hippocampal volumes than HCs without exhibiting any differences from PD-MCI [254]. Early-onset PDD patients exhibited more severe atrophy in the left anterior cingulate and right inferior temporal gyrus associated with a significant volume reduction in substantia innominata [255]. Memory impairment was associated with frontal and hippocampal diffusivity also in the left cingulum [180,256,257]. There was a positive correlation between MMSE scores and cortical thickness in the anterior temporal, dorsolateral prefrontal, posterior cingulate, temporal fusiform and occipitotemporal cortices. Discriminant analysis showed that mean cortical thickness and hippocampal volume had 80% accuracy in identifying PDD patients, which could be characterized by a specific pattern of cortical thinning [258]. Thalamic dorsomedial nucleus free water (FW) correlated with the Montreal Cognitive Assessment scale changes over both 1 and 3 years, respectively, as did FW changes in the NBM, and baseline hippocampal FW was also associated with CI at 3 years [259].
Cluster analysis of multimodal imaging data identified three PD subtypes based in the prominent GM patterns: one group with widespread cortical and subcortical GMV and WM FA reduction, associated with considerable cognitive deficits, a second group with cortical atrophy limited to orbitofrontal and temporal regions with specific neuropsychiatric impairment, and a third subtype without increased detectable GM atrophy, lack of considerable CI and earlier disease onset [260].
Visual impairment in PD, along with a heterogenous profile of CI, contributed to the development of FOG that was due to impairment of executive control [261] and decreased contrast sensitivity related to volume decrease in the cuneus, the lingual and posterior cingulate gyrus, the superior parietal lobe and middle frontal gyrus, and the central areas of the dorsal and ventral visual information system [262]. FOG converters had reduced regional homogeneity in the bilateral medial superior frontal gyrus, which was negatively correlated with the postural instability and gait difficulty score [263].
6.5. White Matter Lesions in PDD
PDD suffered a significantly higher burden of WMH, especially deeper WMH, than PD-MCI [229,264]. WM abnormalities were widespread [42], involving multiple brain regions with a heterogenous pattern of abnormal diffusivity adjacent to cortical and limbic areas [238]. Thinning of the corpus callosum and microstructural WM abnormalities were associated with changes in the orbitofrontal cortex and contributed to CI by disrupting information transfer across interhemispheric and callosal-cortical projections [218,265]. The strongest of DTI changes occurred in the most anterior and posterior callosal segments, reflecting fronto-striatal and posterior cortical type cognitive deficits [265]. The corpus callosum, cingulum bundle and corticospinal tract contributed to decline of cognitive function [240]. The PDD group showed decreased FA and/or mean diffusivity increase in bilateral cingulate tract [266,267], in genu of corpus callosum [268] and hippocampus [269].
In summary, GM changes in PDD predominantly involve temporal regions including hippocampus, frontal and parietal cortex and subcortical areas including thalamus and NBM, while WM lesions predominantly involve the corpus callosum and cingulate gyrus, inducing dysfunction of cortico-cortical and cortico-subcortical networks [270].
7. Brain Network Studies
7.1. Early PD and PD-MCI
Preliminary analyses revealed reduced functional connectivity (FC) in both PD-NC and PD-MCI groups compared with HCs. The PD-MCI group had a significantly decreased FC within the default mode network (DMN), mainly between the hippocampus and inferior frontal gyrus, posterior cingulate cortex and posterior parietal lobule, and between the anterior temporal lobe and inferior frontal gyrus, as well as a significantly decreased FC in the middle frontal and middle temporal gyri. Decreased FC within the DMN between the anterior temporal lobe and inferior frontal gyrus was positively correlated with attention/working performance, while between the hippocampus and inferior frontal gyrus, it was positively correlated with memory function. The PD-MCI group further showed a significant reduction in FC between the DMN precentral, middle temporal gyrus, insula, anterior parietal lobule and middle frontal cortex [193] (Figure 1).

Figure 1.
Schematic overview of functional connectivity in some major networks in Parkinson disease with mild cognitive impairment (PD-MCI). Gray lines: hypocommunication, black lines: hypercommunication. L, R: left, right hemisphere. Network abbreviations: VAN: ventral attention; BGN: basal ganglia; LN: limbic; PFC: prefrontal cortex; FPN: frontoparietal; OFN: orbitofrontal; FTN: frontotemporal; DMN: default mode; CN: cerebellar; SAN: salience; SCN: subcortical; SMA: supplementary motor area; SMN: sensorimotor.
In PD patients with probable RBD, widespread abnormal FC within the most relevant neurocognitive networks, in particular between the left and right frontoparietal network (FPN), the ventrolateral and dorsolateral prefrontal cortex, and between the dorsolateral prefrontal cortex and inferior frontal gyrus, was present [194]. These and other FC changes were already detectable in drug-naive PD patients, even in the absence of clinically overt CI, namely abnormal intrinsic FC within the DMN, executive control network and salience network (SAN), and functional decoupling between the left and right FPN, potentially leading to impairment in cognitive processing and integration or to fronto-striatal maladaptive compensatory mechanisms [195]. PD-NC and PD-MCI patients showed decreased DMN connectivity of the bilateral prefrontal cortex within the FPN, which correlated with some cognitive parameters but not with clinical variables. This suggested that altered DMN connectivity characterizes PD patients, regardless of cognitive status, whereas a functional disconnection of the FPN was associated with MCI in the absence of detectable structural changes [196]. Disruption of the network between the bilateral superior medial frontal gyrus and anterior/middle cingulate cortex appeared relevant for self-awareness of cognition and error processes [201]. In early PD with MCI, a subnetwork predominantly linking temporo-parietal-occipital lobes decreased in both expression and flexibility, reflecting the degree of CI [271].
Disruption of the WM connectivity in frontal and posterior regions was associated with early CI development [80,202]. While PD-MCI patients showed decreased cortical thickness in the right lingual region, widespread changes in the left superior temporal, left fusiform, right insula and right fusiform areas resulting in decreased FC between these affected regions, and dysfunction of resting-state FC, contributed to cognitive decline in PD [219].
Network-based statistics analysis revealed decreased FC in the sensorimotor network (SMN) and DMN, and increased connections in the SAN and the FPN, indicating that the topological organization of GM networks was disrupted in early-stage PD [198], in which altered FC involved the FPN. Similar connections were involved in the DMN and cerebellar network (CN) in both PD-NC and PD-MCI, to a greater extent in the latter, indicating that DMN and CN disruption characterize PD-MCI [197].
Resting-state functional MRI (rsfMRI) analysis in PD-MCI compared to PD-NC revealed altered FC in the right frontal and bilateral parietal areas, which affected broader areas after longer disease duration, while decreased FC in the right frontal area was correlated with shorter disease duration. Decreased FC between substantia innominata, which is significantly correlated with cognitive performance, and the frontal area may be associated with early-onset MCI, suggesting that cholinergic deficits in frontal brain areas might play an important role in the acceleration of cognitive decline in PD [204,255]. Disrupted topology in high-order functional connectivity—FPN, visual, SMN and subcortical networks (in the precuneus, putamen, lingual and supramarginal gyrus, motor area, postcentral and inferior occipital gyrus)—were identified prior to clinical symptoms of CI [205].
rsfMRI analyses revealed abnormally increased and decreased amplitude of low-frequency intrinsic fluctuations (ALFF) and regional homogeneity (ReHo) in PD-NC individuals within the DMN (posterior cingulate, inferior parietal, entorhinal and somatosensory cortex, parahippocampus, basal ganglia and posterior cerebellar lobule VII), which mediates cognition [206]. The ReHo value in the DMN was closely related to PD cognitive function, and the DMN was affected before the development of CI and continuously deteriorated with disease progression [207].
PD-MCI was associated with reduced FC of the mediodorsal thalamus with the paracingulate cortex and breakdown in the connectivity of the mediodorsal thalamus with the para- and posterior cingulate cortex, respectively, while there was an increased FC of the mediodorsal thalamus and posterior cingulate cortex (anterior cingulate, dorsolateral prefrontal, and dorsal posterior parietal cortex) [200]. FOG converters exhibited diminished FC in the basal ganglia, limbic area and cognitive control cortex [263], and they changed subthalamic nucleus activity, causing FOG [272].
PD-MCI patients further showed decreased FC between the striatal networks. This was partly due to atrophy within the SAN. The seed analysis detected relations between higher MCI scores and lower connectivity between the left caudate head, dorsal anterior cingulate and left middle frontal cortex as well as between the right caudate head and anterior cingulate cortex, precuneus and left supramarginal gyrus, but there was increased FC to the left hippocampus and right cerebellar hemisphere [192].
In conclusion, both early and PD-MCI are characterized by reduced FC in the DMN and SAN, the inferior frontal and anterior temporal cortex, the left and right FPN, and the bilateral superior medial frontal cortices, with increased FC in the SAN and FPN, but there was a breakdown of the connection between the mediodorsal thalamus and the cingulate cortex, as well as the striatal network and its cortical connections, indicating a dysfunction of multiple attentional, cognitive control and other essential networks.
7.2. Connectivity Changes in PDD (Figure 2)
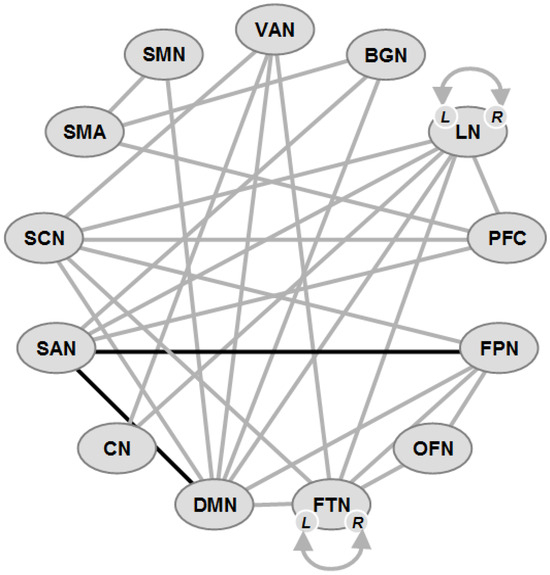
Figure 2.
Schematic overview of functional connectivity in some major networks in Parkinson disease dementia (PDD). Gray lines: hypocommunication, black lines: hypercommunication. L, R: left, right hemisphere. Network abbreviations: VAN: ventral attention; BGN: basal ganglia; LN: limbic; PFC: prefrontal cortex; FPN: frontoparietal; OFN: orbitofrontal; FTN: frontotemporal; DMN: default mode; CN: cerebellar; SAN: salience; SCN: subcortical; SMA: supplementary motor area; SMN: sensorimotor.
High-level cognitive processes are related to intrinsic functional brain networks, specifically the DMN, FPN, and SAN, with the SAN playing a role in modulating DMN and FPN activity [273], and a link between SAN and DMN related to cognitive test scores. The SAN is composed of the midcingulate cortex and insula and is integrating responses to salient stimuli [274]; its control over the DMN and FPN is dysregulated in PD and aging [275,276]. The SAN is a key neural substrate of CI in PD [277]. The basal ganglia network (BGN) is also important, because dopamine deficiency can alter functional brain networks [278,279,280]. However, the reliability of SAN dysfunction and its relationship to other brain networks as well as BGN connectivity with other cortical networks (DMN, FPN and SAN) and their impact on CI in PD are unclear [277,281].
Large-scale connectivity studies found that SMNs decreased their FC in the continuum of PD-associated CI [282]. Disturbed FC was associated with attention, EF, language, memory, learning, and visuospatial and global cognition in the bilateral hippocampus, left putamen, olfactory cortex and bilateral anterior temporal poles, suggesting that the cognitive domain-specific networks are distinct from each other and specific for different cognitive phenotypes, which may be related to specific changes in both FC and brain network topology [216,283,284].
Reduced cognitive performance was associated with FC in the dorsal insular cortex with the DMN [285]. Tracts between the dorsal anterior insular cortex and anterior cingulate cortex showing lower FA and higher diffusivity were related with lower EF and working memory, indicating structural damage in the dorsal limb of the SAN due to the loss of an interconnecting anterior insular and anterior cingulate cortex [286]. The FC between two distinct DMN systems (left-to-right hippocampus) was associated with global and domain-specific CI, while that from the medial prefrontal cortex to the posterior cingulate cortex was related to global and episodic memory impairment, indicating a functionally distinct role of the hippocampal subsystems within the DMN resting state network [287]. Reduced hippocampal FA associated with disrupted cortical FC [288,289], frontal cortical disruption and altered temporal FC were associated with global cognitive decline [269,290]. The severity of EF was correlated with lower dynamic FC between deep gray matter regions (DGMs) and the FPN, declining EF with increasing static DGM-FPN connectivity, and changes in the dorsal attention network [291]. Connectivity between the middle (associative) striatum and precuneus negatively correlated with EFs in PD [292]. Frontal/executive, visual memory/visuospatial, and attention/working memory functions were related to disrupted WM connectivity in the frontal and posterior cortical regions [202].
A decreased claustral structural network, linked to cognitive dysfunctions [293,294], attention or spatial navigation [295], memory and executive task alterations in PD [251], showed significantly decreased FC between the left claustrum and the sensorimotor cortical regions including the postcentral gyrus [296].
Recent rsfMRI studies, using the Parkinson’s Progression Markers Initiative (PPMI), a high-quality database of a large number of PD patients, reported several important findings. (1) There was reduced intra-FPN FC with alterations on posterior parietal nodes in the FPN in PD-CI [277], which was possibly due to reduced parietal metabolism [297], whereas there was no evidence of altered SAN-FPN or DMN-FPN connectivity predicting CI [196,298]. (2) PD-CI patients had dysfunctional SAN-BGN and SAN-DMN FC [277]. The SAN, according to some, included the left and right prefrontal cortex [277,299,300], whereas others did not [274,298,301]. These findings indicate a link between SAN FC with other large-scale brain networks and CI in PD, suggesting that positive FC between these networks is necessary for the SAN to disengage the DMN during cognitive control. Confirming reduced SAN-DMN FC with CI in PD [191,275], they support important relationships between intra-FPN, SAN-BGN and SAN-DMN FC and cognition in PD.
In conclusion, as shown in Supplement Table S3 and Figure 2, the FC changes in PDD were much more extended than in PD-MCI, involving practically all functional networks related to global cognition, attention, memory and other domains, with a breakdown of FCs in the DMN, SAN, FPN and BGN as well as the hippocampus, indicating a widespread breakdown not only of the brain networks that are responsible for global cognition and related functions.
8. Neurophysiological Studies in PD-CI
Neurophysiological methods offer the advantage of observing oscillatory patterns, whose regional and temporal profiles may elucidate how cognitive changes relate to neuronal mechanisms. Aberrant brain oscillations are a hallmark of PD pathophysiology, and cognitive deficits are thought to be related to altered functional brain connectivity. FC disruptions correlate with cognitive phenotypes in PD-MCI and PDD groups, typically in the α band, and EEG data indicate that FC decreases with the worsening of cognitive performance, and the loss of frontotemporal connectivity may be a promising neuromarker for PD-CI [302].
Both PD-NC and MCI patients had diminished δ and α phase; however, electrophysiological abnormalities were more pronounced in PD-MCI over frontal, central, parietal and temporal locations in almost all frequency bands, which was accompanied by bilateral thalamus, hippocampus and right putaminal atrophy [254]. Visual networks were more severely affected in PD-MCI, which was possibly related to dysfunctional subcortical modulating centers [303].
The evaluation of dynamic FC and the stability of functional networks in PD-MCI compared with early PD without MCI found that the α band as well as the functional network stability (FNS) of the central region, right frontal, parietal, occipital and left temporal lobes were abnormally increased, and the dynamic connectivity fluctuations in these regions were significantly decreased. In the γ band, PD-MCI patients showed decreased FNS in the central, left frontal and right temporal lobes, and there were active dynamic fluctuations in the left frontal, temporal and parietal lobes. These network changes were negatively correlated with cognitive function in early PD [304]. A significant joint effect of interventions on EF and task-based changes for the negative correlation between attention and θ power, a positive correlation between EF and α power, and a significant negative relationship between attention and θ power were found over time [305]. Cognitive deficits were further underlined by alterations at the brain network level, including higher δ and θ activity, lower α and β activity, increased connectivity, and segregated network organization [306].
PD-MCI patients showed altered left and right posterior middle frontal gyrus (PMFG)-based FC in the θ frequency bands. Poorer visuospatial function was associated with higher right PMFG FC under closed eyes and poorer attention function with higher left and right PMFG-based FC, which were independent risk factors for CI [307]. Oscillatory power changes in the cortico-striato-thalamo-cortical circuits (α frequencies in the caudate nucleus and α and θ in the dorsolateral prefrontal cortex) contributed to CI in PD [308].
PD-MCI patients compared to HCs were characterized by more frequent posterior topography of the δ-θ phase-amplitude coupling (PAC) and reversed δ-low frequency α PAC direction, i.e., posterior-to-anterior rather than anterior-to-posterior. This suggested that they showed abnormal neurophysiological oscillatory mechanisms mainly led by δ frequencies underpinning FC from frontal to parietal cortical areas [309].
Both the caudate nucleus and dorsolateral prefrontal cortex displayed decreased β oscillations during memory encoding in individuals with CI suggesting potential dysfunctions in the cortico-striatal circuit. Further analyses revealed differences in the α band in both regions and in θ in the dorsolateral prefrontal cortex, highlighting the involvement of the medial and lateral prefrontal cortex in motivation and cognitive control [310]. Low-frequency mid-frontal neural activity was associated with cognitive dysfunction [311,312,313], while others did not find any relationship between DMN FC and cognition in PD [277], which may be attributed to the vast heterogeneity of PD [281].
Aberrant neurophysiological signals were also associated with speech impairments in PD, in particular in the left inferior frontal cortex, the FC of this region with somatomotor cortices mediating the influence of cognitive decline on speech deficits [314].
Decreased θ, α and β activities were found in both the dorsolateral prefrontal cortex and caudate nucleus during working memory and correlating with cognitive dysfunction in PD patients [308]. The latter was associated with increased response latencies and decreased mid-frontal δ power across all tasks [315]. In general, cognition-related changes in EEG activities were observed over multiple spectral rhythms [316]. PDD patients showed decreased θ frequency in the temporal right-frontal left and temporal right-frontal-right regions while in the α frequency band. The most significant decrease was shown in the occipital right-frontal left and occipital left-frontal right areas. Significant asymmetry was seen in the frontal lobes [317]. There were also significant differences in the θ frequency range between many other areas [318].
PD-NC individuals demonstrated reduced P300 event-related amplitudes compared to HCs; PD-MCI patients had lower P300 amplitudes than both other groups and reduced volumes of the putamen. Both P300 amplitudes and putamen volumes showed moderate associations with EFs [319]. Early PD patients with cognitive deficits exhibited significantly larger P300 and N200 components and smaller amplitude N200 components, indicating impaired neuronal processing [320]. A meta-analysis showed prolonged N200 and P300 latencies in PD non-demented (PD-ND) individuals, compared to HCs, and prolonged P300 latency at the Cz electrode site in PDD patients compared to PD-ND ones [321].
Magnetoencephalography showed higher power in lower frequency bands (δ and θ) along with more severe CI and associated with memory, language, attention, and global cognition. Widespread group differences were found in the β band with significant changes between normal cognition and MCI groups. Moreover, bilateral frontal and left hemispheric regions were affected in the other frequencies as cognitive decline became more pronounced. These data suggested that PD-MCI and PDD are qualitatively distinct cognitive phenotypes, and most neurophysiological changes may occur during the time of this transition [322].
9. Involvement of Neuromodulatory Systems in PD-CI
Recent brain imaging studies have provided insight into the dysfunction of multiple neuromodulatory studies that are involved in PD-CI. These were not limited to dopaminergic (DAergic) dysfunction but rather demonstrated the parallel alteration of DAergic, cholinergic and noradrenergic systems [323].
9.1. Mapping Dopaminergic Modulation
Striatal and extrastriatal DA dysfunction in early PD [324] was associated with cognitive deficits due to abnormal processing in the cortico-basal ganglia circuit with reduced prefrontal and parietal metabolism [186] in the SAN and medial temporal lobe [325]. Drug-naive PD patients showed associations between striatal dopamine loss, WMHs and cognition [326], whereas no correlation was found between neuropsychological scores and DAT availability in those without MCI [327]. Reduced presynaptic DA uptake in the striatum resulted in abnormal processing in the frontostriatal circuit with reduced prefrontal and parietal metabolism [328], whereas mesocortical DA transmission appeared to be preserved [297]. Selective DA loss in the anterior putamen is a big risk factor for developing PDD [329,330].
The degradation of DAergic afferents to the anterior cingulate cortex played an essential role in cognition, as it is a key node of the SAN [331]. The depletion of dopamine increased signal variability in the SAN, which in turn lead to decreased corticostriatal connectivity [279,280]; this was supported by altered SAN-BGN FC associated with PD-CI.
PD-MCI patients compared to PD-NC ones showed a more widespread degeneration of dopamine terminals in the dorsal caudate nucleus, while other DAergic systems remained relatively preserved. In contrast, PDD patients showed a severe loss of lateral DAergic systems in frontal, temporal and parietal systems [332]. Degeneration of the medial substantia nigra (SN) and nuclei of other ascending pathways caused dysfunction of the striato-subfrontal and mesocortico-limbic loop [333]. Recent multivariate longitudinal analyses confirmed the impact of the dopamine system on long-term CI in PD [334]. MRI radiomics derived from the caudate nucleus were also highly associated with cognitive decline in PD [136].
9.2. Cholinergic Modulation
Striatal dopamine release can be triggered not only by the ascending activity of SN DAergic neurons but also by the activity of striatal cholinergic interneurons [335,336]. Cholinergic forebrain atrophy predicted cognitive decline in de novo PD [337], and PD patients with a smaller NBM volume had a 3.5-fold greater risk of developing MCI within 5 years [250]. Early cognitive deficits in PD without dementia were more closely related to structural MRI measures of cholinergic forebrain degeneration than hippocampal degeneration [338]. Early degeneration of the NBM and the nucleus of the vertical limb of the diagonal band of Broca (nvlDBB) and their projections preceded/predicted CI in PD [250,339], whereas sreuctural and microstructural alterations in the entorhinal cortex, amygdala, hippocampus, insula, and thalamus were not predictive of the development of cognitive impairment in PD [340]. In PD-MCI patients, the volume of the NBM was positively correlated with cortical thickness in the bilateral posterior cingulate, parietal, frontal gyri and the left insular region [341].
DTI-based diffusion measures were inferior to standard volumetric assessments for capturing cognition-relevant changes in PD-NC [337]. Volume loss, elevated water content and structural metrics in the cholinergic forebrain and NBM were associated with CI in PD [342,343,344]. The PD-MCI group showed imbalanced associations between different cholinergic basal forebrain nuclei (putamen) and cortical regions (middle frontal gyrus, paracingulate and cingulate gyri), revealing that basal forebrain changes were frequency specific for different cognitive scores [345]. PD-MCI converters had significantly greater mean diffusivity in both NBM tracts compared to PD-NC; trends were identified between the lateral tract mean diffusivity and poorer visuospatial performance, working memory decline, and medial tract mean diffusivity and reduced psychomotor speed [346].
