The Involvement of LAG-3positive Plasma Cells in the Development of Multiple Myeloma
Abstract
:1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics of Patients with Plasma Cell Dyscrasia
2.2. CD138+ Plasma Cell Characterization
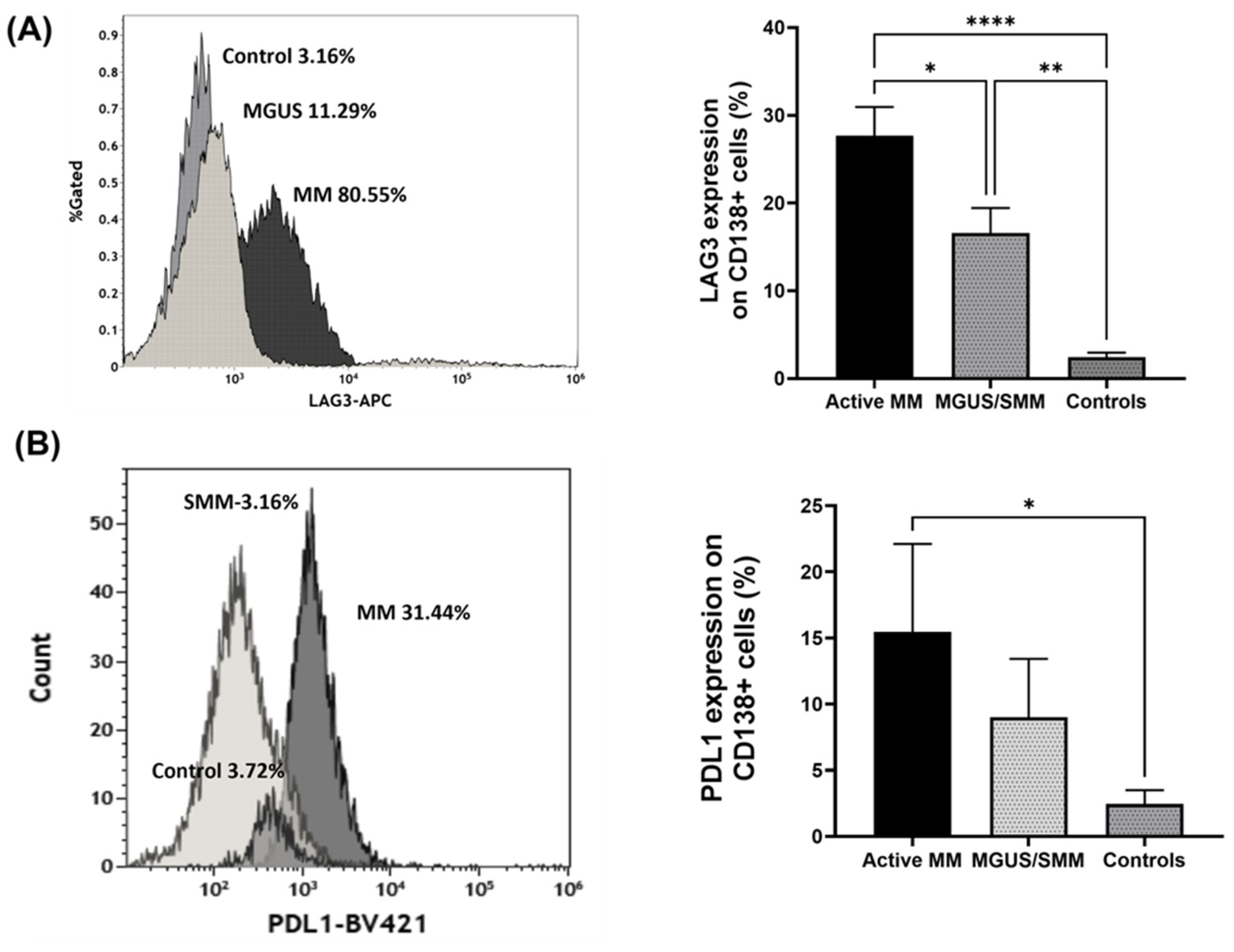