WM lesions in the cholinergic projections from the NBM to the cortex, hippocampus and amygdala were associated with CI over a 5-year period in previous PD-NC [250,347,348]. The loss of basal forebrain cholinergic projections to the hippocampus correlated with memory deficits and conversion to PDD [249,257].
PDD was associated with the selective destruction of corticostriatal resting FC [288]. It further showed a global cortical decrease in 123I-IBVM (vesicular acetylcholinesterase transporter/VAChT) binding in the frontal and posterior cingulate cortex, while reduced IBVM-binding in posterior brain areas was related to cognitive decline [349]. A loss of hippocampal cholinergic fibers occurred in PD-MCI patients, whereas those with PDD were affected by a subsequent increase in αSyn deposition and the dysfunction of both basal forebrain and hippocampal cholinergic systems [339,350].
9.3. Noradrenergic Modulation
The locus ceruleus (LC) is a primary source of ascending noradrenergic innervation in the CNS. Its structural integrity can be measured in vivo using neuromelanin-sensitive MRI that was reduced in PD-MCI patients [351,352]. Noradrenaline has been linked to attentional shifting and response inhibition, which were mediated by prefrontal operations [353]. PD patients showed significantly decreased levels of CSF noradrenaline and 3-methoxy-4-hydroxyphenylglycol as well as noradrenaline transporter availability in the hippocampus, indicating significant noradrenergic dysfunction [354].
A severe loss of claustrum dopamine and noradrenaline (serotonin levels unchanged) may interact with the cortico-claustro-cortical information processing mechanisms by passing via the basal ganglia-thalamo-cortical circuits [355]. In vivo neuroimaging studies have shown that acetylcholine and noradrenalin relate not only to gait control but interact closely with cognition. They go beyond the dual-syndrome hypothesis, suggesting that fronto-subcortical noradrenaline and serotonin deficiency are responsible for EF in PD-NC patients. The relationship between posterior cortical cholinergic dysfunction and visuospatial function and semantic fluency as well as the relationship of the noradrenergic LC, serotonergic dorsal raphe nucleus and ventral tegmental area with certain cognitive domains need further investigation [4,356,357,358].
In summary, non-DAergic systems interact closely with the striatal dopamine system, the activity of which is modulated by cholinergic interneurons and other transmitter systems [359]. Neuroimaging and neuropathological studies have demonstrated the role of parallel alteration of cholinergic, noradrenergic and serotonergic systems in cognition in PD.
10. Brain Positron Emission Tomography Studies in PD-CI
18F-fluorodeoxyglucose PET (FDG-PET) studies in PD-MCI patients showed reduced glucose metabolism in posterior cortical regions, especially in the parietal and cingulate cortex, which were not affected in PD-NC individuals [187,188]. Hypometabolism in parietal, precuneus, hippocampus and occipital lobes was obvious in PD with incident dementia [186,187]. PD-MCI patients exhibited hypometabolism in the frontal and parietal regions compared with PD-NC ones, while it was higher in PDD patients than in those with MCI, mainly in the posterior cortical areas [360,361]. Correlations of reduced metabolism in the areas with event-related potentials confirmed the important role of EF disorders [362]. Patients with atypical brain hypometabolism showed a higher incidence of dementia (60% vs. 3%) and hallucinations (56% vs. 7%) [363].
Decreased glucose metabolism in the left prefrontal cortex and posterior cortex was related to executive and memory dysfunctions, while changes in visuospatial function were associated with hypometabolism in the right and left occipital cortex, suggesting that cognitive function assessment can indirectly reflect the level of glucose metabolism in the relevant brain regions [190]. PD MAPT H1/H1 carriers without dementia exhibited hypometabolism in the frontal cortex, parahippocampal, and cingulate gyrus, in the caudate and globus pallidus, and worse performance in attention than MAPT H1/H2 carriers [364]. In none of these PDD cases was depression/anxiety mentioned, which is also associated with hypometabolism in the frontal cortex [365].
PDD brains showed altered metabolism from several pathways, including glucose and purine metabolism as well as decreases in proline, serine and deoxyguanosine that reflected the progression of αSyn Braak staging [366].
PD-MCI patients exhibited significantly reduced N-acetyl aspartate (NAA), total NAA, choline (Cho), glutathione (GSH), glutamate + glutamine (Glx) and total creatine (tCr), but elevated levels of myo-inositol (Ins) and Ins/tCr ratio as well as reduced NAA/Ins ratio. These findings suggested PD-NC individuals with low NAA and tCr in the posterior cingulate cortex may be at risk of preclinical MCI [367].
18F-florbetapir PET showed a higher degree of Aβ uptake in PD patients at higher risk of developing CI [208,368], contributing to memory impairment and a faster rate of cognitive decline [209]. In PD-NC patients, the target regions for Aβ deposition were in the cingulate, middle occipital and middle temporal gyri, while changes in multiple brain regions were correlated to the performance of different cognitive domains [211]. There was a significant negative association between Aβ deposition and language and memory in the PD group [369]. Older PET studies reported an Aβ deposition prevalence of 0.05 in MCI and 0.34 in PDD cases [370], whereas others did not find an association between Aβ deposition and CI [210,371] and no significant differences between PD-MCI, PD-NC and age-matched controls [210,372].
A more recent study detected higher Aβ deposition in the frontal, temporal lobe and prefrontal cortex in PD-MCI [373]. The Aβ-positive group with more aMCI had lower dopamine activities in the left ventral striatum, suggesting AD-related cognitive changes [374,375], although, in general, PDD patients have a lower incidence and severity of Aβ deposition than AD [374,376,377] or DLB patients [378,379]. In patients with PDD, low CSF Aβ-42 level at baseline was not associated with a specific cognitive profile; memory domains and long-delay free recall were not different from those with increased CSF Aβ levels [380].
18F-flortaucipir PET showed a gradient of tau binding from PD-NC (none or minimal) via PD-MCI (minimal) and PDD (low/modest) to DLB (intermediate/strong) and finally to AD (highest) [381], tau uptake in PDD being intermediate between PD-ND and AD [213]. Likewise, 18F-florzolotau uptake was significantly higher in the cortical regions of PDD patients compared to PD-NC especially in the temporal lobe; uptake in the occipital lobe showed significant correlation with CI [382]. In conclusion, CI in PD is primary linked to biomarkers associated with both AD and PD.
11. Neuropathology of PD-MCI
There are few detailed neuropathological studies of PD-MCI. Among 698 autopsy-proven cases of PD, 16 had met the clinical criteria of MCI [383,384]. They included seven naMCI cases (mean age 80 years, 50% brainstem predominant, 31% brainstem–limbic, and 19% neocortical LB stages, with Braak NFT (neurofibrillary tangle) stage 0–4 (mean 2.1), two with neuritic plaques and four with Aβ plaques) and eight aMCI cases (Braak NFT stage 1–4 (mean 2.7), four with neuritic plaques, and three with Aβ plaques). One aMCI multiple domain case, aged 75 years, was LB brainstem type with Braak NFT stage II, few neuritic and many Aβ plaques. One case each also showed mild cerebral amyloid angiopathy (CAA); most of them had a mild lacunar state in the basal ganglia. The neuritic Braak stage in aCMI was higher than in naMCI, mild to moderate neuritic plaques were present in 43%, mild CAA was present in 11%, lacunar state was present in 25%, and old cerebral infarcts were present in 12%. A recent autopsy study of 159 PD cases included 25 MCI (56% aMCI and 44% naMCI with no significant differences in age, gender and disease duration). All naMCI were brainstem–limbic LB stage 3, while in the aMCI group, only 22% were neocortical LB stage 4. Concomitant non-AD tauopathies were present in nine PD-MCI cases, both aging-related tau astrogliopathy (ARTAG) and argyrophilic grains were seen in five cases; two aMCI cases met the pathological criteria of progressive supranuclear palsy. No differences were found in the neuritic plaque stage, total Aβ score, WM rarefication, cerebral infarct volume, CAA score and APOE carrier frequency. There were some differences between the MCI subtypes; aMCI cases had a slightly higher Braak NFT stage, while naMCI-PD cases had an increase in LB pathology [385]. In conclusion, the available autopsy data for PD-MCI revealed a heterogenous picture with the presence of various types of pathological changes similar to MCI in other diseases [386,387], in which the role of other pathologies should be further explored.
12. Neuropathology of PDD
The majority of autopsy reports of PDD tried to evaluate the convergence and interactions between basic PD (Lewy) pathology and other cortical proteinopathies (αSyn, tau and Aβ) and their contribution to dementia pathogenesis [33,34,357,388,389,390,391,392,393,394,395] and the role of co-pathologies on cognition [203,389,395,396,397].
The morphological substrate of PDD is heterogenous and includes the following: (1) Lewy body (αSyn) pathology (LBP) in cortical, limbic and subcortical/brainstem structures, (2) AD-related neuropathological changes (ADNC)—diffuse Aβ and neuritic plaques, NFTs and CAA, and (3) a variable combination of these pathologies that has been differently related to the severity of CI [357,390,394,395,398]. Limbic and neocortical LBP were significantly higher in PDD than in MCI, CI often correlating with the severity of LBP in the frontal cortex, hippocampus and periamygdaloid cortex, causing a disruption of the limbic loop similar to that in AD [399]. LBP severity in the CA 2/3 region of the hippocampus was related to episodic memory loss [400]. Mice injected with αSyn fibrils developed progressive spatial learning and memory deficits associated with hippocampal and cortical αSyn pathology with extensive neurodegeneration of the CA 2/3 hippocampal subfield at 6 month post-injection and, therefore, it can be used as a model for Lewy-related cognitive dysfunction [401]. However, recent studies revealed that PD-MCI patients had smaller bilateral CA1 volumes than PD-NC, although the same was observed in a de novo PD cohort, providing evidence that CA1 changes may be the earliest indicator of emerging CI [402]. LBP in PDD was higher in subcortical regions than in PD-NC, and its density in the amygdala and hippocampus correlated with dementia severity [403,404]. In a large autopsy study, the severity of neocortical LBP correlated best with dementia severity [405] and more rapid decline in all cognitive domains, whereas limbic LBP was associated with a more rapid decline in visuospatial skills, which was not modified by coexistent ADNC [406]. In another autopsy study, combined LBP and ADNC showed worse deficits in the executive/visuospatial domain than LBP only when LBs were confined to the brainstem, but there were no differences when LBs extended to the limbic or cerebral cortical regions. That means that the impact of co-occurring ADNC on antemortem cognitive deficits varies not only by domain but also on the pathological stage of LBP [407].
It should be considered, however, that not all patients with cortical LBP may develop dementia [408,409] despite its more severe increase in inferior frontal gyrus in PDD [410]. The association between cortical LBP and dementia was challenged by some studies that reported that 15–44.7% of PD-NC patients were associated with severe neocortical LB burden [390,398,409,411], while a small study described PD cases without dementia despite considerable cortical and limbic LBP [408]. On the other hand, in 14.7% of PDD cases, LBP was confined to the brainstem without cortical involvement [411]. A large autopsy study showed that in PD patients, the Braak LB stage was associated with dementia, whereas the severity of ADNC and small vessel pathology did not; thus, neocortical LBP very often, but not always, paralleled dementia in PD, while it also appeared in PD patients without dementia [394]. In a recent personal autopsy study of 210 PD cases, 47.6% with dementia, PDD cases were significantly older at death than PD-NC ones (mean 83.9 vs. 77.9 years, p < 0.05) and had a shorter disease duration (mean 9.2 vs. 14.5 years). Their Braak LB score and NFT stage were significantly higher (mean 4.2 vs. 4.1, and 4.4 vs. 2.3, p < 0.01), as were the Aβ phase (mean 3.0 vs. 1.8) and CAA frequency and intensity (50% vs. 24%, and 0.7 vs. 0.3, all p < 0.01) [34].
This and other autopsy studies have confirmed the co-existence of αSyn, Aβ and tau pathologies in PD with CI and its impact for dementia development [357,388,398,412,413]. ADNC, severe enough for a pathological diagnosis of AD, was present in about 10% of PD-ND patients, 35% of PD-MCI patients and over 50% of PDD patients [28,395,405]. In large autopsy series, around 50% of PD cases showed Braak LB stages 4–6 and NFT stages 4–6 [34,390], while others suggested a significant relationship between cortical LBP and severity of dementia [208,395,414]. Four large autopsy studies of PD showed consistent results: while co-morbid ADNC was present in 19–31.5% of total PD cases, the rate of co-morbid ADNC in PDD ranged from 21.5% to 89.4% [405,413,415], tau pathology being more severe in the prefrontal than in the temporal cortex [416]. Some studies indicated that dementia in PD was related to co-morbid ADNC, the spread of tau being similar to that in typical AD, although in some cases, neocortical tau was variable and the medial temporal lobe was relatively spared [417,418]. A recent meta-analysis stated that the four clinical core features were more common in PD-MCI than in AD-MCI/stable MCI [419].
Despite some differences between study groups, Aβ deposition was not specifically related to dementia in PD, but it may be linked with more rapid cognitive deterioration and earlier mortality [404,420,421,422,423]. On the other hand, significant Aβ burden was found in 15–45.4% of PD cases without CI [398,405,409], while other studies found considerable αSyn, Aβ and tau pathologies in elderly PD patients without CI [424], which could be explained by there being a higher cognitive reserve in these patients [425].
In conclusion, there is increasing evidence for a synergistic effect of αSyn (Lewy) and tau (AD) pathologies as the main driver of cognitive decline/dementia in PD, the relative impact of which is under discussion. A recent study on the disease-specific patterns of αSyn destabilization revealed differences of the cytosolic unfolded, monomeric form of αSyn (αSU) and the cytosolic helically folded, multimeric form (αSH) equilibrium comparing demented and cognitively intact PD patients [426]. These data indicate that the region-specific susceptibility of LBP is a major component for the development of CI in PD but not always, which was confirmed by recent autopsy studies [394].
13. Cognitive Reserve and Resilience in PD
Long-time life experiences, such as education, occupational attainment, leisure activities, modifications of lifestyle, and bilinguism, have been considered proxies of cognitive reserve (CR), which is considered as a modulator of a more favorable cognitive trajectory, less disability and better quality of life. Increased physical activity has been shown to attenuate APOE ε4-related vulnerability to early cognitive decline in patients with PD. This protective effect did not appear to be mediated by striatal DAergic function [427].
It was hypothesized that higher CR would correlate with better performance and is associated with a mild increase in performance with regard to verbal learning, wording memory, and problems in understanding the pragmatic aspects of language experience by patients with PD [428]. There is strong evidence that higher CR was associated with a mild increase in performance with regard to verbal fluency, learning and recognition, working memory and visuoconstructive ability. This suggested that CR moderates the effects of brain morphology and functions on cognition in PD. Multiple CR-related processes may offer resilience against functional decline, among which CR refers to the adaptability of cognitive processes. Network analyses demonstrated that relative to PD patients experiencing cognitive decline, the FPN in cognitively stable individuals was significantly more resilient to network perturbation. This suggested that the topological robustness of the FPN is associated with less cognitive decline in PD [429]. Compared to individuals with high CR, those with low CR had reduced posterior DMN FC in the anterior cingulate gyrus and basal ganglia as well as bilateral reduced connectivity in fronto-parietal regions within the prefrontal networks, while hyper-connectivity was detected within medial prefrontal regions. This suggests that CR may exert a modulatory effect on FC in the basal ganglia and executive–attentional fronto-parietal networks in PD patients with low CR. Attentional control networks seem to be downregulated, whereas a higher recruitment of medial frontal regions suggests compensation via upregulation mechanisms that may contribute to maintaining cognitive functioning when posterior control mechanisms are reduced [430].
Resilience is defined as the process of adapting well in the face of adversary, threats, trauma, tragedy or significant sources of stress. High levels of social support were associated with increased resilience, which in turn was associated with reduced mental health symptoms [431], and correlated with less disability and better quality of life but not with PD severity [432]. Exercise regulates the neuroendocrine system, whose primary role is to respond to stress, maintain homeostasis and improve resilience in aging and neurodegeneration. Exercise in PD has been shown to exert some cognitive and behavioral-induced control over their disease [433].
Stress susceptibility and resilience were mediated by the noradrenergic regulation of DAergic neurons [434]. Recent studies have shown that a genetic resilience score can modify the penetrance of PD risk factors and thereby protect individuals carrying high-risk genetic burden for developing PD. Although no single locus reached genome-wide significance, multimarker analysis of genomic annotation (MAGMA) gene-based analyses nominated TBCA as a putative gene associated with resilience in PD [435]. LRRK2-PD patients showed a significantly higher basal forebrain volume compared to iPD and developed no cognitive changes over a 4-year follow-up period, potentially reflecting a compensatory cholinergic state that could prevent cognitive decline [436], which also might partially be attributed to a slower increase in NfL levels [437]. The LRRK2-G2019S mutation that causes late-onset PD affects striatal neuron function and alters synaptic plasticity, thus promoting resilience to chronic social stress and supporting cognitive function [438]. ATG8 proteins, co-factors for DAergic neuronal transcriptional control, have been proposed as autophagy gene transcriptional co-factors for stress protection of ventral midbrain DAergic neurons and neuronal resilience in PD [439].
In conclusion, CR is suggested to play a role in the presentation of CI, but it is not protective against general cognitive decline, although there is evidence that it protected several cognitive domains from further decline. CR has been documented to modulate the extent of cognitive decline or to protect language fluency and other cognitive domains from further decline. Likewise, resilience is correlated with less disability and cognitive decline, although it shows no correlation with PD severity.
14. Impact of Other Co-Pathologies in Cognition in PD
A number of other neuropathologies, particularly those associated with age, can influence the course and the severity of CI in PD. In addition to the high prevalence of ADNC, TAR DNA-binding protein 43 (TDP-43) and cerebrovascular pathologies, due to their high prevalence and clinical impact on PD patients, show both cross-sectional and longitudinal clinical importance [440]. The extent and severity of cerebral small vessel disease (CSVD) has considerable impact on the severity of CI. A meta-analysis of its influence on cognitive function in PD showed different effects—most on EF, memory and overall cognitive function [441], whereas others found no correlation between CSVD pathology and dementia [442,443]. Lacunes were independently associated with worse attention and processing speed and may accelerate the course of PD [444]. Increased perivascular space in the basal ganglia and WMH was associated with cognitive decline in PD [203,445], as were cerebral microbleeds (CMB), which were both related to hypertension [397]. CMBs were significantly associated with PDD [396,443], while others associated PDD with amyloid-related CMBs and reduced hippocampal volume [446]. The association of severe CAA with PDD has been reported repeatedly [34,398,405], whereas, according to others, cerebrovascular disease and TDP-43 pathologies had no definite impact on the development of PDD [395]. Argyrophilic grain disease, an age-related 4-R tauopathy affecting the medial temporal lobe, although a rare co-pathology, may be another important factor inducing dementia in PD [447], whereas others did not find such a combination [25,405]. Most of these and other age-related co-pathologies make it difficult to disentangle their individual contribution to cognitive decline [398,412,417], but in general, a close cooperation of various neuropathologies is responsible for the development and progression of cognitive and other clinical deficits in PD [389,448].
15. Conclusions and Outlook
PD, a common and heterogenous neurodegenerative disease, is characterized by a combination of motor and non-motor symptoms including CI involving multiple cognitive domains. The morphological and molecular/biochemical basis of CI is heterogenous, and modern neuroimaging studies revealed widespread changes in cerebral GM and WM, involving multiple brain areas and causing the disruption of FC for many critical neuronal networks involved in cognitive, attentional, memory, and behavioral functions. PD patients exhibiting “AD-like” patterns of brain atrophy are at greater risk of developing cognitive decline than PD-NC individuals and HCs [449]. The majority of autopsy-based studies support a strong association between corticolimbic LBP and ADNC resulting from the complex interaction of αSyn, Aβ, tau and other pathological proteins (like PTD-43) resulting in the complex interplay of progressive motor and cognitive decline in PD. It has been proposed that the development of cognitive decline prior to or simultaneous with the manifestation of motor symptoms should be included in the diagnostic procedures of PD [450,451]. The prospective assessment and validation of PD-CI and a deeper understanding of the interaction of multiple pathogenic factors will be achieved by modern fluid biomarkers (reduced αSyn and Aβ-42 levels, increased p-tau 181, glial fibrillary acidic protein/GFAP/and NfL) as well as in post-mortem brain tissue [452,453,454,455,456,457,458] that will also enable a differentiation between PD-CI and AD. In order to elucidate these and other open questions, a combined assessment of in vivo neuroimaging and liquid biomarkers in multicentric longitudinal clinico-pathological studies is warranted that also may contribute to the development of meaningful disease-modifying therapies and preventive measures to slow the progression of PD and associated cognitive deterioration.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms25010498/s1. References [42,55,80,140,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,218,219,221,224,229,237,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,262,264,265,266,267,269,275,277,281,282,283,284,285,286,287,288,290,291,293,296,310,328,333,346,355,374,376,377,381,382,459] are cited in the supplementary materials.
Funding
The study was funded by the Society for the Promotion of Research in Experimental Neurology, Vienna, Austria.
Acknowledgments
The author thanks E. Mitter-Ferstl for secretarial and editorial work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AD | Alzheimer disease |
| ADNC | AD-related neuropathological changes |
| aMCI | amnestic mild cognitive impairment |
| Aβ | amyloid-β |
| αSyn | α-synuclein |
| BGN | basal ganglia network |
| CAA | cerebral amyloid angiopathy |
| CI | cognitive impairment |
| CN | cerebellar network |
| CR | cognitive reserve |
| CSF | cerebrospinal fluid |
| DLB | dementia with Lewy bodies |
| DMN | default mode network |
| DTI | diffusion tensor imaging |
| EF | executive function |
| FA | fractional anisotrophy |
| FC | functional connectivity |
| FOG | freezing of gait |
| FPN | frontoparietal network |
| GBA | glucocerebrosidase |
| GDNF | glial cell line-derived neurotrophic factor |
| GM | gray matter |
| GMV | gray matter volume |
| HCs | healthy controls |
| LBs | Lewy bodies |
| MCI | mild cognitive impairment |
| MDS | Movement Disorder Society |
| naMCI | non-amnestic mild cognitive impairment |
| NBM | nucleus basalis of Meynert |
| NfL | neurofilament light |
| NFT | neurofibrillary tangle |
| PD | Parkinson disease |
| PDD | Parkinson disease dementia |
| PD-MCI | Parkinson disease with mild cognitive impairment |
| PD-NC | Parkinson disease with normal cognition |
| PD-ND | Parkinson disease non-demented |
| RBD | REM sleep behavior disorder |
| rsfMRI | resting-state functional MRI |
| SAN | salience network |
| SCD | subtle cognitive decline |
| SMN | sensorimotor network |
| WM | white matter |
| WMH | white matter hyperintensity |
References
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Neuropathology of sporadic Parkinson’s disease: Evaluation and changes of concepts. Mov. Disord. 2012, 27, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Koros, C.; Stefanis, L.; Scarmeas, N. Parkinsonism and dementia. J. Neurol. Sci. 2022, 433, 120015. [Google Scholar] [CrossRef]
- Ye, H.; Robak, L.A.; Yu, M.; Cykowski, M.; Shulman, J.M. Genetics and pathogenesis of Parkinson’s Syndrome. Annu. Rev. Pathol. 2023, 18, 95–121. [Google Scholar] [CrossRef]
- Zaman, V.; Shields, D.C.; Shams, R.; Drasites, K.P.; Matzelle, D.; Haque, A.; Banik, N.L. Cellular and molecular pathophysiology in the progression of Parkinson’s disease. Metab. Brain Dis. 2021, 36, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.; Guevara, C.A.; Del Valle, P.; Gupta, S.; Benson, D.L.; Huntley, G.W. Non-motor symptoms of Parkinson’s disease: The neurobiology of early psychiatric and cognitive dysfunction. Neuroscientist 2023, 29, 97–116. [Google Scholar] [CrossRef]
- Dickson, D.W.; Fujishiro, H.; Orr, C.; DelleDonne, A.; Josephs, K.A.; Frigerio, R.; Burnett, M.; Parisi, J.E.; Klos, K.J.; Ahlskog, J.E. Neuropathology of non-motor features of Parkinson disease. Park. Relat. Disord. 2009, 15 (Suppl. 3), S1–S5. [Google Scholar] [CrossRef]
- Tolosa, E.; Santamaria, J.; Gaig, C.; Compta, Y. Nonmotor aspects of Parkinson’s disease. In Movement Disorders 4; Schapira, A.H.V., Lang, A.E.T., Fahn, S., Eds.; Saunders-Elsevier: Philadelphia, PA, USA, 2010; pp. 229–251. [Google Scholar]
- Tremblay, C.; Achim, A.M.; Macoir, J.; Monetta, L. The heterogeneity of cognitive symptoms in Parkinson’s disease: A meta-analysis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1265–1272. [Google Scholar] [CrossRef]
- Charcot, J.-M. De la Paralysie Agitante. Oeuvres Complétes: Leçons sur les Maladies du Systéme Nerveux; On Parkinson’s disease. Lectures on the diseases of the nervous system; Sigerson, G., Translator; Bureaux du Progrés Mèdical: Paris, France; New Sydenham Society: London, UK, 1877; Volume 1. [Google Scholar]
- Flores-Torres, M.H.; Bjornevik, K.; Hung, A.Y.; Healy, B.C.; Schwarzschild, M.A.; Blacker, D.; Ascherio, A. Subjective cognitive decline in women with features suggestive of prodromal Parkinson’s disease. Mov. Disord. 2023, 38, 1473–1482. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef]
- Oedekoven, C.; Egeri, L.; Jessen, F.; Wagner, M.; Dodel, R. Subjective cognitive decline in idiopathic Parkinson’s disease: A systematic review. Ageing Res. Rev. 2022, 74, 101508. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yuan, X.; Chen, L.; Hu, B.; Jiang, L.; Shi, T.; Wang, H.; Huang, W. Subjective cognitive decline in patients with Parkinson’s disease: An updated review. Front. Aging Neurosci. 2023, 15, 1117068. [Google Scholar] [CrossRef] [PubMed]
- Ophey, A.; Krohm, F.; Kalbe, E.; Greuel, A.; Drzezga, A.; Tittgemeyer, M.; Timmermann, L.; Jessen, F.; Eggers, C.; Maier, F. Neural correlates and predictors of subjective cognitive decline in patients with Parkinson’s disease. Neurol. Sci. 2022, 43, 3153–3163. [Google Scholar] [CrossRef] [PubMed]
- Pike, K.E.; Cavuoto, M.G.; Li, L.; Wright, B.J.; Kinsella, G.J. Subjective cognitive decline: Level of risk for future dementia and mild cognitive impairment, a meta-analysis of longitudinal studies. Neuropsychol. Rev. 2022, 32, 703–735. [Google Scholar] [CrossRef] [PubMed]
- Litvan, I.; Goldman, J.G.; Troster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 2012, 27, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.F.; Larsen, J.P.; Tysnes, O.B.; Alves, G. Natural course of mild cognitive impairment in Parkinson disease: A 5-year population-based study. Neurology 2017, 88, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, J.; Boel, J.A.; de Bie, R.M.A.; Geskus, R.B.; Schmand, B.A.; Dalrymple-Alford, J.C.; Marras, C.; Adler, C.H.; Goldman, J.G.; Tröster, A.I.; et al. Mild cognitive impairment as a risk factor for Parkinson’s disease dementia. Mov. Disord. 2017, 32, 1056–1065. [Google Scholar] [CrossRef]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007, 22, 1689–1707. [Google Scholar] [CrossRef]
- Goetz, C.G.; Emre, M.; Dubois, B. Parkinson’s disease dementia: Definitions, guidelines, and research perspectives in diagnosis. Ann. Neurol. 2008, 64 (Suppl. 2), S81–S92. [Google Scholar] [CrossRef]
- Kiesmann, M.; Chanson, J.B.; Godet, J.; Vogel, T.; Schweiger, L.; Chayer, S.; Kaltenbach, G. The Movement Disorders Society criteria for the diagnosis of Parkinson’s disease dementia: Their usefulness and limitations in elderly patients. J. Neurol. 2013, 260, 2569–2579. [Google Scholar] [CrossRef]
- Svenningsson, P.; Westman, E.; Ballard, C.; Aarsland, D. Cognitive impairment in patients with Parkinson’s disease: Diagnosis, biomarkers, and treatment. Lancet Neurol. 2012, 11, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Fengler, S.; Liepelt-Scarfone, I.; Brockmann, K.; Schäffer, E.; Berg, D.; Kalbe, E. Cognitive changes in prodromal Parkinson’s disease: A review. Mov. Disord. 2017, 32, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Carceles-Cordon, M.; Weintraub, D.; Chen-Plotkin, A.S. Cognitive heterogeneity in Parkinson’s disease: A mechanistic view. Neuron 2023, 111, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Meireles, J.; Massano, J. Cognitive impairment and dementia in Parkinson’s disease: Clinical features, diagnosis, and management. Front. Neurol. 2012, 3, 88. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Morphological basis of Parkinson disease-associated cognitive impairment: An update. J. Neural Transm. 2022, 129, 977–999. [Google Scholar] [CrossRef] [PubMed]
- Oosterveld, L.P.; Allen, J.C., Jr.; Reinoso, G.; Seah, S.H.; Tay, K.Y.; Au, W.L.; Tan, L.C. Prognostic factors for early mortality in Parkinson’s disease. Park. Relat. Disord. 2015, 21, 226–230. [Google Scholar] [CrossRef]
- Chandler, J.M.; Nair, R.; Biglan, K.; Ferries, E.A.; Munsie, L.M.; Changamire, T.; Patel, N. Characteristics of Parkinson’s disease in patients with and without cognitive impairment. J. Park. Dis. 2021, 11, 1381–1392. [Google Scholar] [CrossRef]
- Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Khoo, T.K.; Breen, D.P.; Barker, R.A.; Collerton, D.; Taylor, J.P.; Burn, D.J. Severity of mild cognitive impairment in early Parkinson’s disease contributes to poorer quality of life. Park. Relat. Disord. 2014, 20, 1071–1075. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Tovar-Rios, D.A.; Alves, G.; Dalen, I.; Williams-Gray, C.H.; Camacho, M.; Forsgren, L.; Bäckström, D.; Lawson, R.A.; Macleod, A.D.; et al. Cognitive and motor decline in dementia with Lewy bodies and Parkinson’s disease dementia. Mov. Disord. Clin. Pract. 2023, 10, 980–986. [Google Scholar] [CrossRef]
- Jellinger, K.A. Dementia with Lewy bodies and Parkinson’s disease-dementia: Current concepts and controversies. J. Neural Transm. 2018, 125, 615–650. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Morphological characteristics differentiate dementia with Lewy bodies from Parkinson disease with and without dementia. J. Neural Transm. 2023, 130, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A.; Korczyn, A.D. Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med. 2018, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.; Pishva, E.; Chouliaras, L.; Lunnon, K. Elucidating distinct molecular signatures of Lewy body dementias. Neurobiol. Dis. 2023, 188, 106337. [Google Scholar] [CrossRef] [PubMed]
- Rongve, A.; Aarsland, D. The Lewy body dementias: Dementia with Lewy bodies and Parkinson’s disease dementia. In Oxford Textbook of Old Age Psychiatry, 3rd ed.; Dening, T., Thomas, A., Stewart, R., Taylor, J.-P., Eds.; Oxford University Press: Oxford, UK, 2021; pp. 495–512. [Google Scholar]
- Habich, A.; Wahlund, L.O.; Westman, E.; Dierks, T.; Ferreira, D. (Dis-)connected dots in dementia with Lewy bodies—A systematic review of connectivity studies. Mov. Disord. 2023, 38, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; Helmer, C.; Foubert-Samier, A.; Auriacombe, S.; Dartigues, J.F.; Tison, F. Risk of dementia in an elderly population of Parkinson’s disease patients: A 15-year population-based study. Alzheimer’s Dement. 2012, 8, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Hely, M.A.; Reid, W.G.; Adena, M.A.; Halliday, G.M.; Morris, J.G. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef]
- Marder, K. Cognitive impairment and dementia in Parkinson’s disease. Mov. Disord. 2010, 25 (Suppl. 1), S110–S116. [Google Scholar] [CrossRef]
- Hall, J.M.; Lewis, S.J.G. Neural correlates of cognitive impairment in Parkinson’s disease: A review of structural MRI findings. Int. Rev. Neurobiol. 2019, 144, 1–28. [Google Scholar]
- Aarsland, D.; Bronnick, K.; Williams-Gray, C.; Weintraub, D.; Marder, K.; Kulisevsky, J.; Burn, D.; Barone, P.; Pagonabarraga, J.; Allcock, L.; et al. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology 2010, 75, 1062–1069. [Google Scholar] [CrossRef]
- Aarsland, D.; Zaccai, J.; Brayne, C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov. Disord. 2005, 20, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Severiano, E.S.C.; Alarcão, J.; Pavão Martins, I.; Ferreira, J.J. Frequency of dementia in Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. Sci. 2022, 432, 120077. [Google Scholar] [CrossRef] [PubMed]
- Rongve, A.; Aarsland, D. Dementia in Parkinson’s disease and dementia with Lewy bodies. In Oxford Textbook of Old Age Psychiatry 2e; Dening, T., Thomas, A., Eds.; Oxford Univ. Press: Oxford, UK, 2013; pp. 469–478. [Google Scholar]
- Bock, M.A.; Tanner, C.M. The epidemiology of cognitive function in Parkinson’s disease. Prog. Brain Res. 2022, 269, 3–37. [Google Scholar] [PubMed]
- Galtier, I.; Nieto, A.; Mata, M.; Lorenzo, J.N.; Barroso, J. Specific pattern of linguistic impairment in Parkinson’s disease patients with subjective cognitive decline and mild cognitive impairment predicts dementia. J. Int. Neuropsychol. Soc. 2023, 29, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Lee, P.H. Subjective cognitive complaints in cognitively normal patients with Parkinson’s disease: A systematic review. J. Mov. Disord. 2023, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Purri, R.; Brennan, L.; Rick, J.; Xie, S.X.; Deck, B.L.; Chahine, L.M.; Dahodwala, N.; Chen-Plotkin, A.; Duda, J.E.; Morley, J.F.; et al. Subjective cognitive complaint in Parkinson’s disease patients with normal cognition: Canary in the coal mine? Mov. Disord. 2020, 35, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.P.; Mendonça, M.D.; Caetano, A.P.; Lampreia, T.M.; Miguel, R.; Bugalho, P.M. Cognitive complaints in Parkinson’s disease patients: From subjective cognitive complaints to dementia and affective disorders. J. Neural Transm. 2019, 126, 1329–1335. [Google Scholar] [CrossRef]
- Dujardin, K.; Duhamel, A.; Delliaux, M.; Thomas-Antérion, C.; Destée, A.; Defebvre, L. Cognitive complaints in Parkinson’s disease: Its relationship with objective cognitive decline. J. Neurol. 2010, 257, 79–84. [Google Scholar] [CrossRef]
- Erro, R.; Santangelo, G.; Barone, P.; Picillo, M.; Amboni, M.; Longo, K.; Giordano, F.; Moccia, M.; Allocca, R.; Pellecchia, M.T.; et al. Do subjective memory complaints herald the onset of mild cognitive impairment in Parkinson disease? J. Geriatr. Psychiatry Neurol. 2014, 27, 276–281. [Google Scholar] [CrossRef]
- Galtier, I.; Nieto, A.; Lorenzo, J.N.; Barroso, J. Subjective cognitive decline and progression to dementia in Parkinson’s disease: A long-term follow-up study. J. Neurol. 2019, 266, 745–754. [Google Scholar] [CrossRef]
- Hong, J.Y.; Lee, J.E.; Sohn, Y.H.; Lee, P.H. Neurocognitive and atrophic patterns in Parkinson’s disease based on subjective memory complaints. J. Neurol. 2012, 259, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, M.; Trojano, L.; De Micco, R.; Sant’Elia, V.; Giordano, A.; Russo, A.; Passamonti, L.; Tedeschi, G.; Chiorri, C.; Tessitore, A. Correlates of the discrepancy between objective and subjective cognitive functioning in non-demented patients with Parkinson’s disease. J. Neurol. 2021, 268, 3444–3455. [Google Scholar] [CrossRef] [PubMed]
- Erro, R.; Santangelo, G.; Picillo, M.; Vitale, C.; Amboni, M.; Longo, K.; Costagliola, A.; Pellecchia, M.T.; Allocca, R.; De Rosa, A.; et al. Link between non-motor symptoms and cognitive dysfunctions in de novo, drug-naive PD patients. J. Neurol. 2012, 259, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, S.; Wang, S. Application of periventricular white matter hyperintensities combined with homocysteine into predicting mild cognitive impairment in Parkinson’s disease. Int. J. Gen. Med. 2023, 16, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pourzinal, D.; Byrne, G.J.; McMahon, K.L.; Copland, D.A.; O’Sullivan, J.D.; Mitchell, L.; Dissanayaka, N.N. Global assessment, cognitive profile, and characteristics of mild cognitive impairment in Parkinson’s disease. Int. J. Geriatr. Psychiatry 2023, 38, e5955. [Google Scholar] [CrossRef] [PubMed]
- Monastero, R.; Cicero, C.E.; Baschi, R.; Davì, M.; Luca, A.; Restivo, V.; Zangara, C.; Fierro, B.; Zappia, M.; Nicoletti, A. Mild cognitive impairment in Parkinson’s disease: The Parkinson’s disease cognitive study (PACOS). J. Neurol. 2018, 265, 1050–1058. [Google Scholar] [CrossRef]
- Nicoletti, A.; Luca, A.; Baschi, R.; Cicero, C.E.; Mostile, G.; Davì, M.; Pilati, L.; Restivo, V.; Zappia, M.; Monastero, R. Incidence of mild cognitive impairment and dementia in Parkinson’s disease: The Parkinson’s disease cognitive impairment study. Front. Aging Neurosci. 2019, 11, 21. [Google Scholar] [CrossRef]
- Bernard, B.A.; Carns, D.; Stebbins, G.T.; Goldman, J.G.; Goetz, C.G. Relationship of Movement Disorders Society—Unified Parkinson’s Disease Rating Scale nonmotor symptoms to cognitive functioning in patients with Parkinson’s disease. Mov. Disord. Clin. Pract. 2020, 7, 279–283. [Google Scholar] [CrossRef]
- Petkus, A.J.; Filoteo, J.V.; Schiehser, D.M.; Gomez, M.E.; Hui, J.S.; Jarrahi, B.; McEwen, S.; Jakowec, M.W.; Petzinger, G.M. Mild cognitive impairment, psychiatric symptoms, and executive functioning in patients with Parkinson’s disease. Int. J. Geriatr. Psychiatry 2020, 35, 396–404. [Google Scholar] [CrossRef]
- Barone, P.; Aarsland, D.; Burn, D.; Emre, M.; Kulisevsky, J.; Weintraub, D. Cognitive impairment in nondemented Parkinson’s disease. Mov. Disord. 2011, 26, 2483–2495. [Google Scholar] [CrossRef]
- Poletti, M.; Frosini, D.; Pagni, C.; Baldacci, F.; Nicoletti, V.; Tognoni, G.; Lucetti, C.; Del Dotto, P.; Ceravolo, R.; Bonuccelli, U. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2012, 83, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Domellöf, M.E.; Ekman, U.; Forsgren, L.; Elgh, E. Cognitive function in the early phase of Parkinson’s disease, a five-year follow-up. Acta Neurol. Scand. 2015, 132, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Baiano, C.; Barone, P.; Trojano, L.; Santangelo, G. Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: A meta-analysis. Mov. Disord. 2020, 35, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Knopman, D.S. Classification and epidemiology of MCI. Clin. Geriatr. Med. 2013, 29, 753–772. [Google Scholar] [CrossRef] [PubMed]
- Galtier, I.; Nieto, A.; Lorenzo, J.N.; Barroso, J. Mild cognitive impairment in Parkinson’s disease: Diagnosis and progression to dementia. J. Clin. Exp. Neuropsychol. 2016, 38, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Caviness, J.N.; Driver-Dunckley, E.; Connor, D.J.; Sabbagh, M.N.; Hentz, J.G.; Noble, B.; Evidente, V.G.; Shill, H.A.; Adler, C.H. Defining mild cognitive impairment in Parkinson’s disease. Mov. Disord. 2007, 22, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.G.; Weis, H.; Stebbins, G.; Bernard, B.; Goetz, C.G. Clinical differences among mild cognitive impairment subtypes in Parkinson’s disease. Mov. Disord. 2012, 27, 1129–1136. [Google Scholar] [CrossRef]
- Gagnon, J.F.; Bertrand, J.A.; Génier Marchand, D. Cognition in rapid eye movement sleep behavior disorder. Front. Neurol. 2012, 3, 82. [Google Scholar] [CrossRef]
- Jozwiak, N.; Postuma, R.B.; Montplaisir, J.; Latreille, V.; Panisset, M.; Chouinard, S.; Bourgouin, P.A.; Gagnon, J.F. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep 2017, 40, zsx101. [Google Scholar] [CrossRef]
- Nagy, A.V.; Leschziner, G.; Eriksson, S.H.; Lees, A.; Noyce, A.J.; Schrag, A. Cognitive impairment in REM-sleep behaviour disorder and individuals at risk of Parkinson’s disease. Park. Relat. Disord. 2023, 109, 105312. [Google Scholar] [CrossRef]
- Dalrymple-Alford, J.C.; Livingston, L.; MacAskill, M.R.; Graham, C.; Melzer, T.R.; Porter, R.J.; Watts, R.; Anderson, T.J. Characterizing mild cognitive impairment in Parkinson’s disease. Mov. Disord. 2011, 26, 629–636. [Google Scholar] [CrossRef]
- Goldman, J.G.; Litvan, I. Mild cognitive impairment in Parkinson’s disease. Minerva Medica 2011, 102, 441–459. [Google Scholar] [PubMed]
- Sousa, N.M.F.; Brucki, S.M.D. Addenbrooke’s cognitive examination III: Diagnostic utility for detecting mild cognitive impairment and dementia in Parkinson’s disease. Arq. Neuro-Psiquiatr. 2023, 81, 155–163. [Google Scholar]
- Saredakis, D.; Collins-Praino, L.E.; Gutteridge, D.S.; Stephan, B.C.M.; Keage, H.A.D. Conversion to MCI and dementia in Parkinson’s disease: A systematic review and meta-analysis. Park. Relat. Disord. 2019, 65, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Hobson, P.; Meara, J. Mild cognitive impairment in Parkinson’s disease and its progression onto dementia: A 16-year outcome evaluation of the Denbighshire cohort. Int. J. Geriatr. Psychiatry 2015, 30, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Yoo, H.S.; Lee, Y.H.; Lee, H.S.; Ye, B.S.; Sohn, Y.H.; Kwon, H.; Lee, P.H. Frontal atrophy as a marker for dementia conversion in Parkinson’s disease with mild cognitive impairment. Hum. Brain Mapp. 2019, 40, 3784–3794. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, D.; Wang, Q.; Bai, C.; Li, Y.; Guo, X.; Chen, B.; Zhang, L.; Yuan, J. Predictors of cognitive impairment in newly diagnosed Parkinson’s disease with normal cognition at baseline: A 5-year cohort study. Front. Aging Neurosci. 2023, 15, 1142558. [Google Scholar] [CrossRef]