2.2.1. The Expression of Regulatory Receptors on CD138+ Plasma Cells
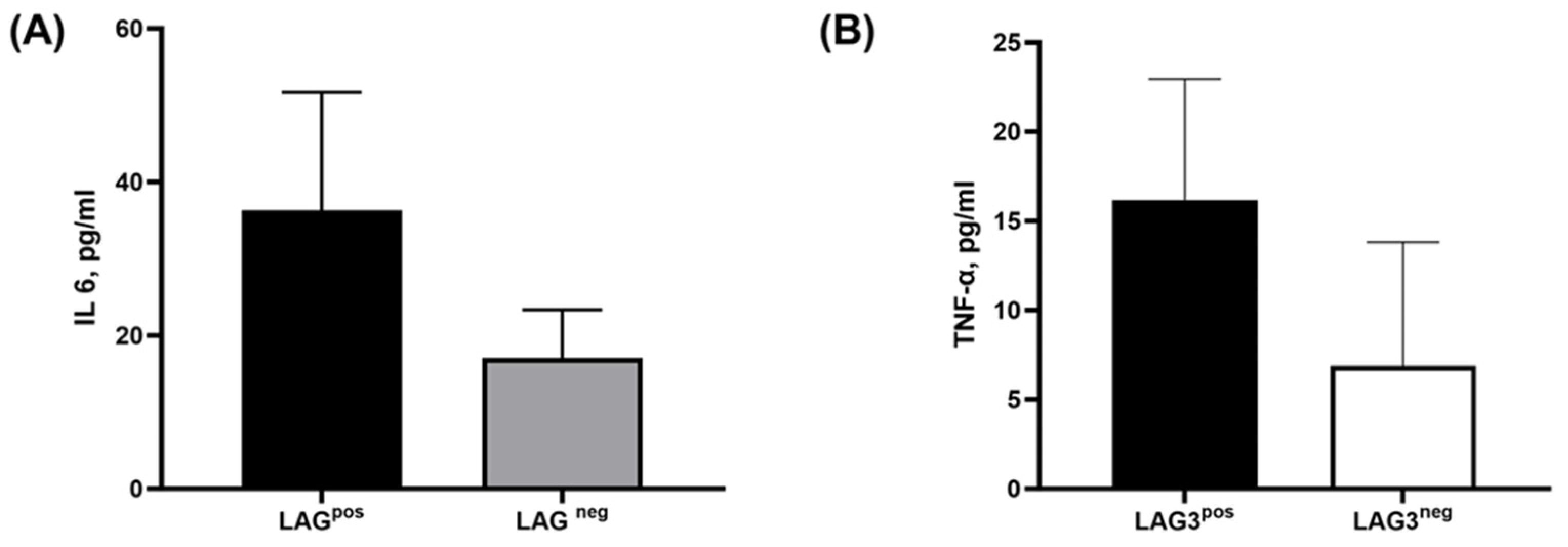
2.2.2. The Secretion of Tumor-Related Pro-Inflammatory Cytokines from CD138+ Plasma Cells
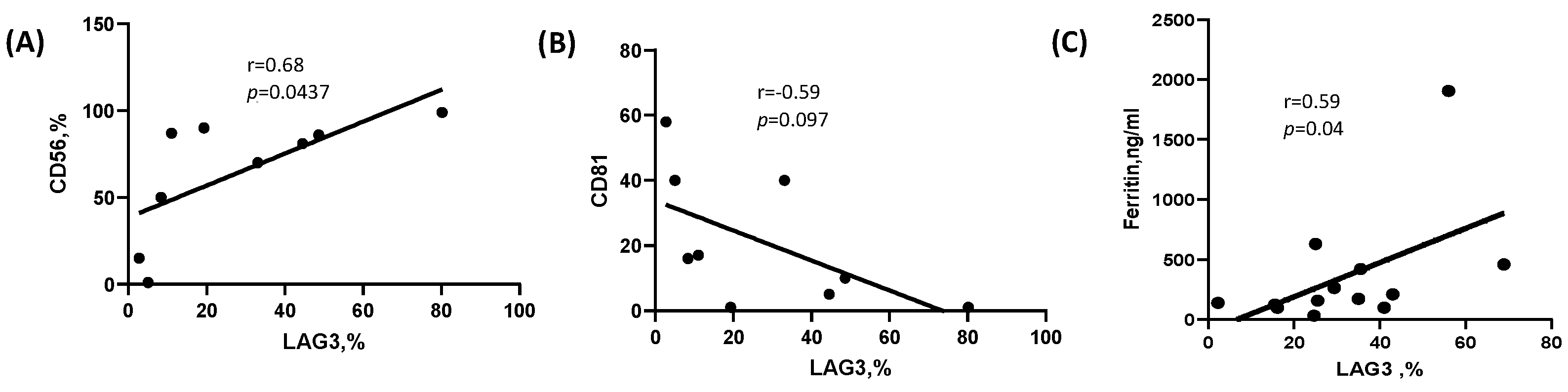