- Aarsland, D.; Bronnick, K.; Ehrt, U.; De Deyn, P.P.; Tekin, S.; Emre, M.; Cummings, J.L. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: Frequency, profile and associated care giver stress. J. Neurol. Neurosurg. Psychiatry 2007, 78, 36–42. [Google Scholar] [CrossRef]
- Lee, A.H.; Weintraub, D. Psychosis in Parkinson’s disease without dementia: Common and comorbid with other non-motor symptoms. Mov. Disord. 2012, 27, 858–863. [Google Scholar] [CrossRef]
- Verbaan, D.; van Rooden, S.M.; Visser, M.; Marinus, J.; Emre, M.; van Hilten, J.J. Psychotic and compulsive symptoms in Parkinson’s disease. Mov. Disord. 2009, 24, 738–744. [Google Scholar] [CrossRef]
- Santos-García, D.; de Deus Fonticoba, T.; Cores Bartolomé, C.; Feal Painceiras, M.J.; García Díaz, I.; Íñiguez Alvarado, M.C.; Paz, J.M.; Jesús, S.; Cosgaya, M.; García Caldentey, J.; et al. Cognitive impairment and dementia in young onset Parkinson’s disease. J. Neurol. 2023, 270, 5793–5812. [Google Scholar] [CrossRef] [PubMed]
- Kenney, L.E.; Ratajska, A.M.; Lopez, F.V.; Marsiske, M.; Bowers, D. Early rapid eye movement sleep behavior disorder predicts incident cognitive impairment in Parkinson’s disease across ages. Park. Relat. Disord. 2023, 110, 105392. [Google Scholar] [CrossRef] [PubMed]
- Periñán, M.T.; Macías-García, D.; Jesús, S.; Martín-Rodríguez, J.F.; Muñoz-Delgado, L.; Jimenez-Jaraba, M.V.; Buiza-Rueda, D.; Bonilla-Toribio, M.; Adarmes-Gómez, A.D.; Gómez-Garre, P.; et al. Homocysteine levels, genetic background, and cognitive impairment in Parkinson’s disease. J. Neurol. 2023, 270, 477–485. [Google Scholar] [CrossRef]
- Park, J.; Choi, S.; Kim, R. Association between prediabetes and cognitive function in Parkinson’s disease. Brain Behav. 2023, 13, e2838. [Google Scholar] [CrossRef] [PubMed]
- Grant, H.; Anderton, R.; Gasson, N.; Lawrence, B.J. The gut microbiome and cognition in Parkinson’s disease: A systematic review. Nutr. Neurosci. 2023, 26, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cen, K.; Cui, Y.; Feng, X.; Hou, X. Uric acid levels and their association with vascular dementia and Parkinson’s disease dementia: A meta-analysis. Neurol. Sci. 2023, 44, 2017–2024. [Google Scholar] [CrossRef]
- Qu, Y.; Qin, Q.X.; Wang, D.L.; Li, J.T.; Zhao, J.W.; An, K.; Li, J.Y.; Mao, Z.J.; Min, Z.; Xiong, Y.J.; et al. Estimated glomerular filtration rate is a biomarker of cognitive impairment in Parkinson’s disease. Front. Aging Neurosci. 2023, 15, 1130833. [Google Scholar] [CrossRef]
- Bocti, C.; Pépin, F.; Tétreault, M.; Cossette, P.; Langlois, F.; Imbeault, H.; Duval, N.; Lacombe, G.; Fulop, T. Orthostatic hypotension associated with executive dysfunction in mild cognitive impairment. J. Neurol. Sci. 2017, 382, 79–83. [Google Scholar] [CrossRef]
- Loureiro, D.; Bilbao, R.; Bordet, S.; Grasso, L.; Otero-Losada, M.; Capani, F.; Ponzo, O.J.; Perez-Lloret, S. A systematic review and meta-analysis on the association between orthostatic hypotension and mild cognitive impairment and dementia in Parkinson’s disease. Neurol. Sci. 2023, 44, 1211–1222. [Google Scholar] [CrossRef]
- Ruiz Barrio, I.; Miki, Y.; Jaunmuktane, Z.T.; Warner, T.; De Pablo-Fernandez, E. Association between orthostatic hypotension and dementia in patients with Parkinson disease and multiple system atrophy. Neurology 2023, 100, e998–e1008. [Google Scholar] [CrossRef]
- Monaghan, A.S.; Gordon, E.; Graham, L.; Hughes, E.; Peterson, D.S.; Morris, R. Cognition and freezing of gait in Parkinson’s disease: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2023, 147, 105068. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Xie, H.; Qin, G.; Wu, D.; Shan, M.; Hu, T.; Yin, Z.; An, Q.; Ma, R.; Wang, S.; et al. Association between cognitive impairment and freezing of gait in patients with Parkinson’s disease. J. Clin. Med. 2023, 12, 2799. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, J.; Chen, Y.; Qin, Q.; Wang, D.; Zhao, J.; Yang, Q.; Mao, Z.; Xiong, Y.; Min, Z.; et al. Freezing of gait is a risk factor for cognitive decline in Parkinson’s disease. J. Neurol. 2023, 270, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Malkiewicz, J.J.; Kasprzyk, A.G.; Waksmundzki, D.; Wegrzynek, J.; Chmiela, T.; Siuda, J. Risk factors for dementia in Parkinson’s disease—The overuse of anticholinergic drugs. Neurol. Neurochir. Pol. 2023, 57, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Dong, Z.; Zhong, J.; Pan, P.; Xu, G.; Zhang, Z.; Zhang, X.; Shi, H. Effect of cerebral small vessel disease on cognitive impairment in Parkinson’s disease. Acta Neurol. Belg. 2023, 123, 487–495. [Google Scholar] [CrossRef]
- Schrag, A.; Bohlken, J.; Dammertz, L.; Teipel, S.; Hermann, W.; Akmatov, M.K.; Bätzing, J.; Holstiege, J. Widening the spectrum of risk factors, comorbidities, and prodromal features of Parkinson disease. JAMA Neurol. 2023, 80, 161–171. [Google Scholar] [CrossRef]
- Darweesh, S.K.L.; Wolters, F.J.; Postuma, R.B.; Stricker, B.H.; Hofman, A.; Koudstaal, P.J.; Ikram, M.K.; Ikram, M.A. Association between poor cognitive functioning and risk of incident parkinsonism: The Rotterdam Study. JAMA Neurol. 2017, 74, 1431–1438. [Google Scholar] [CrossRef]
- Gasser, T.; Hardy, J.; Mizuno, Y. Milestones in PD genetics. Mov. Disord. 2011, 26, 1042–1048. [Google Scholar] [CrossRef]
- Martin, I.; Dawson, V.L.; Dawson, T.M. Recent advances in the genetics of Parkinson’s disease. Annu. Rev. Genom. Hum. Genet. 2011, 12, 301–325. [Google Scholar] [CrossRef]
- Piredda, R.; Desmarais, P.; Masellis, M.; Gasca-Salas, C. Cognitive and psychiatric symptoms in genetically determined Parkinson’s disease: A systematic review. Eur. J. Neurol. 2020, 27, 229–234. [Google Scholar] [CrossRef]
- Wise, A.H.; Alcalay, R.N. Genetics of cognitive dysfunction in Parkinson’s disease. Prog. Brain Res. 2022, 269, 195–226. [Google Scholar] [PubMed]
- Xu, J.; Li, J.; Sun, Y.J.; Quan, W.; Liu, L.; Zhang, Q.H.; Qin, Y.D.; Pei, X.C.; Su, H.; Chen, J.J. Identification of key genes and signaling pathways associated with dementia with Lewy bodies and Parkinson’s disease dementia using bioinformatics. Front. Neurol. 2023, 14, 1029370. [Google Scholar] [CrossRef] [PubMed]
- Fagan, E.S.; Pihlstrøm, L. Genetic risk factors for cognitive decline in Parkinson’s disease: A review of the literature. Eur. J. Neurol. 2017, 24, 561-e20. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhou, G.; Wang, Y.; Zhang, R.; Guo, Z.; Zhou, H.; Zheng, H.; Sun, Y.; Ma, C.; Lu, M.; et al. Association of GBA genotype with motor and cognitive decline in Chinese Parkinson’s disease patients. Front. Aging Neurosci. 2023, 15, 1091919. [Google Scholar] [CrossRef] [PubMed]
- De Michele, G.; Palmieri, G.R.; Pane, C.; Valente, E.M.; Palmieri, I.; Dello Iacovo, C.D.P.; Cuomo, N.; Giglio, A.; De Lucia, N.; Fico, T.; et al. Motor and non-motor features in Parkinson’s Disease patients carrying GBA gene mutations. Acta Neurol. Belg. 2023, 123, 221–226. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, Y.; Zhang, P.; Han, C.; Lu, Y.; Mo, Z.; Zhang, Z.; Li, X.; Zhao, S.; Cai, F.; et al. Characterization of a pathogenic variant in GBA for Parkinson’s disease with mild cognitive impairment patients. Mol. Brain 2020, 13, 102. [Google Scholar] [CrossRef]
- Xiao, B.; Deng, X.; Ng, E.Y.; Lo, Y.L.; Xu, Z.; Tay, K.Y.; Au, W.L.; Ng, A.; Tan, L.C.S.; Tan, E.K. Parkinson’s disease genome-wide association study-linked PARK16 variant is associated with a lower risk of cognitive impairment: A 4-year observational study. Eur. J. Neurol. 2023, 30, 2874–2878. [Google Scholar] [CrossRef]
- Ortega, R.A.; Wang, C.; Raymond, D.; Bryant, N.; Scherzer, C.R.; Thaler, A.; Alcalay, R.N.; West, A.B.; Mirelman, A.; Kuras, Y.; et al. Association of dual LRRK2 G2019S and GBA variations with Parkinson disease progression. JAMA Netw. Open 2021, 4, e215845. [Google Scholar] [CrossRef]
- Tan, M.M.X.; Lawton, M.A.; Jabbari, E.; Reynolds, R.H.; Iwaki, H.; Blauwendraat, C.; Kanavou, S.; Pollard, M.I.; Hubbard, L.; Malek, N.; et al. Genome-wide association studies of cognitive and motor progression in Parkinson’s disease. Mov. Disord. 2021, 36, 424–433. [Google Scholar] [CrossRef]
- Liu, J.Y.; Ma, L.Z.; Wang, J.; Cui, X.J.; Sheng, Z.H.; Fu, Y.; Li, M.; Ou, Y.N.; Yu, J.T.; Tan, L.; et al. Age-related association between APOE epsilon4 and cognitive progression in de novo Parkinson’s disease. J. Alzheimer’s Dis. 2023, 91, 1121–1132. [Google Scholar] [CrossRef]
- Jo, S.; Kim, S.O.; Park, K.W.; Lee, S.H.; Hwang, Y.S.; Chung, S.J. The role of APOE in cognitive trajectories and motor decline in Parkinson’s disease. Sci. Rep. 2021, 11, 7819. [Google Scholar] [CrossRef] [PubMed]
- Real, R.; Martinez-Carrasco, A.; Reynolds, R.H.; Lawton, M.A.; Tan, M.M.X.; Shoai, M.; Corvol, J.C.; Ryten, M.; Bresner, C.; Hubbard, L.; et al. Association between the LRP1B and APOE loci and the development of Parkinson’s disease dementia. Brain 2023, 146, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Szwedo, A.A.; Dalen, I.; Pedersen, K.F.; Camacho, M.; Bäckström, D.; Forsgren, L.; Tzoulis, C.; Winder-Rhodes, S.; Hudson, G.; Liu, G.; et al. GBA and APOE impact cognitive decline in Parkinson’s disease: A 10-year population-based study. Mov. Disord. 2022, 37, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Dai, S.; Jin, C.; Si, X.; Gu, L.; Song, Z.; Gao, T.; Chen, Y.; Yan, Y.; Yin, X.; et al. Aquaporin-4 polymorphisms are associated with cognitive performance in Parkinson’s disease. Front. Aging Neurosci. 2022, 13, 740491. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.Y.; Ma, C.C.; Chen, F.F.; Zhou, X.Y.; Li, X.; Tang, C.X.; Zhang, L.; Gao, D.S. Possible role of glial cell line-derived neurotrophic factor for predicting cognitive impairment in Parkinson’s disease: A case-control study. Neural Regen. Res. 2021, 16, 885–892. [Google Scholar] [PubMed]
- Aarsland, D.; Andersen, K.; Larsen, J.P.; Perry, R.; Wentzel-Larsen, T.; Lolk, A.; Kragh-Sorensen, P. The rate of cognitive decline in Parkinson disease. Arch. Neurol. 2004, 61, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Williams-Gray, C.H.; Evans, J.R.; Goris, A.; Foltynie, T.; Ban, M.; Robbins, T.W.; Brayne, C.; Kolachana, B.S.; Weinberger, D.R.; Sawcer, S.J.; et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain 2009, 132, 2958–2969. [Google Scholar] [CrossRef]
- Kobak Tur, E.; Ari, B.C. Mild cognitive impairment in patients with Parkinson’s disease and the analysis of associated factors. Neurol. Res. 2023, 45, 1161–1168. [Google Scholar] [CrossRef]
- Johnson, D.K.; Langford, Z.; Garnier-Villarreal, M.; Morris, J.C.; Galvin, J.E. Onset of mild cognitive impairment in Parkinson disease. Alzheimer Dis. Assoc. Disord. 2016, 30, 127–133. [Google Scholar] [CrossRef]
- Cholerton, B.; Johnson, C.O.; Fish, B.; Quinn, J.F.; Chung, K.A.; Peterson-Hiller, A.L.; Rosenthal, L.S.; Dawson, T.M.; Albert, M.S.; Hu, S.C.; et al. Sex differences in progression to mild cognitive impairment and dementia in Parkinson’s disease. Park. Relat. Disord. 2018, 50, 29–36. [Google Scholar] [CrossRef]
- Bock, M.A.; Vittinghoff, E.; Bahorik, A.L.; Leng, Y.; Fink, H.; Yaffe, K. Cognitive and functional trajectories in older adults with prediagnostic Parkinson disease. Neurology 2023, 100, e1386–e1394. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; McDonald, W.M.; Vitek, J.L.; Evatt, M.; Freeman, A.; Haber, M.; Bakay, R.A.; Triche, S.; Sirockman, B.; DeLong, M.R. Cognitive impairments in advanced PD without dementia. Neurology 2002, 59, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Roberts, R.O.; Knopman, D.S.; Boeve, B.F.; Geda, Y.E.; Ivnik, R.J.; Smith, G.E.; Jack, C.R., Jr. Mild cognitive impairment: Ten years later. Arch. Neurol. 2009, 66, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, M.; De Micco, R.; Russo, A.G.; Esposito, F.; Sant’Elia, V.; Ricciardi, L.; Morgante, F.; Russo, A.; Goldman, J.G.; Chiorri, C.; et al. Memory phenotypes in early, de novo Parkinson’s disease patients with mild cognitive impairment. Mov. Disord. 2023, 38, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Muslimovic, D.; Post, B.; Speelman, J.D.; Schmand, B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 2005, 65, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.S.; Leverenz, J.B. Profile of cognitive impairment in Parkinson’s disease. Brain Pathol. 2010, 20, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Muslimovic, D.; Schmand, B.; Speelman, J.D.; de Haan, R.J. Course of cognitive decline in Parkinson’s disease: A meta-analysis. J. Int. Neuropsychol. Soc. 2007, 13, 920–932. [Google Scholar] [CrossRef]
- Pedersen, K.F.; Larsen, J.P.; Tysnes, O.B.; Alves, G. Prognosis of mild cognitive impairment in early Parkinson disease: The Norwegian ParkWest study. JAMA Neurol. 2013, 70, 580–586. [Google Scholar] [CrossRef]
- Pigott, K.; Rick, J.; Xie, S.X.; Hurtig, H.; Chen-Plotkin, A.; Duda, J.E.; Morley, J.F.; Chahine, L.M.; Dahodwala, N.; Akhtar, R.S.; et al. Longitudinal study of normal cognition in Parkinson disease. Neurology 2015, 85, 1276–1282. [Google Scholar] [CrossRef]
- McKinlay, A.; Grace, R.C.; Dalrymple-Alford, J.C.; Roger, D. Characteristics of executive function impairment in Parkinson’s disease patients without dementia. J. Int. Neuropsychol. Soc. 2010, 16, 268–277. [Google Scholar] [CrossRef]
- Hannaway, N.; Zarkali, A.; Leyland, L.A.; Bremner, F.; Nicholas, J.M.; Wagner, S.K.; Roig, M.; Keane, P.A.; Toosy, A.; Chataway, J.; et al. Visual dysfunction is a better predictor than retinal thickness for dementia in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2023, 94, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; Eom, J.; Park, K.S.; Park, Y.W.; Chung, S.J.; Kim, Y.J.; Ahn, S.S.; Kim, J.; Lee, P.H.; Sohn, Y.H.; et al. An interpretable multiparametric radiomics model of basal ganglia to predict dementia conversion in Parkinson’s disease. NPJ Park. Dis. 2023, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Uribe, C.; Bunch, J.; Thomas, K.R. Beyond PD-MCI: Objectively defined subtle cognitive decline predicts future cognitive and functional changes. J. Neurol. 2021, 268, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Kwon, H.; Chung, S.J.; Sohn, Y.H.; Lee, J.M.; Lee, P.H. Neural correlates of self-awareness of cognitive deficits in non-demented patients with Parkinson’s disease. Eur. J. Neurol. 2021, 28, 4022–4030. [Google Scholar] [CrossRef] [PubMed]
- Gasca-Salas, C.; Duff-Canning, S.; McArthur, E.; Armstrong, M.J.; Fox, S.; Meaney, C.A.; Tang-Wai, D.F.; Gill, D.; Eslinger, P.J.; Zadikoff, C.; et al. Predictors of cognitive change in Parkinson disease: A 2-year follow-up study. Alzheimer Dis. Assoc. Disord. 2023, 37, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Cho, K.H.; Song, S.K.; Kim, H.J.; Lee, H.S.; Sohn, Y.H.; Lee, P.H. Exploratory analysis of neuropsychological and neuroanatomical correlates of progressive mild cognitive impairment in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2014, 85, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Kim, S.H.; Park, C.W.; Lee, H.S.; Kim, Y.J.; Lee, P.H.; Jeong, Y.; Sohn, Y.H. Is the cingulate island sign a marker for early dementia conversion in Parkinson’s disease? Eur. J. Neurol. 2023, 30, 3732–3740. [Google Scholar] [CrossRef]
- Wallace, E.R.; Segerstrom, S.C.; van Horne, C.G.; Schmitt, F.A.; Koehl, L.M. Meta-analysis of cognition in Parkinson’s disease mild cognitive impairment and dementia progression. Neuropsychol. Rev. 2022, 32, 149–160. [Google Scholar] [CrossRef]
- Goldman, J.G.; Holden, S.; Ouyang, B.; Bernard, B.; Goetz, C.G.; Stebbins, G.T. Diagnosing PD-MCI by MDS Task Force criteria: How many and which neuropsychological tests? Mov. Disord. 2015, 30, 402–406. [Google Scholar] [CrossRef]
- Dubois, B.; Burn, D.; Goetz, C.; Aarsland, D.; Brown, R.G.; Broe, G.A.; Dickson, D.; Duyckaerts, C.; Cummings, J.; Gauthier, S.; et al. Diagnostic procedures for Parkinson’s disease dementia: Recommendations from the movement disorder society task force. Mov. Disord. 2007, 22, 2314–2324. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Martinez-Martin, P.; Falup-Pecurariu, C.; Rodriguez-Blazquez, C.; Serrano-Duenas, M.; Carod Artal, F.J.; Rojo Abuin, J.M.; Aarsland, D. Dementia associated with Parkinson’s disease: Applying the Movement Disorder Society Task Force criteria. Park. Relat. Disord. 2011, 17, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.; Grabli, D.; Bernard, B.; Czernecki, V.; Goldman, J.G.; Stebbins, G.; Dubois, B.; Goetz, C.G. Clinical validation of Movement Disorder Society-recommended diagnostic criteria for Parkinson’s disease with dementia. Mov. Disord. 2012, 27, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, M.E.; Giustini, P.; Bernardi, S.; Stirpe, P.; Vanacore, N.; Meco, G. A simplified algorithm may lead to overestimate dementia in PD. A clinical and epidemiological study using criteria for PD-D proposed by the Movement Disorders Task Force. J. Neural Transm. 2011, 118, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.N.; Souza, C.P.; Foss, M.P.; Tumas, V. An analysis of the cognitive items of the movement disorders society checklist for the diagnosis of dementia in patients with Parkinson’s disease. Park. Relat. Disord. 2015, 21, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Isella, V.; Mapelli, C.; Siri, C.; De Gaspari, D.; Pezzoli, G.; Antonini, A.; Poletti, M.; Bonuccelli, U.; Vista, M.; Appollonio, I.M. Validation and attempts of revision of the MDS-recommended tests for the screening of Parkinson’s disease dementia. Park. Relat. Disord. 2014, 20, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Rocha, N.P.; Carreira, E.X.; Prado, A.C.A.; Tavares, F.; Tavares, M.; Cardoso, F.; Jaeger, A.; Souza, L.C.; Teixeira, A.L. Cognitive evaluation in Parkinson’s disease: Applying the Movement Disorder Society recommendations in a population with a low level of formal education. Arq. Neuro-Psiquiatr. 2023, 81, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.R.; Wieler, M.; Gee, M.; Camicioli, R. Temporal lobe changes in early, untreated Parkinson’s disease. Mov. Disord. 2009, 24, 1949–1954. [Google Scholar] [CrossRef]
- Pereira, J.B.; Svenningsson, P.; Weintraub, D.; Brønnick, K.; Lebedev, A.; Westman, E.; Aarsland, D. Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology 2014, 82, 2017–2025. [Google Scholar] [CrossRef]
- Apostolova, L.; Alves, G.; Hwang, K.S.; Babakchanian, S.; Bronnick, K.S.; Larsen, J.P.; Thompson, P.M.; Chou, Y.Y.; Tysnes, O.B.; Vefring, H.K.; et al. Hippocampal and ventricular changes in Parkinson’s disease mild cognitive impairment. Neurobiol. Aging 2012, 33, 2113–2124. [Google Scholar] [CrossRef][Green Version]
- Gao, H.L.; Qu, Y.; Chen, S.C.; Yang, Q.M.; Li, J.Y.; Tao, A.Y.; Mao, Z.J.; Xue, Z. Third ventricular width by transcranial sonography is associated with cognitive impairment in Parkinson’s disease. CNS Neurosci. Ther. 2023. [Google Scholar] [CrossRef]
- Gao, Y.; Nie, K.; Huang, B.; Mei, M.; Guo, M.; Xie, S.; Huang, Z.; Wang, L.; Zhao, J.; Zhang, Y. Changes of brain structure in Parkinson’s disease patients with mild cognitive impairment analyzed via VBM technology. Neurosci. Lett. 2017, 658, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.X.; Kang, D.Z.; Chen, F.Y.; Liu, Y.; Wu, G.; Li, X.; Yu, L.H.; Lin, Y.X.; Lin, Z.Y. Gray matter atrophy associated with mild cognitive impairment in Parkinson’s disease. Neurosci. Lett. 2016, 617, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Kunst, J.; Marecek, R.; Klobusiakova, P.; Balazova, Z.; Anderkova, L.; Nemcova-Elfmarkova, N.; Rektorova, I. Patterns of grey matter atrophy at different stages of Parkinson’s and Alzheimer’s diseases and relation to cognition. Brain Topogr. 2019, 32, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Segura, B.; Baggio, H.C.; Marti, M.J.; Valldeoriola, F.; Compta, Y.; Garcia-Diaz, A.I.; Vendrell, P.; Bargallo, N.; Tolosa, E.; Junque, C. Cortical thinning associated with mild cognitive impairment in Parkinson’s disease. Mov. Disord. 2014, 29, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Devignes, Q.; Lopes, R.; Dujardin, K. Neuroimaging outcomes associated with mild cognitive impairment subtypes in Parkinson’s disease: A systematic review. Park. Relat. Disord. 2022, 95, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.; Hakun, J.; Lewis, M.M.; De Jesus, S.; Du, G.; Eslinger, P.J.; Kong, L.; Huang, X. Frontostriatal and limbic contributions to cognitive decline in Parkinson’s disease. J. Neuroimaging 2023, 33, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Pletcher, C.; Dabbs, K.; Barzgari, A.; Pozorski, V.; Haebig, M.; Wey, S.; Krislov, S.; Theisen, F.; Okonkwo, O.; Cary, P.; et al. Cerebral cortical thickness and cognitive decline in Parkinson’s disease. Cereb. Cortex Commun. 2023, 4, tgac044. [Google Scholar] [CrossRef]
- Donzuso, G.; Monastero, R.; Cicero, C.E.; Luca, A.; Mostile, G.; Giuliano, L.; Baschi, R.; Caccamo, M.; Gagliardo, C.; Palmucci, S.; et al. Neuroanatomical changes in early Parkinson’s disease with mild cognitive impairment: A VBM study; the Parkinson’s Disease Cognitive Impairment Study (PaCoS). Neurol. Sci. 2021, 42, 3723–3731. [Google Scholar] [CrossRef]
- Mihaescu, A.S.; Masellis, M.; Graff-Guerrero, A.; Kim, J.; Criaud, M.; Cho, S.S.; Ghadery, C.; Valli, M.; Strafella, A.P. Brain degeneration in Parkinson’s disease patients with cognitive decline: A coordinate-based meta-analysis. Brain Imaging Behav. 2019, 13, 1021–1034. [Google Scholar] [CrossRef]
- Weintraub, D.; Doshi, J.; Koka, D.; Davatzikos, C.; Siderowf, A.D.; Duda, J.E.; Wolk, D.A.; Moberg, P.J.; Xie, S.X.; Clark, C.M. Neurodegeneration across stages of cognitive decline in Parkinson disease. Arch. Neurol. 2011, 68, 1562–1568. [Google Scholar] [CrossRef]
- Melzer, T.R.; Watts, R.; MacAskill, M.R.; Pitcher, T.L.; Livingston, L.; Keenan, R.J.; Dalrymple-Alford, J.C.; Anderson, T.J. Grey matter atrophy in cognitively impaired Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2012, 83, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Canu, E.; Donzuso, G.; Stojkovic, T.; Basaia, S.; Stankovic, I.; Tomic, A.; Markovic, V.; Petrovic, I.; Stefanova, E.; et al. Tracking cortical changes throughout cognitive decline in Parkinson’s disease. Mov. Disord. 2020, 35, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Chen, C.; Song, W.; Yi, Z.; Zhao, P.; Zhong, J.; Dai, Z.; Shi, H.; Pan, P. Regional gray matter reductions associated with mild cognitive impairment in Parkinson’s disease: A meta-analysis of voxel-based morphometry studies. Behav. Brain Res. 2019, 371, 111973. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Granert, O.; Timmers, M.; Pilotto, A.; Van Nueten, L.; Roeben, B.; Salvadore, G.; Galpern, W.R.; Streffer, J.; Scheffler, K.; et al. Association of hippocampal subfields, CSF biomarkers, and cognition in patients with Parkinson disease without dementia. Neurology 2021, 96, e904–e915. [Google Scholar] [CrossRef] [PubMed]
- Foo, H.; Mak, E.; Chander, R.J.; Ng, A.; Au, W.L.; Sitoh, Y.Y.; Tan, L.C.; Kandiah, N. Associations of hippocampal subfields in the progression of cognitive decline related to Parkinson’s disease. Neuroimage Clin. 2016, 14, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, Y.; Wang, L.; Zeng, X.; Xu, Y.; Wang, Z. Role of hippocampal subfields in neurodegenerative disease progression analyzed with a multi-scale attention-based network. Neuroimage Clin. 2023, 38, 103370. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.J.; Amin, M.; Tanner, J.J.; Ding, M.; Mareci, T.A.; Price, C.C. Free water fraction predicts cognitive decline for individuals with idiopathic Parkinson’s disease. Park. Relat. Disord. 2022, 104, 72–77. [Google Scholar] [CrossRef]
- Foo, H.; Mak, E.; Yong, T.T.; Wen, M.C.; Chander, R.J.; Au, W.L.; Sitoh, Y.Y.; Tan, L.C.; Kandiah, N. Progression of subcortical atrophy in mild Parkinson’s disease and its impact on cognition. Eur. J. Neurol. 2017, 24, 341–348. [Google Scholar] [CrossRef]
- Choi, S.A.; Evidente, V.G.; Caviness, J.N.; Shill, H.A.; Sabbagh, M.N.; Connor, D.J.; Hentz, J.G.; Adler, C.H.; Beach, T.G. Are there differences in cerebral white matter lesion burdens between Parkinson’s disease patients with or without dementia? (Correspondence). Acta Neuropathol. 2010, 119, 147–149. [Google Scholar] [CrossRef][Green Version]
- Zhao, W.; Cheng, B.; Zhu, T.; Cui, Y.; Shen, Y.; Fu, X.; Li, M.; Feng, Y.; Zhang, S. Effects of white matter hyperintensity on cognitive function in PD patients: A meta-analysis. Front. Neurol. 2023, 14, 1203311. [Google Scholar] [CrossRef]
- Carvalho de Abreu, D.C.; Pieruccini-Faria, F.; Sarquis-Adamson, Y.; Black, A.; Fraser, J.; Van Ooteghem, K.; Cornish, B.; Grimes, D.; Jog, M.; Masellis, M.; et al. White matter hyperintensity burden predicts cognitive but not motor decline in Parkinson’s disease: Results from the Ontario Neurodegenerative Diseases Research Initiative. Eur. J. Neurol. 2023, 30, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Dunet, V.; Fartaria, M.J.; Deverdun, J.; Le Bars, E.; Maury, F.; Castelnovo, G.; Kober, T.; Cuadra, M.B.; Geny, C.; Marechal, B.; et al. Episodic memory decline in Parkinson’ s disease: Relation with white matter hyperintense lesions and influence of quantification method. Brain Imaging Behav. 2019, 13, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Agosta, F.; Canu, E.; Stefanova, E.; Sarro, L.; Tomic, A.; Špica, V.; Comi, G.; Kostic, V.S.; Filippi, M. Mild cognitive impairment in Parkinson’s disease is associated with a distributed pattern of brain white matter damage. Hum. Brain Mapp. 2014, 35, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Orimo, S.; Aoki, S.; Ito, K.; Abe, O.; Amano, A.; Sato, R.; Sakai, K.; Mizusawa, H. Cognitive status correlates with white matter alteration in Parkinson’s disease. Hum. Brain Mapp. 2012, 33, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Melzer, T.R.; Watts, R.; MacAskill, M.R.; Pitcher, T.L.; Livingston, L.; Keenan, R.J.; Dalrymple-Alford, J.C.; Anderson, T.J. White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology 2013, 80, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Shen, X.; Huang, M.; Li, Z.; Zeng, X.; Wang, R.; Shen, G.; Yu, H. Assessment of white matter lesions in Parkinson’s disease: Voxel-based analysis and tract-based spatial statistics analysis of Parkinson’s disease with mild cognitive impairment. Curr. Neurovasc. Res. 2020, 17, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yuan, Y.; Sang, T.; Yu, L.; Yu, Y.; Liu, X.; Zhou, W.; Zeng, Q.; Wang, J.; Peng, G.; et al. Local white matter abnormalities in Parkinson’s disease with mild cognitive impairment: Assessed with neurite orientation dispersion and density imaging. J. Neurosci. Res. 2023, 101, 1154–1169. [Google Scholar] [CrossRef]
- Stewart, S.A.; Pimer, L.; Fisk, J.D.; Rusak, B.; Leslie, R.A.; Eskes, G.; Schoffer, K.; McKelvey, J.R.; Rolheiser, T.; Khan, M.N.; et al. Olfactory function and diffusion tensor imaging as markers of mild cognitive impairment in early stages of Parkinson’s disease. Clin. EEG Neurosci. 2023, 54, 91–97. [Google Scholar] [CrossRef]
- Kübler, D.; Kobylecki, C.; McDonald, K.R.; Anton-Rodriguez, J.M.; Herholz, K.; Carter, S.F.; Hinz, R.; Thompson, J.C.; Al-Fatly, B.; Gerhard, A. Structural and metabolic correlates of neuropsychological profiles in multiple system atrophy and Parkinson’s disease. Park. Relat. Disord. 2023, 107, 105277. [Google Scholar] [CrossRef]
- Yu, Z.; Pang, H.; Yu, H.; Wu, Z.; Ding, Z.; Fan, G. Segmental disturbance of white matter microstructure in predicting mild cognitive impairment in idiopathic Parkinson’s disease: An individualized study based on automated fiber quantification tractography. Park. Relat. Disord. 2023, 115, 105802. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Koeppe, R.A.; Minoshima, S.; Giordani, B.; Albin, R.L.; Frey, K.A.; Kuhl, D.E. Cerebral glucose metabolic features of Parkinson disease and incident dementia: Longitudinal study. J. Nucl. Med. 2011, 52, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Homenko, J.G.; Susin, D.S.; Kataeva, G.V.; Irishina, J.A.; Zavolokov, I.G. Characteristics of cerebral glucose metabolism in patients with cognitive impairment in Parkinson’s disease. Zhurnal Nevrol. Psikhiatrii Im. SS Korsakova 2017, 117, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Siddiqui, U.F.; Anastasiou, Z.; Weintraub, D.; Schott, J.M. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson’s disease: A cohort study. Lancet Neurol. 2017, 16, 66–75. [Google Scholar] [CrossRef] [PubMed]
- González-Redondo, R.; García-García, D.; Clavero, P.; Gasca-Salas, C.; García-Eulate, R.; Zubieta, J.L.; Arbizu, J.; Obeso, J.A.; Rodríguez-Oroz, M.C. Grey matter hypometabolism and atrophy in Parkinson’s disease with cognitive impairment: A two-step process. Brain 2014, 137, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Zhihui, S.; Yinghua, L.; Hongguang, Z.; Yuyin, D.; Xiaoxiao, D.; Lulu, G.; Yi, L.; Kangli, F.; Ying, Z. Correlation analysis between (18)F-fluorodeoxyglucose positron emission tomography and cognitive function in first diagnosed Parkinson’s disease patients. Front. Neurol. 2023, 14, 1195576. [Google Scholar] [CrossRef]
- Aracil-Bolaños, I.; Sampedro, F.; Marín-Lahoz, J.; Horta-Barba, A.; Martínez-Horta, S.; Botí, M.; Pérez-Pérez, J.; Bejr-Kasem, H.; Pascual-Sedano, B.; Campolongo, A.; et al. A divergent breakdown of neurocognitive networks in Parkinson’s Disease mild cognitive impairment. Hum. Brain Mapp. 2019, 40, 3233–3242. [Google Scholar] [CrossRef]
- Lang, S.; Yoon, E.J.; Kibreab, M.; Kathol, I.; Cheetham, J.; Hammer, T.; Sarna, J.; Ismail, Z.; Monchi, O. Mild behavioral impairment in Parkinson’s disease is associated with altered corticostriatal connectivity. Neuroimage Clin. 2020, 26, 102252. [Google Scholar] [CrossRef]
- Hou, Y.; Yang, J.; Luo, C.; Song, W.; Ou, R.; Liu, W.; Gong, Q.; Shang, H. Dysfunction of the default mode network in drug-naive Parkinson’s disease with mild cognitive impairments: A resting-state fMRI study. Front. Aging Neurosci. 2016, 8, 247. [Google Scholar] [CrossRef]
- Liu, J.; Zou, X.; Gu, J.; Yu, Q.; Dong, Z.; Zuo, H.; Chen, X.; Du, X.; Zou, D.; Han, Y.; et al. Altered connectivity in the cognitive control-related prefrontal cortex in Parkinson’s disease with rapid eye movement sleep behavior disorder. Brain Imaging Behav. 2023, 17, 702–714. [Google Scholar] [CrossRef]
- De Micco, R.; Piramide, N.; Di Nardo, F.; Siciliano, M.; Cirillo, M.; Russo, A.; Silvestro, M.; Tedeschi, G.; Esposito, F.; Tessitore, A. Resting-state network connectivity changes in drug-naive Parkinson’s disease patients with probable REM sleep behavior disorder. J. Neural Transm. 2023, 130, 43–51. [Google Scholar] [CrossRef]
- Amboni, M.; Tessitore, A.; Esposito, F.; Santangelo, G.; Picillo, M.; Vitale, C.; Giordano, A.; Erro, R.; de Micco, R.; Corbo, D.; et al. Resting-state functional connectivity associated with mild cognitive impairment in Parkinson’s disease. J. Neurol. 2015, 262, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Suo, X.; Lei, D.; Li, N.; Li, J.; Peng, J.; Li, W.; Yang, J.; Qin, K.; Kemp, G.J.; Peng, R.; et al. Topologically convergent and divergent morphological gray matter networks in early-stage Parkinson’s disease with and without mild cognitive impairment. Hum. Brain Mapp. 2021, 42, 5101–5112. [Google Scholar] [CrossRef] [PubMed]
- Suo, X.; Lei, D.; Li, N.; Li, W.; Kemp, G.J.; Sweeney, J.A.; Peng, R.; Gong, Q. Disrupted morphological grey matter networks in early-stage Parkinson’s disease. Brain Struct. Funct. 2021, 226, 1389–1403. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Mei, M.; Gao, Y.; Huang, B.; Qiu, Y.; Zhang, Y.; Wang, L.; Zhao, J.; Huang, Z.; Nie, K. Changes of brain structural network connection in Parkinson’s disease patients with mild cognitive dysfunction: A study based on diffusion tensor imaging. J. Neurol. 2020, 267, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Owens-Walton, C.; Jakabek, D.; Power, B.D.; Walterfang, M.; Hall, S.; van Westen, D.; Looi, J.C.L.; Shaw, M.; Hansson, O. Structural and functional neuroimaging changes associated with cognitive impairment and dementia in Parkinson’s disease. Psychiatry Res. Neuroimaging 2021, 312, 111273. [Google Scholar] [CrossRef] [PubMed]
- Maier, F.; Greuel, A.; Hoock, M.; Kaur, R.; Tahmasian, M.; Schwartz, F.; Csoti, I.; Jessen, F.; Drzezga, A.; van Eimeren, T.; et al. Impaired self-awareness of cognitive deficits in Parkinson’s disease relates to cingulate cortex dysfunction. Psychol. Med. 2023, 53, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Kim, Y.J.; Jung, J.H.; Lee, H.S.; Ye, B.S.; Sohn, Y.H.; Jeong, Y.; Lee, P.H. Association between white matter connectivity and early dementia in patients with Parkinson disease. Neurology 2022, 98, e1846–e1856. [Google Scholar] [CrossRef]
- Chen, H.; Wan, H.; Zhang, M.; Wardlaw, J.M.; Feng, T.; Wang, Y. Perivascular space in Parkinson’s disease: Association with CSF amyloid/tau and cognitive decline. Park. Relat. Disord. 2022, 95, 70–76. [Google Scholar] [CrossRef]
- Kim, I.; Shin, N.Y.; Yunjin, B.; Hyu Lee, P.; Lee, S.K.; Mee Lim, S. Early-onset mild cognitive impairment in Parkinson’s disease: Altered corticopetal cholinergic network. Sci. Rep. 2017, 7, 2381. [Google Scholar] [CrossRef]
- Shang, S.; Zhu, S.; Wu, J.; Xu, Y.; Chen, L.; Dou, W.; Yin, X.; Chen, Y.C.; Shen, D.; Ye, J. Topological disruption of high-order functional networks in cognitively preserved Parkinson’s disease. CNS Neurosci. Ther. 2023, 29, 566–576. [Google Scholar] [CrossRef]
- Harrington, D.L.; Shen, Q.; Castillo, G.N.; Filoteo, J.V.; Litvan, I.; Takahashi, C.; French, C. Aberrant intrinsic activity and connectivity in cognitively normal Parkinson’s disease. Front. Aging Neurosci. 2017, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Jin, W.; Li, N.; Gao, J.; Wang, J.; Chang, Y.; Yin, K.; Chen, Y.; Zhang, S.; Wang, T. Brain activity alterations in patients with Parkinson’s disease with cognitive impairment based on resting-state functional MRI. Neurosci. Lett. 2021, 747, 135672. [Google Scholar] [CrossRef] [PubMed]
- Petrou, M.; Bohnen, N.I.; Muller, M.L.; Koeppe, R.A.; Albin, R.L.; Frey, K.A. Abeta-amyloid deposition in patients with Parkinson disease at risk for development of dementia. Neurology 2012, 79, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Tommasini, L.; Aghakhanyan, G.; Frosini, D.; Giuntini, M.; Tognoni, G.; Bonuccelli, U.; Volterrani, D.; Ceravolo, R. Clinical correlates of cerebral amyloid deposition in Parkinson’s disease dementia: Evidence from a PET study. J. Alzheimer’s Dis. 2019, 70, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Melzer, T.R.; Stark, M.R.; Keenan, R.J.; Myall, D.J.; MacAskill, M.R.; Pitcher, T.L.; Livingston, L.; Grenfell, S.; Horne, K.L.; Young, B.N.; et al. Beta amyloid deposition is not associated with cognitive impairment in Parkinson’s disease. Front. Neurol. 2019, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chen, P.H.; Tsai, C.C.; Chiang, H.F.; Hsieh, C.C.; Chen, T.L.; Liao, W.H.; Chen, Y.L.; Wang, J.J. Diffusion and structural MRI as potential biomarkers in people with Parkinson’s disease and cognitive impairment. Eur. Radiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, D.G.; Phillips, J.S.; Roll, E.; Peterson, C.; Lobrovich, R.; Rascovsky, K.; Ungrady, M.; Wolk, D.A.; Das, S.; Weintraub, D.; et al. Multimodal in vivo and postmortem assessments of tau in Lewy body disorders. Neurobiol. Aging 2020, 96, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Gomperts, S.N.; Locascio, J.J.; Makaretz, S.J.; Schultz, A.; Caso, C.; Vasdev, N.; Sperling, R.; Growdon, J.H.; Dickerson, B.C.; Johnson, K. Tau positron emission tomographic imaging in the Lewy body diseases. JAMA Neurol. 2016, 73, 1334–1341. [Google Scholar] [CrossRef]
- Kantarci, K.; Lowe, V.J.; Boeve, B.F.; Senjem, M.L.; Tosakulwong, N.; Lesnick, T.G.; Spychalla, A.J.; Gunter, J.L.; Fields, J.A.; Graff-Radford, J.; et al. AV-1451 tau and beta-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann. Neurol. 2017, 81, 58–67. [Google Scholar] [CrossRef]
- Marquie, M.; Verwer, E.E.; Meltzer, A.C.; Kim, S.J.W.; Aguero, C.; Gonzalez, J.; Makaretz, S.J.; Siao Tick Chong, M.; Ramanan, P.; Amaral, A.C.; et al. Lessons learned about [F-18]-AV-1451 off-target binding from an autopsy-confirmed Parkinson’s case. Acta Neuropathol. Commun 2017, 5, 75. [Google Scholar] [CrossRef]
- Wylie, K.P.; Kluger, B.M.; Medina, L.D.; Holden, S.K.; Kronberg, E.; Tregellas, J.R.; Buard, I. Hippocampal, basal ganglia and olfactory connectivity contribute to cognitive impairments in Parkinson’s disease. Eur. J. Neurosci. 2023, 57, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xu, M.; Yu, H.; He, J.; Li, Y.; Song, D.; Fan, G.G. Detection of mild cognitive impairment in Parkinson’s disease using gradient boosting decision tree models based on multilevel DTI indices. J. Transl. Med. 2023, 21, 310. [Google Scholar] [CrossRef]
- Owens-Walton, C.; Adamson, C.; Walterfang, M.; Hall, S.; van Westen, D.; Hansson, O.; Shaw, M.; Looi, J.C.L. Midsagittal corpus callosal thickness and cognitive impairment in Parkinson’s disease. Eur. J. Neurosci. 2022, 55, 1859–1872. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, B.; Zhou, C.; Gao, C.; Hu, Y.; Yin, W.F.; Yin, K.; Jiang, G.; Ren, H.; Pang, A.; et al. Cortical atrophy is associated with cognitive impairment in Parkinson’s disease: A combined analysis of cortical thickness and functional connectivity. Brain Imaging Behav. 2022, 16, 2586–2600. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, Z.; Yang, T.; Li, Y.; Gao, S.; Wu, G.; Jiang, T.; Liang, P. Entorhinal cortex atrophy in early, drug-naive Parkinson’s disease with mild cognitive impairment. Aging Dis 2019, 10, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, A.; Bedetti, C.; Degroot, C.; Mejia-Constain, B.; Lafontaine, A.L.; Soland, V.; Chouinard, S.; Bruneau, M.A.; Mellah, S.; Belleville, S.; et al. Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson’s disease longitudinally. Brain 2014, 137, 1120–1129. [Google Scholar] [CrossRef]
- Yazdan Panah, M.; Mokary, Y.; Shah, S.; Thapa, S.; Chand, S.; Shaygannejad, V.; Mirmosayyeb, O. Comparing the hippocampal volumetric atrophy between demented and nondemented individuals with Parkinson’s disease: A systematic review and meta-analysis. Health Sci. Rep. 2023, 6, e1514. [Google Scholar] [CrossRef]
- Camicioli, R.; Sabino, J.; Gee, M.; Bouchard, T.; Fisher, N.; Hanstock, C.; Emery, D.; Martin, W.R. Ventricular dilatation and brain atrophy in patients with Parkinson’s disease with incipient dementia. Mov. Disord. 2011, 26, 1443–1450. [Google Scholar] [CrossRef]
- Weintraub, D.; Dietz, N.; Duda, J.E.; Wolk, D.A.; Doshi, J.; Xie, S.X.; Davatzikos, C.; Clark, C.M.; Siderowf, A. Alzheimer’s disease pattern of brain atrophy predicts cognitive decline in Parkinson’s disease. Brain 2012, 135, 170–180. [Google Scholar] [CrossRef]
- Sivaranjini, S.; Sujatha, C.M. Morphological analysis of subcortical structures for assessment of cognitive dysfunction in Parkinson’s disease using multi-atlas based segmentation. Cogn. Neurodyn. 2021, 15, 835–845. [Google Scholar] [CrossRef]
- Sampedro, F.; Puig-Davi, A.; Martinez-Horta, S.; Pagonabarraga, J.; Horta-Barba, A.; Aracil-Bolaños, I.; Kulisevsky, J. Cortical macro and microstructural correlates of cognitive and neuropsychiatric symptoms in Parkinson’s disease. Clin. Neurol. Neurosurg. 2023, 224, 107531. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.W.; Firbank, M.J.; Yarnall, A.J.; Khoo, T.K.; Brooks, D.J.; Barker, R.A.; Burn, D.J.; O’Brien, J.T. Gray and white matter imaging: A biomarker for cognitive impairment in early Parkinson’s disease? Mov. Disord. 2016, 31, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Rektor, I.; Svátková, A.; Vojtíšek, L.; Zikmundová, I.; Vanícek, J.; Király, A.; Szabó, N. White matter alterations in Parkinson’s disease with normal cognition precede grey matter atrophy. PLoS ONE 2018, 13, e0187939. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Deng, B.; Xie, F.; Yang, X.; Xie, Z.; Chen, Y.; Yang, Z.; Huang, X.; Zhu, S.; Wang, Q. The influence of white matter hyperintensity on cognitive impairment in Parkinson’s disease. Ann. Clin. Transl. Neurol. 2021, 8, 1917–1934. [Google Scholar] [CrossRef] [PubMed]
- Dadar, M.; Zeighami, Y.; Yau, Y.; Fereshtehnejad, S.M.; Maranzano, J.; Postuma, R.B.; Dagher, A.; Collins, D.L. White matter hyperintensities are linked to future cognitive decline in de novo Parkinson’s disease patients. Neuroimage Clin. 2018, 20, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Scamarcia, P.G.; Agosta, F.; Spinelli, E.G.; Basaia, S.; Stojkovic, T.; Stankovic, I.; Sarasso, E.; Canu, E.; Markovic, V.; Petrovic, I.; et al. Longitudinal white matter damage evolution in Parkinson’s disease. Mov. Disord. 2022, 37, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.; Kamtchum-Tatuene, J.; Khan, K.; Shuaib, A.; Jickling, G.C.; Miyasaki, J.M.; Smith, E.E.; Camicioli, R. White matter hyperintensities in patients with Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. Sci. 2021, 426, 117481. [Google Scholar] [CrossRef] [PubMed]
- Hanning, U.; Teuber, A.; Lang, E.; Trenkwalder, C.; Mollenhauer, B.; Minnerup, H. White matter hyperintensities are not associated with cognitive decline in early Parkinson’s disease—The DeNoPa cohort. Park. Relat. Disord. 2019, 69, 61–67. [Google Scholar] [CrossRef]
- Yarnall, A.J.; Breen, D.P.; Duncan, G.W.; Khoo, T.K.; Coleman, S.Y.; Firbank, M.J.; Nombela, C.; Winder-Rhodes, S.; Evans, J.R.; Rowe, J.B.; et al. Characterizing mild cognitive impairment in incident Parkinson disease: The ICICLE-PD study. Neurology 2014, 82, 308–316. [Google Scholar] [CrossRef]
- Dalaker, T.O.; Larsen, J.P.; Dwyer, M.G.; Aarsland, D.; Beyer, M.K.; Alves, G.; Bronnick, K.; Tysnes, O.B.; Zivadinov, R. White matter hyperintensities do not impact cognitive function in patients with newly diagnosed Parkinson’s disease. Neuroimage 2009, 47, 2083–2089. [Google Scholar] [CrossRef]
- Schröter, N.; Bormann, T.; Rijntjes, M.; Blazhenets, G.; Berti, R.; Sajonz, B.E.A.; Urbach, H.; Weiller, C.; Meyer, P.T.; Rau, A.; et al. Cognitive deficits in Parkinson’s disease are associated with neuronal dysfunction and not white matter lesions. Mov. Disord. Clin. Pract. 2023, 10, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Sarasso, E.; Agosta, F.; Piramide, N.; Filippi, M. Progression of grey and white matter brain damage in Parkinson’s disease: A critical review of structural MRI literature. J. Neurol. 2021, 268, 3144–3179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Burock, M.A. Diffusion tensor imaging in Parkinson’s disease and parkinsonian syndrome: A systematic review. Front. Neurol. 2020, 11, 531993. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Zhang, Y.; Wang, L.; Peng, K.; Han, L.; Nie, K.; Yang, H.; Zhang, L.; Wang, J. Diffusion tensor imaging reveals white matter changes associated with cognitive status in patients with Parkinson’s disease. Am. J. Alzheimer’s Dis. Other Dement. 2013, 28, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Sang, T.; He, J.; Wang, J.; Zhang, C.; Zhou, W.; Zeng, Q.; Yuan, Y.; Yu, L.; Feng, Y. Alterations in white matter fiber in Parkinson disease across different cognitive stages. Neurosci. Lett. 2022, 769, 136424. [Google Scholar] [CrossRef] [PubMed]
- Ay, U.; Yildirim, Z.; Erdogdu, E.; Kiçik, A.; Ozturk-Isik, E.; Demiralp, T.; Gurvit, H. Shrinkage of olfactory amygdala connotes cognitive impairment in patients with Parkinson’s disease. Cogn. Neurodyn. 2023, 17, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Park, Y.H.; Yoo, H.S.; Lee, Y.H.; Ye, B.S.; Sohn, Y.H.; Lee, J.M.; Lee, P.H. Mild cognitive impairment reverters have a favorable cognitive prognosis and cortical integrity in Parkinson’s disease. Neurobiol. Aging 2019, 78, 168–177. [Google Scholar] [CrossRef]
- Zhou, C.; Guan, X.J.; Guo, T.; Zeng, Q.L.; Gao, T.; Huang, P.Y.; Xuan, M.; Gu, Q.Q.; Xu, X.J.; Zhang, M.M. Progressive brain atrophy in Parkinson’s disease patients who convert to mild cognitive impairment. CNS Neurosci. Ther. 2020, 26, 117–125. [Google Scholar] [CrossRef]
- Gasca-Salas, C.; García-Lorenzo, D.; Garcia-Garcia, D.; Clavero, P.; Obeso, J.A.; Lehericy, S.; Rodríguez-Oroz, M.C. Parkinson’s disease with mild cognitive impairment: Severe cortical thinning antedates dementia. Brain Imaging Behav. 2019, 13, 180–188. [Google Scholar] [CrossRef]
- Sunwoo, M.K.; Jeon, S.; Ham, J.H.; Hong, J.Y.; Lee, J.E.; Lee, J.M.; Sohn, Y.H.; Lee, P.H. The burden of white matter hyperintensities is a predictor of progressive mild cognitive impairment in patients with Parkinson’s disease. Eur. J. Neurol. 2014, 21, 922-e50. [Google Scholar] [CrossRef]
- Xia, J.; Miu, J.; Ding, H.; Wang, X.; Chen, H.; Wang, J.; Wu, J.; Zhao, J.; Huang, H.; Tian, W. Changes of brain gray matter structure in Parkinson’s disease patients with dementia. Neural Regen. Res. 2013, 8, 1276–1285. [Google Scholar] [PubMed]
- Xu, Y.; Yang, J.; Hu, X.; Shang, H. Voxel-based meta-analysis of gray matter volume reductions associated with cognitive impairment in Parkinson’s disease. J. Neurol. 2016, 263, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Borroni, B.; Premi, E.; Formenti, A.; Turrone, R.; Alberici, A.; Cottini, E.; Rizzetti, C.; Gasparotti, R.; Padovani, A. Structural and functional imaging study in dementia with Lewy bodies and Parkinson’s disease dementia. Park. Relat. Disord. 2015, 21, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.B.; Hall, S.; Jalakas, M.; Grothe, M.J.; Strandberg, O.; Stomrud, E.; Westman, E.; van Westen, D.; Hansson, O. Longitudinal degeneration of the basal forebrain predicts subsequent dementia in Parkinson’s disease. Neurobiol. Dis. 2020, 139, 104831. [Google Scholar] [CrossRef] [PubMed]
- Ray, N.J.; Bradburn, S.; Murgatroyd, C.; Toseeb, U.; Mir, P.; Kountouriotis, G.K.; Teipel, S.J.; Grothe, M.J. In vivo cholinergic basal forebrain atrophy predicts cognitive decline in de novo Parkinson’s disease. Brain 2018, 141, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Calamuneri, A.; Milardi, D.; Mormina, E.; Gaeta, M.; Corallo, F.; Lo Buono, V.; Chillemi, G.; Marino, S.; Cacciola, A.; et al. Claustral structural connectivity and cognitive impairment in drug naïve Parkinson’s disease. Brain Imaging Behav. 2019, 13, 933–944. [Google Scholar] [CrossRef]
- Nikolenko, V.N.; Rizaeva, N.A.; Beeraka, N.M.; Oganesyan, M.V.; Kudryashova, V.A.; Dubovets, A.A.; Borminskaya, I.D.; Bulygin, K.V.; Sinelnikov, M.Y.; Aliev, G. The mystery of claustral neural circuits and recent updates on its role in neurodegenerative pathology. Behav. Brain Funct. 2021, 17, 8. [Google Scholar] [CrossRef]
- Pan, P.L.; Shi, H.C.; Zhong, J.G.; Xiao, P.R.; Shen, Y.; Wu, L.J.; Song, Y.Y.; He, G.X.; Li, H.L. Gray matter atrophy in Parkinson’s disease with dementia: Evidence from meta-analysis of voxel-based morphometry studies. Neurol. Sci. 2013, 34, 613–619. [Google Scholar] [CrossRef]
- Hünerli-Gündüz, D.; Özbek Isbitiren, Y.; Uzunlar, H.; Çavusoglu, B.; Çolakoglu, B.D.; Ada, E.; Güntekin, B.; Yener, G.G. Reduced power and phase-locking values were accompanied by thalamus, putamen, and hippocampus atrophy in Parkinson’s disease with mild cognitive impairment: An event-related oscillation study. Neurobiol. Aging 2023, 121, 88–106. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Cho, K.H.; Lee, J.J.; Ham, J.H.; Ye, B.S.; Lee, S.K.; Lee, J.M.; Sohn, Y.H.; Lee, P.H. Cognitive and neuroanatomical correlates in early versus late onset Parkinson’s disease dementia. J. Alzheimer’s Dis. 2017, 55, 485–495. [Google Scholar] [CrossRef]
- Carlesimo, G.A.; Piras, F.; Assogna, F.; Pontieri, F.E.; Caltagirone, C.; Spalletta, G. Hippocampal abnormalities and memory deficits in Parkinson disease: A multimodal imaging study. Neurology 2012, 78, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, F.; Gallea, C.; Mongin, M.; Pyatigorskaya, N.; Valabregue, R.; Ewenczyk, C.; Sarazin, M.; Yahia-Cherif, L.; Vidailhet, M.; Lehéricy, S. Multimodal magnetic resonance imaging investigation of basal forebrain damage and cognitive deficits in Parkinson’s disease. Mov. Disord. 2019, 34, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Ibarretxe-Bilbao, N.; Compta, Y.; Hough, M.; Junque, C.; Bargallo, N.; Tolosa, E.; Martí, M.J. Cortical thinning is associated with disease stages and dementia in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2013, 84, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Guttuso, T., Jr.; Sirica, D.; Tosun, D.; Zivadinov, R.; Pasternak, O.; Weintraub, D.; Baglio, F.; Bergsland, N. Thalamic dorsomedial nucleus free water correlates with cognitive decline in Parkinson’s disease. Mov. Disord. 2022, 37, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Inguanzo, A.; Sala-Llonch, R.; Segura, B.; Erostarbe, H.; Abos, A.; Campabadal, A.; Uribe, C.; Baggio, H.C.; Compta, Y.; Marti, M.J.; et al. Hierarchical cluster analysis of multimodal imaging data identifies brain atrophy and cognitive patterns in Parkinson’s disease. Park. Relat. Disord. 2021, 82, 16–23. [Google Scholar] [CrossRef]
- Bayot, M.; Dujardin, K.; Gérard, M.; Braquet, A.; Tard, C.; Betrouni, N.; Defebvre, L.; Delval, A. The contribution of executive control dysfunction to freezing of gait in Parkinson’s disease. Clin. Neurophysiol. 2023, 152, 75–89. [Google Scholar] [CrossRef]
- Alenikova, O.A.; Dymkovskaya, M.N. Features of visual, cognitive and neuroimaging changes in Parkinson’s disease patients with freezing of gait. Zhurnal Nevrol. Psikhiatrii Im. SS Korsakova 2023, 123, 59–66. [Google Scholar] [CrossRef]
- Bao, Y.; Ya, Y.; Liu, J.; Zhang, C.; Wang, E.; Fan, G. Regional homogeneity and functional connectivity of freezing of gait conversion in Parkinson’s disease. Front. Aging Neurosci. 2023, 15, 1179752. [Google Scholar] [CrossRef]
- Beyer, M.K.; Aarsland, D.; Greve, O.J.; Larsen, J.P. Visual rating of white matter hyperintensities in Parkinson’s disease. Mov. Disord. 2006, 21, 223–229. [Google Scholar] [CrossRef]
- Bledsoe, I.O.; Stebbins, G.T.; Merkitch, D.; Goldman, J.G. White matter abnormalities in the corpus callosum with cognitive impairment in Parkinson disease. Neurology 2018, 91, e2244–e2255. [Google Scholar] [CrossRef]
- Kamagata, K.; Motoi, Y.; Tomiyama, H.; Abe, O.; Ito, K.; Shimoji, K.; Suzuki, M.; Hori, M.; Nakanishi, A.; Sano, T.; et al. Relationship between cognitive impairment and white-matter alteration in Parkinson’s disease with dementia: Tract-based spatial statistics and tract-specific analysis. Eur. Radiol. 2013, 23, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Nishinaka, K.; Oda, M.; Niikawa, H.; Kubori, T.; Udaka, F. Dementia in Parkinson’s disease: Diffusion tensor imaging. Acta Neurol. Scand. 2007, 116, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Chondrogiorgi, M.; Astrakas, L.G.; Zikou, A.K.; Weis, L.; Xydis, V.G.; Antonini, A.; Argyropoulou, M.I.; Konitsiotis, S. Multifocal alterations of white matter accompany the transition from normal cognition to dementia in Parkinson’s disease patients. Brain Imaging Behav. 2019, 13, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Fan, G.G.; Liu, H.; Wang, S. Changes in anatomical and functional connectivity of Parkinson’s disease patients according to cognitive status. Eur. J. Radiol. 2015, 84, 1318–1324. [Google Scholar] [CrossRef]
- Colon-Perez, L.M.; Tanner, J.J.; Couret, M.; Goicochea, S.; Mareci, T.H.; Price, C.C. Cognition and connectomes in nondementia idiopathic Parkinson’s disease. Netw Neurosci 2018, 2, 106–124. [Google Scholar] [CrossRef]
- Chu, C.; Zhang, Z.; Wang, J.; Wang, L.; Shen, X.; Bai, L.; Li, Z.; Dong, M.; Liu, C.; Yi, G.; et al. Evolution of brain network dynamics in early Parkinson’s disease with mild cognitive impairment. Cogn. Neurodyn. 2023, 17, 681–694. [Google Scholar] [CrossRef]
- Georgiades, M.J.; Shine, J.M.; Gilat, M.; McMaster, J.; Owler, B.; Mahant, N.; Lewis, S.J.G. Subthalamic nucleus activity during cognitive load and gait dysfunction in Parkinson’s disease. Mov. Disord. 2023, 38, 1549–1554. [Google Scholar] [CrossRef]
- Bressler, S.L.; Menon, V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cogn. Sci. 2010, 14, 277–290. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef]
- Putcha, D.; Ross, R.S.; Cronin-Golomb, A.; Janes, A.C.; Stern, C.E. Salience and default mode network coupling predicts cognition in aging and Parkinson’s disease. J. Int. Neuropsychol. Soc. 2016, 22, 205–215. [Google Scholar] [CrossRef]
- Chand, G.B.; Wu, J.; Hajjar, I.; Qiu, D. Interactions of the salience network and its subsystems with the default-mode and the central-executive networks in normal aging and mild cognitive impairment. Brain Connect. 2017, 7, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Yeager, B.E.; Twedt, H.P.; Bruss, J.; Schultz, J.; Narayanan, N.S. Salience network and cognitive impairment in Parkinson’s disease. medRxiv 2023. [Google Scholar] [CrossRef]
- Obeso, J.A.; Rodríguez-Oroz, M.C.; Rodríguez, M.; Lanciego, J.L.; Artieda, J.; Gonzalo, N.; Olanow, C.W. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci. 2000, 23, S8–S19. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, G.; Zeighami, Y.; Clark, C.A.; Coull, J.T.; Nagano-Saito, A.; Leyton, M.; Dagher, A.; Mišic, B. Dopamine signaling modulates the stability and integration of intrinsic brain networks. Cereb. Cortex 2019, 29, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Shima, A.; Inano, R.; Tabu, H.; Okada, T.; Nakamoto, Y.; Takahashi, R.; Sawamoto, N. Altered functional connectivity associated with striatal dopamine depletion in Parkinson’s disease. Cereb. Cortex Commun. 2023, 4, tgad004. [Google Scholar] [CrossRef] [PubMed]
- Badea, L.; Onu, M.; Wu, T.; Roceanu, A.; Bajenaru, O. Exploring the reproducibility of functional connectivity alterations in Parkinson’s disease. PLoS ONE 2017, 12, e0188196. [Google Scholar] [CrossRef]
- Ay, U.; Gürvit, I.H. Alterations in large-scale intrinsic connectivity networks in the Parkinson’s disease-associated cognitive impairment continuum: A systematic review. Arch. Neuropsychiatry 2022, 59, S57–S66. [Google Scholar] [CrossRef]
- Rucco, R.; Lardone, A.; Liparoti, M.; Lopez, E.T.; De Micco, R.; Tessitore, A.; Granata, C.; Mandolesi, L.; Sorrentino, G.; Sorrentino, P. Brain networks and cognitive impairment in Parkinson’s disease. Brain Connect. 2022, 12, 465–475. [Google Scholar] [CrossRef]
- Lopes, R.; Delmaire, C.; Defebvre, L.; Moonen, A.J.; Duits, A.A.; Hofman, P.; Leentjens, A.F.; Dujardin, K. Cognitive phenotypes in Parkinson’s disease differ in terms of brain-network organization and connectivity. Hum. Brain Mapp. 2017, 38, 1604–1621. [Google Scholar] [CrossRef]
- Fathy, Y.Y.; Hepp, D.H.; de Jong, F.J.; Geurts, J.J.G.; Foncke, E.M.J.; Berendse, H.W.; van de Berg, W.D.J.; Schoonheim, M.M. Anterior insular network disconnection and cognitive impairment in Parkinson’s disease. Neuroimage Clin. 2020, 28, 102364. [Google Scholar] [CrossRef]
- Jonkman, L.E.; Fathy, Y.Y.; Berendse, H.W.; Schoonheim, M.M.; van de Berg, W.D.J. Structural network topology and microstructural alterations of the anterior insula associate with cognitive and affective impairment in Parkinson’s disease. Sci. Rep. 2021, 11, 16021. [Google Scholar] [CrossRef] [PubMed]
- Zarifkar, P.; Kim, J.; La, C.; Zhang, K.; YorkWilliams, S.; Levine, T.F.; Tian, L.; Borghammer, P.; Poston, K.L. Cognitive impairment in Parkinson’s disease is associated with Default Mode Network subsystem connectivity and cerebrospinal fluid Aß. Park. Relat. Disord. 2021, 83, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Seibert, T.M.; Murphy, E.A.; Kaestner, E.J.; Brewer, J.B. Interregional correlations in Parkinson disease and Parkinson-related dementia with resting functional MR imaging. Radiology 2012, 263, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Rektorova, I.; Krajcovicova, L.; Marecek, R.; Mikl, M. Default mode network and extrastriate visual resting state network in patients with Parkinson’s disease dementia. Neurodegener. Dis. 2012, 10, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Fiorenzato, E.; Strafella, A.P.; Kim, J.; Schifano, R.; Weis, L.; Antonini, A.; Biundo, R. Dynamic functional connectivity changes associated with dementia in Parkinson’s disease. Brain 2019, 142, 2860–2872. [Google Scholar] [CrossRef] [PubMed]
- Boon, L.I.; Hepp, D.H.; Douw, L.; van Geenen, N.; Broeders, T.A.A.; Geurts, J.J.G.; Berendse, H.W.; Schoonheim, M.M. Functional connectivity between resting-state networks reflects decline in executive function in Parkinson’s disease: A longitudinal fMRI study. Neuroimage Clin. 2020, 28, 102468. [Google Scholar] [CrossRef]
- Anderkova, L.; Barton, M.; Rektorova, I. Striato-cortical connections in Parkinson’s and Alzheimer’s diseases: Relation to cognition. Mov. Disord. 2017, 32, 917–922. [Google Scholar] [CrossRef]
- Krimmel, S.R.; White, M.G.; Panicker, M.H.; Barrett, F.S.; Mathur, B.N.; Seminowicz, D.A. Resting state functional connectivity and cognitive task-related activation of the human claustrum. Neuroimage 2019, 196, 59–67. [Google Scholar] [CrossRef]
- Kalaitzakis, M.E.; Pearce, R.K.; Gentleman, S.M. Clinical correlates of pathology in the claustrum in Parkinson’s disease and dementia with Lewy bodies. Neurosci. Lett. 2009, 461, 12–15. [Google Scholar] [CrossRef]
- Jankowski, M.M.; O’Mara, S.M. Dynamics of place, boundary and object encoding in rat anterior claustrum. Front. Behav. Neurosci. 2015, 9, 250. [Google Scholar] [CrossRef]
- Ayyildiz, S.; Velioglu, H.A.; Ayyildiz, B.; Sutcubasi, B.; Hanoglu, L.; Bayraktaroglu, Z.; Yildirim, S.; Atasever, A.; Yulug, B. Differentiation of claustrum resting-state functional connectivity in healthy aging, Alzheimer’s disease, and Parkinson’s disease. Hum. Brain Mapp. 2023, 44, 1741–1750. [Google Scholar] [CrossRef]
- Huang, C.; Mattis, P.; Perrine, K.; Brown, N.; Dhawan, V.; Eidelberg, D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology 2008, 70, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Putcha, D.; Ross, R.S.; Cronin-Golomb, A.; Janes, A.C.; Stern, C.E. Altered intrinsic functional coupling between core neurocognitive networks in Parkinson’s disease. Neuroimage Clin. 2015, 7, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Almdahl, I.S.; Martinussen, L.J.; Ousdal, O.T.; Kraus, M.; Sowa, P.; Agartz, I.; Korsnes, M.S. Task-based functional connectivity reveals aberrance with the salience network during emotional interference in late-life depression. Aging Ment. Health 2023, 27, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Cermakova, P.; Chlapecka, A.; Csajbók, Z.; Andrýsková, L.; Brázdil, M.; Marecková, K. Parental education, cognition and functional connectivity of the salience network. Sci. Rep. 2023, 13, 2761. [Google Scholar] [CrossRef]
- Marek, S.; Dosenbach, N.U.F. The frontoparietal network: Function, electrophysiology, and importance of individual precision mapping. Dialogues Clin. Neurosci. 2018, 20, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Chaton, L.; Benquet, P.; Delval, A.; Leroy, C.; Plomhause, L.; Moonen, A.J.; Duits, A.A.; Leentjens, A.F.; van Kranen-Mastenbroek, V.; et al. Functional connectivity disruptions correlate with cognitive phenotypes in Parkinson’s disease. Neuroimage Clin. 2017, 14, 591–601. [Google Scholar] [CrossRef]
- Yener, G.G.; Fide, E.; Özbek, Y.; Emek-Savas, D.D.; Aktürk, T.; Çakmur, R.; Güntekin, B. The difference of mild cognitive impairment in Parkinson’s disease from amnestic mild cognitive impairment: Deeper power decrement and no phase-locking in visual event-related responses. Int. J. Psychophysiol. 2019, 139, 48–58. [Google Scholar] [CrossRef]
- Yi, G.; Wang, Y.; Wang, L.; Chu, C.; Wang, J.; Shen, X.; Han, X.; Li, Z.; Bai, L.; Zhang, R.; et al. Capturing the abnormal brain network activity in early Parkinsons disease with mild cognitive impairment based on dynamic functional connectivity. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1238–1247. [Google Scholar] [CrossRef]
- Trenado, C.; Trauberg, P.; Elben, S.; Dimenshteyn, K.; Folkerts, A.K.; Witt, K.; Weiss, D.; Liepelt-Scarfone, I.; Kalbe, E.; Wojtecki, L. Resting state EEG as biomarker of cognitive training and physical activity’s joint effect in Parkinson’s patients with mild cognitive impairment. Neurol. Res. Pract 2023, 5, 46. [Google Scholar] [CrossRef]
- Bar-On, M.; Baharav, S.; Katzir, Z.; Mirelman, A.; Sosnik, R.; Maidan, I. Task-related reorganization of cognitive network in Parkinson’s disease using electrophysiology. Mov. Disord. 2023, 38, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Dang, G.; Su, X.; Zhu, L.; Shi, X.; Che, S.; Lan, X.; Luo, X.; Guo, Y. Identifying mild cognitive impairment in Parkinson’s disease with electroencephalogram functional connectivity. Front. Aging Neurosci. 2021, 13, 701499. [Google Scholar] [CrossRef]
- Paulo, D.L.; Qian, H.; Subramanian, D.; Johnson, G.W.; Zhao, Z.; Hett, K.; Kang, H.; Chris Kao, C.; Roy, N.; Summers, J.E.; et al. Corticostriatal beta oscillation changes associated with cognitive function in Parkinson’s disease. Brain 2023, 146, 3662–3675. [Google Scholar] [CrossRef] [PubMed]
- Bayraktaroglu, Z.; Aktürk, T.; Yener, G.; de Graaf, T.A.; Hanoglu, L.; Yildirim, E.; Hünerli Gündüz, D.; Kiyi, I.; Sack, A.T.; Babiloni, C.; et al. Abnormal cross frequency coupling of brain electroencephalographic oscillations related to visual oddball task in Parkinson’s disease with mild cognitive impairment. Clin. EEG Neurosci. 2023, 54, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Hnazaee, M.F.; Litvak, V. Investigating cortico-striatal beta oscillations in Parkinson’s disease cognitive decline. Brain 2023, 146, 3571–3573. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.S.; Cavanagh, J.F.; Frank, M.J.; Laubach, M. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat. Neurosci. 2013, 16, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Richardson, S.P.; Narayanan, N.; Cavanagh, J.F. Mid-frontal theta activity is diminished during cognitive control in Parkinson’s disease. Neuropsychologia 2018, 117, 113–122. [Google Scholar] [CrossRef]
- Uc, E.; Anjum, F.; Dasgupta, S.; Narayanan, N. Resting-state EEG predicts cognitive impairment in Parkinson’s disease (Abstr-P6-11.015). Neurology 2023, 100 (Suppl. 2),, 4018. [Google Scholar] [CrossRef]
- Wiesman, A.I.; Donhauser, P.W.; Degroot, C.; Diab, S.; Kousaie, S.; Fon, E.A.; Klein, D.; Baillet, S. Aberrant neurophysiological signaling associated with speech impairments in Parkinson’s disease. NPJ Park. Dis. 2023, 9, 61. [Google Scholar] [CrossRef]
- Singh, A.; Cole, R.C.; Espinoza, A.I.; Wessel, J.R.; Cavanagh, J.F.; Narayanan, N.S. Evoked mid-frontal activity predicts cognitive dysfunction in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2023, 94, 945–953. [Google Scholar] [CrossRef]
- Anjum, M.F.; Espinoza, A.; Cole, R.; Singh, A.; May, P.; Uc, E.; Dasgupta, S.; Narayanan, N. Resting-state EEG measures cognitive impairment in Parkinson’s disease. Res. Sq. 2023. preprint. [Google Scholar] [CrossRef]
- Chang, H.; Liu, B.; Zong, Y.; Lu, C.; Wang, X. EEG-based Parkinson’s disease recognition via attention-based sparse graph convolutional neural network. IEEE J. Biomed. Health Inform. 2023, 27, 5216–5224. [Google Scholar] [CrossRef] [PubMed]
- Zawislak-Fornagiel, K.; Ledwon, D.; Bugdol, M.; Romaniszyn-Kania, P.; Malecki, A.; Gorzkowska, A.; Mitas, A.W. Specific patterns of coherence and phase lag index in particular regions as biomarkers of cognitive impairment in Parkinson’s disease. Park. Relat. Disord. 2023, 111, 105436. [Google Scholar] [CrossRef] [PubMed]
- Hünerli, D.; Emek-Savas, D.D.; Çavusoglu, B.; Dönmez Çolakoglu, B.; Ada, E.; Yener, G.G. Mild cognitive impairment in Parkinson’s disease is associated with decreased P300 amplitude and reduced putamen volume. Clin. Neurophysiol. 2019, 130, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Folmer, R.L.; Vachhani, J.J.; Riggins, A. Electrophysiological evidence of auditory and cognitive processing deficits in Parkinson disease. Biomed. Res. Int. 2021, 2021, 6610908. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gu, L.; Zhang, S.; Wu, Y.; Wei, X.; Wang, C.; Xu, Y.; Guo, Y. N200 and P300 component changes in Parkinson’s disease: A meta-analysis. Neurol. Sci. 2022, 43, 6719–6730. [Google Scholar] [CrossRef]
- Simon, O.B.; Rojas, D.C.; Ghosh, D.; Yang, X.; Rogers, S.E.; Martin, C.S.; Holden, S.K.; Kluger, B.M.; Buard, I. Profiling Parkinson’s disease cognitive phenotypes via resting-state magnetoencephalography. J. Neurophysiol. 2022, 127, 279–289. [Google Scholar] [CrossRef]
- Ye, Z. Mapping neuromodulatory systems in Parkinson’s disease: Lessons learned beyond dopamine. Curr. Med. 2022, 1, 15. [Google Scholar] [CrossRef]
- Siepel, F.J.; Bronnick, K.S.; Booij, J.; Ravina, B.M.; Lebedev, A.V.; Pereira, J.B.; Gruner, R.; Aarsland, D. Cognitive executive impairment and dopaminergic deficits in de novo Parkinson’s disease. Mov. Disord. 2014, 29, 1802–1808. [Google Scholar] [CrossRef]
- Christopher, L.; Duff-Canning, S.; Koshimori, Y.; Segura, B.; Boileau, I.; Chen, R.; Lang, A.E.; Houle, S.; Rusjan, P.; Strafella, A.P. Salience network and parahippocampal dopamine dysfunction in memory-impaired Parkinson disease. Ann. Neurol. 2015, 77, 269–280. [Google Scholar] [CrossRef]
- Jeong, S.H.; Lee, H.S.; Jung, J.H.; Baik, K.; Sohn, Y.H.; Chung, S.J.; Lee, P.H. Associations between white matter hyperintensities, striatal dopamine loss, and cognition in drug-naive Parkinson’s disease. Park. Relat. Disord. 2022, 97, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pellecchia, M.T.; Picillo, M.; Santangelo, G.; Longo, K.; Moccia, M.; Erro, R.; Amboni, M.; Vitale, C.; Vicidomini, C.; Salvatore, M.; et al. Cognitive performances and DAT imaging in early Parkinson’s disease with mild cognitive impairment: A preliminary study. Acta Neurol. Scand. 2015, 131, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Ekman, U.; Eriksson, J.; Forsgren, L.; Mo, S.J.; Riklund, K.; Nyberg, L. Functional brain activity and presynaptic dopamine uptake in patients with Parkinson’s disease and mild cognitive impairment: A cross-sectional study. Lancet Neurol. 2012, 11, 679–687. [Google Scholar] [CrossRef]
- Chung, S.J.; Lee, H.S.; Yoo, H.S.; Lee, Y.H.; Lee, P.H.; Sohn, Y.H. Patterns of striatal dopamine depletion in early Parkinson disease: Prognostic relevance. Neurology 2020, 95, e280–e290. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Yoo, H.S.; Oh, J.S.; Kim, J.S.; Ye, B.S.; Sohn, Y.H.; Lee, P.H. Effect of striatal dopamine depletion on cognition in de novo Parkinson’s disease. Park. Relat. Disord. 2018, 51, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q. Salience Network of the Human Brain; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Sasikumar, S.; Strafella, A.P. Imaging mild cognitive impairment and dementia in Parkinson’s disease. Front. Neurol. 2020, 11, 47. [Google Scholar] [CrossRef]
- Martínez-Horta, S.; Kulisevsky, J. Is all cognitive impairment in Parkinson’s disease “mild cognitive impairment”? J. Neural Transm. 2011, 118, 1185–1190. [Google Scholar] [CrossRef]
- Weintraub, D.; Picillo, M.; Cho, H.R.; Caspell-Garcia, C.; Blauwendraat, C.; Brown, E.G.; Chahine, L.M.; Coffey, C.S.; Dobkin, R.D.; Foroud, T.; et al. Impact of the dopamine system on long-term cognitive impairment in Parkinson disease: An exploratory study. Mov. Disord. Clin. Pract. 2023, 10, 943–955. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Xu, H.; Zhou, L.; Jiao, R.; Liu, W.; Zhu, F.; Kang, X.; Liu, B.; Teng, S.; et al. Temporal components of cholinergic terminal to dopaminergic terminal transmission in dorsal striatum slices of mice. J. Physiol. 2014, 592, 3559–3576. [Google Scholar] [CrossRef]
- Threlfell, S.; Lalic, T.; Platt, N.J.; Jennings, K.A.; Deisseroth, K.; Cragg, S.J. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 2012, 75, 58–64. [Google Scholar] [CrossRef]
- Grothe, M.J.; Labrador-Espinosa, M.A.; Jesús, S.; Macías-García, D.; Adarmes-Gómez, A.; Carrillo, F.; Camacho, E.I.; Franco-Rosado, P.; Lora, F.R.; Martín-Rodríguez, J.F.; et al. In vivo cholinergic basal forebrain degeneration and cognition in Parkinson’s disease: Imaging results from the COPPADIS study. Park. Relat. Disord. 2021, 88, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.; Kanel, P.; Dyrba, M.; Storch, A.; Bohnen, N.I.; Teipel, S.; Grothe, M.J. Structural and molecular cholinergic imaging markers of cognitive decline in Parkinson’s disease. Brain 2023, 146, 4964–4973. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.K.L.; Lim, E.J.; Ahmed, I.; Chang, R.C.; Pearce, R.K.B.; Gentleman, S.M. Review: Revisiting the human cholinergic nucleus of the diagonal band of Broca. Neuropathol. Appl. Neurobiol. 2018, 44, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.; Pagano, G.; Fernández Bonfante, J.A.; Wilson, H.; Politis, M. Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson’s disease. Brain 2018, 141, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Li, Y.; Li, B.; Nie, K.; Zhang, P.; Cai, T.; Mei, M.; Wang, L.; Zhang, Y. Meynert nucleus-related cortical thinning in Parkinson’s disease with mild cognitive impairment. Quant. Imaging Med. Surg. 2021, 11, 1554–1566. [Google Scholar] [CrossRef] [PubMed]
- Rogozinski, S.; Klietz, M.; Respondek, G.; Oertel, W.H.; Grothe, M.J.; Pereira, J.B.; Höglinger, G.U. Reduction in volume of nucleus basalis of Meynert is specific to Parkinson’s disease and progressive supranuclear palsy but not to multiple system atrophy. Front. Aging Neurosci. 2022, 14, 851788. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.; de Natale, E.R.; Politis, M. Nucleus basalis of Meynert degeneration predicts cognitive impairment in Parkinson’s disease. Handb. Clin. Neurol. 2021, 179, 189–205. [Google Scholar]
- Ray, N.J.; Lawson, R.A.; Martin, S.L.; Sigurdsson, H.P.; Wilson, J.; Galna, B.; Lord, S.; Alcock, L.; Duncan, G.W.; Khoo, T.K.; et al. Free-water imaging of the cholinergic basal forebrain and pedunculopontine nucleus in Parkinson’s disease. Brain 2023, 146, 1053–1064. [Google Scholar] [CrossRef]
- Zhang, P.; Rong, S.; He, C.; Li, Y.; Li, X.; Chen, Z.; Nie, K.; Wang, L.; Zhang, Y. Cortical connectivity of cholinergic basal forebrain in Parkinson’s disease with mild cognitive impairment. Quant. Imaging Med. Surg. 2023, 13, 2167–2182. [Google Scholar] [CrossRef]
- Crockett, R.A.; Wilkins, K.B.; Aditham, S.; Brontë-Stewart, H.M. No laughing white matter: Reduced integrity of the cortical cholinergic pathways in Parkinson’s disease-related cognitive impairment. Neurobiol. Dis. 2023, 185, 106243. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Albin, R.L.; Muller, M.L.; Petrou, M.; Kotagal, V.; Koeppe, R.A.; Scott, P.J.; Frey, K.A. Frequency of cholinergic and caudate nucleus dopaminergic deficits across the predemented cognitive spectrum of Parkinson disease and evidence of interaction effects. JAMA Neurol. 2015, 72, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Park, H.E.; Park, I.S.; Oh, Y.S.; Yang, D.W.; Lee, K.S.; Choi, H.S.; Ahn, K.J.; Kim, J.S. Subcortical whiter matter hyperintensities within the cholinergic pathways of patients with dementia and parkinsonism. J. Neurol. Sci. 2015, 353, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Shinotoh, H.; Eidelberg, D. Functional brain imaging of cognitive dysfunction in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2012, 83, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.; Reyes, S.; Landeck, N.; Bye, C.; Leanza, G.; Double, K.; Thompson, L.; Halliday, G.; Kirik, D. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain 2014, 137, 2493–2508. [Google Scholar] [CrossRef] [PubMed]
- Prasuhn, J.; Prasuhn, M.; Fellbrich, A.; Strautz, R.; Lemmer, F.; Dreischmeier, S.; Kasten, M.; Münte, T.F.; Hanssen, H.; Heldmann, M.; et al. Association of locus coeruleus and substantia nigra pathology with cognitive and motor functions in patients with Parkinson disease. Neurology 2021, 97, e1007–e1016. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Wang, J.; Zhou, Y.; Ye, F.; Zhang, Y.; Cheng, X.; Huang, Z.; Liu, K.; Fei, G.; et al. Mild cognitive impairment in de novo Parkinson’s disease: A neuromelanin MRI study in locus coeruleus. Mov. Disord. 2019, 34, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Robbins, T.W.; Roberts, A.C. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb. Cortex 2007, 17 (Suppl. 1), i151–i160. [Google Scholar] [CrossRef]
- Lancini, E.; Haag, L.; Bartl, F.; Rühling, M.; Ashton, N.J.; Zetterberg, H.; Düzel, E.; Hämmerer, D.; Betts, M.J. Cerebrospinal fluid and positron-emission tomography biomarkers for noradrenergic dysfunction in neurodegenerative diseases: A systematic review and meta-analysis. Brain Commun. 2023, 5, fcad085. [Google Scholar] [CrossRef]
- Sitte, H.H.; Pifl, C.; Rajput, A.H.; Hörtnagl, H.; Tong, J.; Lloyd, G.K.; Kish, S.J.; Hornykiewicz, O. Dopamine and noradrenaline, but not serotonin, in the human claustrum are greatly reduced in patients with Parkinson’s disease: Possible functional implications. Eur. J. Neurosci. 2017, 45, 192–197. [Google Scholar] [CrossRef]
- Del Tredici, K.; Braak, H. Dysfunction of the locus coeruleus-norepinephrine system and related circuitry in Parkinson’s disease-related dementia. J. Neurol. Neurosurg. Psychiatry 2013, 84, 774–783. [Google Scholar] [CrossRef]
- Halliday, G.M.; Leverenz, J.B.; Schneider, J.S.; Adler, C.H. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov. Disord. 2014, 29, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, Y.; De Deyn, P.P. Targeting the norepinephrinergic system in Parkinson’s disease and related disorders: The locus coeruleus story. Neurochem Int. 2017, 102, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Szot, P.; Franklin, A.; Sikkema, C.; Wilkinson, C.W.; Raskind, M.A. Sequential loss of LC noradrenergic and dopaminergic neurons results in a correlation of dopaminergic neuronal number to striatal dopamine concentration. Front. Pharmacol. 2012, 3, 184. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, D.; Clavero, P.; Gasca Salas, C.; Lamet, I.; Arbizu, J.; Gonzalez-Redondo, R.; Obeso, J.A.; Rodriguez-Oroz, M.C. Posterior parietooccipital hypometabolism may differentiate mild cognitive impairment from dementia in Parkinson’s disease. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Ge, J.; Liu, F.; Wu, P.; Guo, S.; Liu, Z.; Wang, Y.; Ding, Z.; Wu, J.; Zuo, C.; et al. Cerebral metabolic differences associated with cognitive impairment in Parkinson’s disease. PLoS ONE 2016, 11, e0152716. [Google Scholar] [CrossRef] [PubMed]
- Khomenko, I.G.; Pronina, M.V.; Kataeva, G.V.; Kropotov, J.D.; Irishina, Y.A.; Susin, D.S. Combined 18F-fluorodeoxyglucose positron emission tomography and event-related potentials study of the cognitive impairment mechanisms in Parkinson’s disease. J. Clin. Neurosci. 2020, 72, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Imarisio, A.; Pilotto, A.; Premi, E.; Caminiti, S.P.; Presotto, L.; Sala, A.; Zatti, C.; Lupini, A.; Turrone, R.; Paghera, B.; et al. Atypical brain FDG-PET patterns increase the risk of long-term cognitive and motor progression in Parkinson’s disease. Park. Relat. Disord. 2023, 115, 105848. [Google Scholar] [CrossRef]
- Gasca-Salas, C.; Trompeta, C.; López-Aguirre, M.; Rodríguez Rojas, R.; Clarimon, J.; Dols-Icardo, O.; El Bounasri, S.; Guida, P.; Mata-Marín, D.; Hernández-Fernández, F.; et al. Brain hypometabolism in non-demented microtubule-associated protein tau H1 carriers with Parkinson’s disease. J. Neuroimaging 2023, 33, 953–959. [Google Scholar] [CrossRef]
- Mayberg, H.S.; Starkstein, S.E.; Sadzot, B.; Preziosi, T.; Andrezejewski, P.L.; Dannals, R.F.; Wagner, H.N., Jr.; Robinson, R.G. Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson’s disease. Ann. Neurol. 1990, 28, 57–64. [Google Scholar] [CrossRef]
- Scholefield, M.; Church, S.J.; Taylor, G.; Knight, D.; Unwin, R.D.; Cooper, G.J.S. Multi-regional alterations in glucose and purine metabolic pathways in the Parkinson’s disease dementia brain. NPJ Park. Dis. 2023, 9, 66. [Google Scholar] [CrossRef]
- Huang, M.; Yu, H.; Cai, X.; Zhang, Y.; Pu, W.; Gao, B. A comparative study of posterior cingulate metabolism in patients with mild cognitive impairment due to Parkinson’s disease or Alzheimer’s disease. Sci. Rep. 2023, 13, 14241. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Frey, K.A.; Muller, M.L.; Petrou, M.; Kotagal, V.; Koeppe, R.A.; Scott, P.J.; Albin, R.L.; Bohnen, N.I. Striatal and cortical beta-amyloidopathy and cognition in Parkinson’s disease. Mov. Disord. 2016, 31, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Baik, K.; Kim, H.R.; Park, M.; Lee, Y.; Na, H.K.; Sohn, Y.H.; Seong, J.K.; Lee, P.H. Effect of amyloid on cognitive performance in Parkinson’s disease and dementia with lewy bodies. Mov. Disord. 2023, 38, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Petrou, M.; Dwamena, B.A.; Foerster, B.R.; MacEachern, M.P.; Bohnen, N.I.; Muller, M.L.; Albin, R.L.; Frey, K.A. Amyloid deposition in Parkinson’s disease and cognitive impairment: A systematic review. Mov. Disord. 2015, 30, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Katako, A.; Aljuaid, M.; Goertzen, A.L.; Borys, A.; Hobson, D.E.; Kim, S.M.; Lee, C.S. Distinct brain metabolic patterns separately associated with cognition, motor function, and aging in Parkinson’s disease dementia. Neurobiol. Aging 2017, 60, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.R.; Maass, A.; Pressman, P.; Stiver, J.; Schonhaut, D.R.; Baker, S.L.; Kramer, J.; Rabinovici, G.D.; Jagust, W.J. Associations between tau, beta-amyloid, and cognition in Parkinson disease. JAMA Neurol. 2018, 75, 227–235. [Google Scholar] [CrossRef]
- Ghadery, C.; Koshimori, Y.; Christopher, L.; Kim, J.; Rusjan, P.; Lang, A.E.; Houle, S.; Strafella, A.P. The interaction between neuroinflammation and beta-amyloid in cognitive decline in Parkinson’s disease. Mol. Neurobiol. 2020, 57, 492–501. [Google Scholar] [CrossRef]
- Oh, Y.S.; Yoo, S.W.; Lyoo, C.H.; Yoo, J.Y.; Yoon, H.; Ha, S.; Lee, K.S.; Kim, J.S. The association of beta-amyloid with cognition and striatal dopamine in early, non-demented Parkinson’s disease. J. Park. Dis. 2021, 11, 605–613. [Google Scholar]
- Na, S.; Jeong, H.; Park, J.S.; Chung, Y.A.; Song, I.U. The impact of amyloid-beta positivity with 18F-florbetaben PET on neuropsychological aspects in Parkinson’s disease dementia. Metabolites 2020, 10, 380. [Google Scholar] [CrossRef]
- Mashima, K.; Ito, D.; Kameyama, M.; Osada, T.; Tabuchi, H.; Nihei, Y.; Yoshizaki, T.; Noguchi, E.; Tanikawa, M.; Iizuka, T.; et al. Extremely low prevalence of amyloid positron emission tomography positivity in Parkinson’s disease without dementia. Eur. Neurol. 2017, 77, 231–237. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Ong, K.; Mulligan, R.S.; Holl, G.; Pejoska, S.; Jones, G.; O’Keefe, G.; Ackerman, U.; Tochon-Danguy, H.; Chan, J.G.; et al. Amyloid imaging with (18)F-florbetaben in Alzheimer disease and other dementias. J. Nucl. Med. 2011, 52, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, R.S.; Xie, S.X.; Brennan, L.; Pontecorvo, M.J.; Hurtig, H.I.; Trojanowski, J.Q.; Weintraub, D.; Siderowf, A.D. Amyloid-beta positron emission tomography imaging of Alzheimer’s pathology in Parkinson’s disease dementia. Mov. Disord. Clin. Pract. 2016, 3, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Frey, K.A.; Petrou, M. Imaging amyloidopathy in Parkinson disease and parkinsonian dementia syndromes. Clin. Transl. Imaging 2015, 3, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Tufekcioglu, Z.; Lange, J.; Pedersen, K.F.; Tysnes, O.-B.; Alves, G.; Emre, M. Cognitive profile in PD dementia patients with low versus normal CSF amyloid beta. Dement. Geriatr. Cogn. Disord. Extra 2023, 13, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Muller, M.; Frey, K.A. Molecular imaging and updated diagnostic criteria in Lewy body dementias. Curr. Neurol. Neurosci. Rep. 2017, 17, 73. [Google Scholar] [CrossRef]
- Tang, Y.; Li, L.; Hu, T.; Jiao, F.; Han, L.; Li, S.; Xu, Z.; Fan, Y.; Sun, Y.; Liu, F.; et al. In vivo (18) F-florzolotau tau positron emission tomography imaging in Parkinson’s disease dementia. Mov. Disord. 2023, 38, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.H.; Beach, T.G. Variability of diffuse plaques and amyloid angiopathy in Parkinson’s disease with mild cognitive impairment. Acta Neuropathol. 2010, 120, 831–832. [Google Scholar] [CrossRef]
- Jellinger, K.A. Mild cognitive impairment in Parkinson disease: Heterogenous mechanisms. J. Neural Transm. 2013, 120, 157–167. [Google Scholar] [CrossRef]
- Knox, M.G.; Adler, C.H.; Shill, H.A.; Driver-Dunckley, E.; Mehta, S.A.; Belden, C.; Zamrini, E.; Serrano, G.; Sabbagh, M.N.; Caviness, J.N.; et al. Neuropathological findings in Parkinson’s disease with mild cognitive impairment. Mov. Disord. 2020, 35, 845–850. [Google Scholar] [CrossRef]
- Markesbery, W.R. Neuropathologic alterations in mild cognitive impairment: A review. J. Alzheimer’s Dis. 2010, 19, 221–228. [Google Scholar] [CrossRef]
- Takao, M. Neuropathology of mild cognitive impairment. Rinsho Shinkeigaku 2012, 52, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; He, Z. Concomitant protein pathogenesis in Parkinson’s disease and perspective mechanisms. Front. Aging Neurosci. 2023, 15, 1189809. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, D.G.; Irwin, D.J. Neuropathological substrates of cognition in Parkinson’s disease. Prog. Brain Res. 2022, 269, 177–193. [Google Scholar] [PubMed]
- Irwin, D.J.; Lee, V.M.; Trojanowski, J.Q. Parkinson’s disease dementia: Convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Neurobiology of cognitive impairment in Parkinson’s disease. Expert Rev. Neurother. 2012, 12, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzakis, M.E.; Pearce, R.K. The morbid anatomy of dementia in Parkinson’s disease. Acta Neuropathol. 2009, 118, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.K.L.; Chau, T.W.; Lim, E.J.; Ahmed, I.; Chang, R.C.; Kalaitzakis, M.E.; Graeber, M.B.; Gentleman, S.M.; Pearce, R.K.B. Hippocampal CA2 Lewy pathology is associated with cholinergic degeneration in Parkinson’s disease with cognitive decline. Acta Neuropathol. Commun. 2019, 7, 61. [Google Scholar] [CrossRef]
- Martin, W.R.W.; Younce, J.R.; Campbell, M.C.; Racette, B.A.; Norris, S.A.; Ushe, M.; Criswell, S.; Davis, A.A.; Alfradique-Dunham, I.; Maiti, B.; et al. Neocortical Lewy body pathology parallels Parkinson’s dementia, but not always. Ann. Neurol. 2023, 93, 184–195. [Google Scholar] [CrossRef]
- Smith, C.; Malek, N.; Grosset, K.; Cullen, B.; Gentleman, S.; Grosset, D.G. Neuropathology of dementia in patients with Parkinson’s disease: A systematic review of autopsy studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1234–1243. [Google Scholar] [CrossRef]
- Daida, K.; Tanaka, R.; Yamashiro, K.; Ogawa, T.; Oyama, G.; Nishioka, K.; Shimo, Y.; Umemura, A.; Hattori, N. The presence of cerebral microbleeds is associated with cognitive impairment in Parkinson’s disease. J. Neurol. Sci. 2018, 393, 39–44. [Google Scholar] [CrossRef]
- Qin, Q.; Wan, H.; Wang, D.; Li, J.; Yang, Q.; Zhao, J.; Xue, Z. Effect of cerebral microbleeds on cognitive function and quality of life in Parkinson disease. Med. Sci. Monit. 2022, 28, e935026. [Google Scholar] [CrossRef] [PubMed]
- Compta, Y.; Parkkinen, L.; O’Sullivan, S.S.; Vandrovcova, J.; Holton, J.L.; Collins, C.; Lashley, T.; Kallis, C.; Williams, D.R.; de Silva, R.; et al. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: Which is more important? Brain 2011, 134, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.M.; Rinne, J.O.; Helenius, H.; Roytta, M. Neuritic degeneration in the hippocampus and amygdala in Parkinson’s disease in relation to Alzheimer pathology. Acta Neuropathol. 1999, 98, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, D.H.; Roy, S.; Salmon, D.P.; Galasko, D.R.; Hansen, L.A.; Masliah, E.; Gage, F.H. Hippocampal alpha-synuclein in dementia with Lewy bodies contributes to memory impairment and is consistent with spread of pathology. J. Neurosci. 2017, 37, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Dues, D.J.; Nguyen, A.P.T.; Becker, K.; Ma, J.; Moore, D.J. Hippocampal subfield vulnerability to a-synuclein pathology precedes neurodegeneration and cognitive dysfunction. NPJ Park. Dis. 2023, 29, 125. [Google Scholar] [CrossRef]
- Erhardt, E.; Horner, A.; Shaff, N.; Wertz, C.; Nitschke, S.; Vakhtin, A.; Mayer, A.; Adair, J.; Knoefel, J.; Rosenberg, G.; et al. Longitudinal hippocampal subfields, CSF biomarkers, and cognition in patients with Parkinson disease. Clin Park. Relat. Disord. 2023, 9, 100199. [Google Scholar] [CrossRef]
- Apaydin, H.; Ahlskog, J.E.; Parisi, J.E.; Boeve, B.F.; Dickson, D.W. Parkinson disease neuropathology: Later-developing dementia and loss of the levodopa response. Arch. Neurol. 2002, 59, 102–112. [Google Scholar] [CrossRef]
- Halliday, G.M.; Song, Y.J.; Harding, A.J. Striatal beta-amyloid in dementia with Lewy bodies but not Parkinson’s disease. J. Neural Transm. 2011, 118, 713–719. [Google Scholar] [CrossRef]
- Irwin, D.J.; White, M.T.; Toledo, J.B.; Xie, S.X.; Robinson, J.L.; Van Deerlin, V.; Lee, V.M.; Leverenz, J.B.; Montine, T.J.; Duda, J.E.; et al. Neuropathologic substrates of Parkinson disease dementia. Ann. Neurol. 2012, 72, 587–598. [Google Scholar] [CrossRef]
- Schneider, J.A.; Arvanitakis, Z.; Yu, L.; Boyle, P.A.; Leurgans, S.E.; Bennett, D.A. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain 2012, 135, 3005–3014. [Google Scholar] [CrossRef]
- Ryman, S.G.; Yutsis, M.; Tian, L.; Henderson, V.W.; Montine, T.J.; Salmon, D.P.; Galasko, D.; Poston, K.L. Cognition at each stage of Lewy body disease with co-occurring Alzheimer’s disease pathology. J. Alzheimer’s Dis. 2021, 80, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Colosimo, C.; Hughes, A.J.; Kilford, L.; Lees, A.J. Lewy body cortical involvement may not always predict dementia in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2003, 74, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Kempster, P.A.; O’Sullivan, S.S.; Holton, J.L.; Revesz, T.; Lees, A.J. Relationships between age and late progression of Parkinson’s disease: A clinico-pathological study. Brain 2010, 133, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Wills, J.; Jones, J.; Haggerty, T.; Duka, V.; Joyce, J.N.; Sidhu, A. Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson’s disease brains with and without dementia. Exp. Neurol. 2010, 225, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Horvath, J.; Herrmann, F.R.; Burkhard, P.R.; Bouras, C.; Kövari, E. Neuropathology of dementia in a large cohort of patients with Parkinson’s disease. Park. Relat. Disord. 2013, 19, 864–868, discussion 864. [Google Scholar] [CrossRef]
- Irwin, D.J.; Grossman, M.; Weintraub, D.; Hurtig, H.I.; Duda, J.E.; Xie, S.X.; Lee, E.B.; Van Deerlin, V.M.; Lopez, O.L.; Kofler, J.K.; et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: A retrospective analysis. Lancet Neurol. 2017, 16, 55–65. [Google Scholar] [CrossRef]
- Jellinger, K.A.; Seppi, K.; Wenning, G.K.; Poewe, W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson’s disease. J. Neural Transm. 2002, 109, 329–339. [Google Scholar] [CrossRef]
- Biundo, R.; Weis, L.; Antonini, A. Cognitive decline in Parkinson’s disease: The complex picture. NPJ Park. Dis. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Braak, H.; Rüb, U.; Jansen Steur, E.N.; Del Tredici, K.; de Vos, R.A. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 2005, 64, 1404–1410. [Google Scholar] [CrossRef]
- Vermersch, P.; Delacourte, A.; Javoy-Agid, F.; Hauw, J.J.; Agid, Y. Dementia in Parkinson’s disease: Biochemical evidence for cortical involvement using the immunodetection of abnormal Tau proteins. Ann. Neurol. 1993, 33, 445–450. [Google Scholar] [CrossRef]
- Coughlin, D.; Xie, S.X.; Liang, M.; Williams, A.; Peterson, C.; Weintraub, D.; McMillan, C.T.; Wolk, D.A.; Akhtar, R.S.; Hurtig, H.I.; et al. Cognitive and pathological influences of tau pathology in Lewy body disorders. Ann. Neurol. 2019, 85, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Walker, Z.; Moreno, E.; Thomas, A.; Inglis, F.; Tabet, N.; Rainer, M.; Pizzolato, G.; Padovani, A. Clinical usefulness of dopamine transporter SPECT imaging with 123I-FP-CIT in patients with possible dementia with Lewy bodies: Randomised study. Br. J. Psychiatry 2015, 206, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Donaghy, P.C.; Carrarini, C.; Ferreira, D.; Habich, A.; Aarsland, D.; Babiloni, C.; Bayram, E.; Kane, J.P.; Lewis, S.J.; Pilotto, A.; et al. Research diagnostic criteria for mild cognitive impairment with Lewy bodies: A systematic review and meta-analysis. Alzheimer’s Dement. 2023, 19, 3186–3202. [Google Scholar] [CrossRef] [PubMed]
- Compta, Y.; Parkkinen, L.; Kempster, P.; Selikhova, M.; Lashley, T.; Holton, J.L.; Lees, A.J.; Revesz, T. The significance of alpha-synuclein, amyloid-beta and tau pathologies in Parkinson’s disease progression and related dementia. Neurodegener. Dis. 2014, 13, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Kotzbauer, P.T.; Cairns, N.J.; Campbell, M.C.; Willis, A.W.; Racette, B.A.; Tabbal, S.D.; Perlmutter, J.S. Pathologic accumulation of alpha-synuclein and Abeta in Parkinson disease patients with dementia. Arch. Neurol. 2012, 69, 1326–1331. [Google Scholar] [CrossRef]
- Ruffmann, C.; Calboli, F.C.; Bravi, I.; Gveric, D.; Curry, L.K.; de Smith, A.; Pavlou, S.; Buxton, J.L.; Blakemore, A.I.; Takousis, P.; et al. Cortical Lewy bodies and Abeta burden are associated with prevalence and timing of dementia in Lewy body diseases. Neuropathol. Appl. Neurobiol. 2016, 42, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, M.N.; Adler, C.H.; Lahti, T.J.; Connor, D.J.; Vedders, L.; Peterson, L.K.; Caviness, J.N.; Shill, H.A.; Sue, L.I.; Ziabreva, I.; et al. Parkinson disease with dementia: Comparing patients with and without Alzheimer pathology. Alzheimer Dis. Assoc. Disord. 2009, 23, 295–297. [Google Scholar] [CrossRef]
- Parkkinen, L.; Kauppinen, T.; Pirttilä, T.; Autere, J.M.; Alafuzoff, I. Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann. Neurol. 2005, 57, 82–91. [Google Scholar] [CrossRef]
- Hindle, J.V.; Martyr, A.; Clare, L. Cognitive reserve in Parkinson’s disease: A systematic review and meta-analysis. Park. Relat. Disord. 2014, 20, 1–7. [Google Scholar] [CrossRef]
- de Boni, L.; Watson, A.H.; Zaccagnini, L.; Wallis, A.; Zhelcheska, K.; Kim, N.; Sanderson, J.; Jiang, H.; Martin, E.; Cantlon, A.; et al. Brain region-specific susceptibility of Lewy body pathology in synucleinopathies is governed by alpha-synuclein conformations. Acta Neuropathol. 2022, 143, 453–469. [Google Scholar] [CrossRef]
- Kim, R.; Park, S.; Yoo, D.; Jun, J.S.; Jeon, B. Association of physical activity and APOE genotype with longitudinal cognitive change in early Parkinson disease. Neurology 2021, 96, e2429–e2437. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, S.; Mondini, S.; Signorini, M.; Marchetto, A.; Bambini, V.; Arcara, G. Pragmatic language disorder in Parkinson’s disease and the potential effect of cognitive reserve. Front. Psychol. 2019, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Cascone, A.D.; Langella, S.; Sklerov, M.; Dayan, E. Frontoparietal network resilience is associated with protection against cognitive decline in Parkinson’s disease. Commun. Biol. 2021, 4, 1021. [Google Scholar] [CrossRef] [PubMed]
- Di Tella, S.; De Marco, M.; Baglio, F.; Silveri, M.C.; Venneri, A. Resting-state functional connectivity is modulated by cognitive reserve in early Parkinson’s disease. Front. Psychol. 2023, 14, 1207988. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.M.; Henry, R.S.; Perrin, P.B.; Watson, J.; Villaseñor, T.; Lageman, S.K.; Smith, E.R.; Curiel, G.R.; Avila, J.; Jimenez Maldonado, M.E.; et al. Structural equation modeling of Parkinson’s caregiver social support, resilience, and mental health: A strength-based perspective. Neurol. Res. Int. 2020, 2020, 7906547. [Google Scholar] [CrossRef] [PubMed]
- Robottom, B.J.; Gruber-Baldini, A.L.; Anderson, K.E.; Reich, S.G.; Fishman, P.S.; Weiner, W.J.; Shulman, L.M. What determines resilience in patients with Parkinson’s disease? Park. Relat. Disord. 2012, 18, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Luthra, N.S.; Christou, D.D.; Clow, A.; Corcos, D.M. Targeting neuroendocrine abnormalities in Parkinson’s disease with exercise. Front. Neurosci. 2023, 17, 1228444. [Google Scholar] [CrossRef]
- Isingrini, E.; Perret, L.; Rainer, Q.; Amilhon, B.; Guma, E.; Tanti, A.; Martin, G.; Robinson, J.; Moquin, L.; Marti, F.; et al. Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat. Neurosci. 2016, 19, 560–563. [Google Scholar] [CrossRef]
- Liu, H.; Dehestani, M.; Blauwendraat, C.; Makarious, M.B.; Leonard, H.; Kim, J.J.; Schulte, C.; Noyce, A.; Jacobs, B.M.; Foote, I.; et al. Polygenic resilience modulates the penetrance of Parkinson disease genetic risk factors. Ann. Neurol. 2022, 92, 270–278. [Google Scholar] [CrossRef]
- Batzu, L.; Urso, D.; Grothe, M.J.; Veréb, D.; Chaudhuri, K.R.; Pereira, J.B. Increased basal forebrain volumes could prevent cognitive decline in LRRK2 Parkinson’s disease. Neurobiol. Dis. 2023, 183, 106182. [Google Scholar] [CrossRef]
- Yang, D.; Xie, H.; Wu, S.; Ying, C.; Chen, Y.; Ge, Y.; Yao, R.; Li, K.; Jiang, Z.; Chen, G. Neurofilament light chain as a mediator between LRRK2 mutation and dementia in Parkinson’s disease. NPJ Park. Dis. 2023, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Matikainen-Ankney, B.A.; Kezunovic, N.; Menard, C.; Flanigan, M.E.; Zhong, Y.; Russo, S.J.; Benson, D.L.; Huntley, G.W. Parkinson’s disease-linked LRRK2-G2019S mutation alters synaptic plasticity and promotes resilience to chronic social stress in young adulthood. J. Neurosci. 2018, 38, 9700–9711. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Moreno, N.; Lane, J.D. ATG8 proteins are co-factors for human dopaminergic neuronal transcriptional control: Implications for neuronal resilience in Parkinson disease. Autophagy 2023, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.B.; Abdelnour, C.; Weil, R.S.; Ferreira, D.; Rodriguez-Porcel, F.; Pilotto, A.; Wyman-Chick, K.A.; Grothe, M.J.; Kane, J.P.M.; Taylor, A.; et al. Dementia with Lewy bodies: Impact of co-pathologies and implications for clinical trial design. Alzheimer’s Dement. 2023, 19, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Wang, G.; Liu, Q.; Wang, Y. Effect of cerebral small vessel disease on cognitive impairment in Parkinson’s disease: A systematic review and meta-analysis. Ann. Transl. Med. 2022, 10, 288. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Halliday, G.M.; Soh, D.; Cordato, D.J.; Kril, J.J. Impact of small vessel disease on severity of motor and cognitive impairment in Parkinson’s disease. J. Clin. Neurosci. 2018, 58, 70–74. [Google Scholar] [CrossRef]
- Wan, H.; Chen, H.; Zhang, M.; Feng, T.; Wang, Y. Cerebral microbleeds is associated with dementia in Parkinson’s disease. Acta Neurol. Belg. 2023, 123, 407–413. [Google Scholar] [CrossRef]
- Chen, K.; Jin, Z.; Fang, J.; Qi, L.; Liu, C.; Wang, R.; Su, Y.; Yan, H.; Liu, A.; Xi, J.; et al. Lacunes may worsen cognition but not motor function in Parkinson’s disease. Brain Behav. 2023, 13, e2880. [Google Scholar] [CrossRef]
- Donahue, E.K.; Foreman, R.P.; Duran, J.J.; Jakowec, M.W.; O’Neill, J.; Petkus, A.J.; Holschneider, D.P.; Choupan, J.; Van Horn, J.D.; Venkadesh, S.; et al. Increased perivascular space volume in white matter and basal ganglia is associated with cognition in Parkinson’s Disease. Brain Imaging Behav. 2023. [Google Scholar] [CrossRef]
- Tsai, H.H.; Tsai, L.K.; Lo, Y.L.; Lin, C.H. Amyloid related cerebral microbleed and plasma Abeta40 are associated with cognitive decline in Parkinson’s disease. Sci. Rep. 2021, 11, 7115. [Google Scholar] [CrossRef]
- Homma, T.; Mochizuki, Y.; Takahashi, K.; Komori, T. Medial temporal regional argyrophilic grain as a possible important factor affecting dementia in Parkinson’s disease. Neuropathology 2015, 35, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Yu, L.; Wilson, R.S.; Leurgans, S.E.; Nag, S.; Shulman, J.M.; Barnes, L.L.; Schneider, J.A.; Bennett, D.A. Progressive parkinsonism in older adults is related to the burden of mixed brain pathologies. Neurology 2019, 92, e1821–e1830. [Google Scholar] [CrossRef] [PubMed]
- Charissé, D.; Erus, G.; Pomponio, R.; Gorges, M.; Schmidt, N.; Schneider, C.; Liepelt-Scarfone, I.; Riedel, O.; Reetz, K.; Schulz, J.B.; et al. Brain age and Alzheimer’s-like atrophy are domain-specific predictors of cognitive impairment in Parkinson’s disease. Neurobiol. Aging 2022, 109, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Postuma, R.B.; Bloem, B.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.M.; Hardy, J.; Lang, A.E.; et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov. Disord. 2014, 29, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Paolini Paoletti, F.; Gaetani, L.; Bellomo, G.; Chipi, E.; Salvadori, N.; Montanucci, C.; Mancini, A.; Filidei, M.; Nigro, P.; Simoni, S.; et al. CSF neurochemical profile and cognitive changes in Parkinson’s disease with mild cognitive impairment. NPJ Park. Dis. 2023, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, I.; Laansma, M.A.; Lin, C.P.; Hermans, E.J.M.; Bouwman, M.M.A.; Bol, J.G.J.M.; Galis-de Graaf, Y.; Hepp, D.H.; Rozemuller, A.J.M.; Barkhof, F.; et al. Neurofilament light chain is increased in the parahippocampal cortex and associates with pathological hallmarks in Parkinson’s disease dementia. Transl. Neurodegener. 2023, 12, 3. [Google Scholar] [CrossRef]
- Liu, T.; Zuo, H.; Ma, D.; Song, D.; Zhao, Y.; Cheng, O. Cerebrospinal fluid GFAP is a predictive biomarker for conversion to dementia and Alzheimer’s disease-associated biomarkers alterations among de novo Parkinson’s disease patients: A prospective cohort study. J. Neuroinflamm. 2023, 20, 167. [Google Scholar] [CrossRef]
- Mao, S.; Teng, X.; Li, Z.; Zu, J.; Zhang, T.; Xu, C.; Cui, G. Association of serum neurofilament light chain and glial fibrillary acidic protein levels with cognitive decline in Parkinson’s disease. Brain Res. 2023, 1805, 148271. [Google Scholar] [CrossRef]
- Mizutani, Y.; Ohdake, R.; Tatebe, H.; Higashi, A.; Shima, S.; Ueda, A.; Ito, M.; Tokuda, T.; Watanabe, H. Associations of Alzheimer’s-related plasma biomarkers with cognitive decline in Parkinson’s disease. J. Neurol. 2023, 270, 5461–5474. [Google Scholar] [CrossRef]
- Skogseth, R.E.; Bronnick, K.; Pereira, J.B.; Mollenhauer, B.; Weintraub, D.; Fladby, T.; Aarsland, D. Associations between cerebrospinal fluid biomarkers and cognition in early untreated Parkinson’s disease. J. Park. Dis. 2015, 5, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Zhang, Z.W.; Qiu, L.; Lin, Y.; Jiang, M.; Chia, S.Y.; Wei, Y.; Ng, A.S.L.; Reynolds, R.; Tan, E.K.; et al. Increased expression of pathological markers in Parkinson’s disease dementia post-mortem brains compared to dementia with Lewy bodies. BMC Neurosci. 2022, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Nagano-Saito, A.; Kato, T.; Arahata, Y.; Nakamura, A.; Kawasumi, Y.; Hatano, K.; Abe, Y.; Yamada, T.; Kachi, T.; et al. Striatal and extrastriatal dysfunction in Parkinson’s disease with dementia: A 6-[18F]fluoro-L-dopa PET study. Brain 2002, 125, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).