2.3. Correlation of LAG-3 Expression with Laboratory and Clinical Parameters
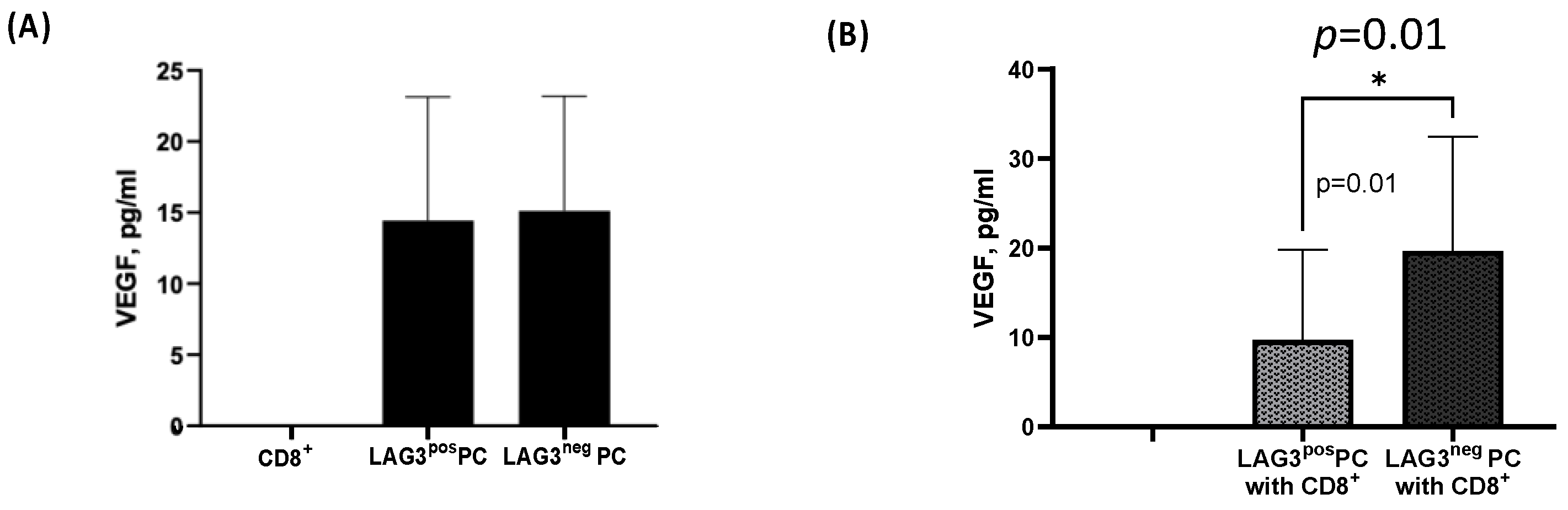
2.4. The Biological Function of LAG-3pos Plasma Cells
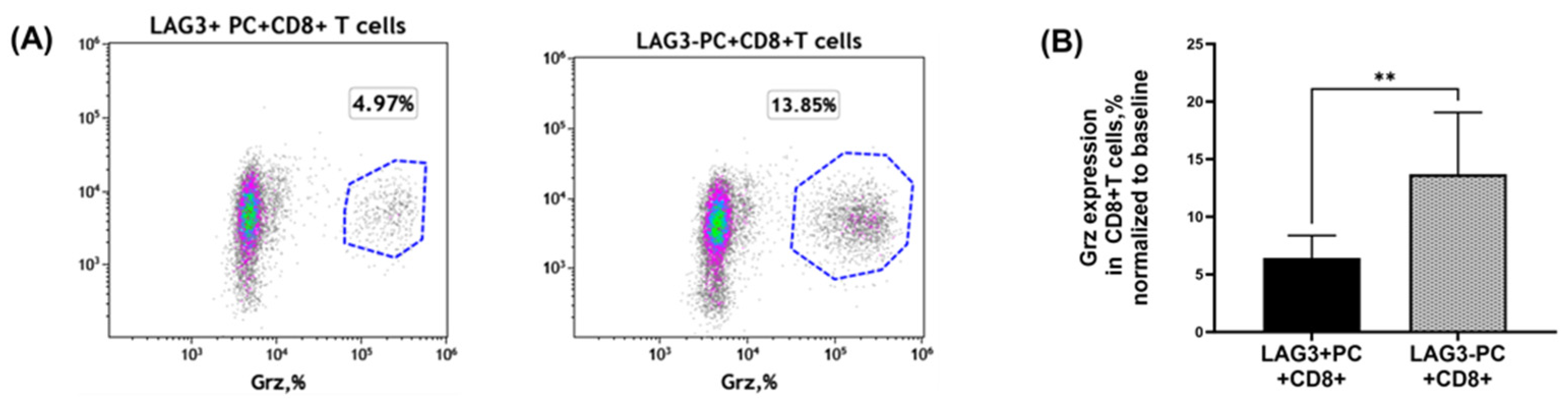
2.4.1. Granzyme Secretion from CD8+T Cells Co-Cultured with Plasma Cells
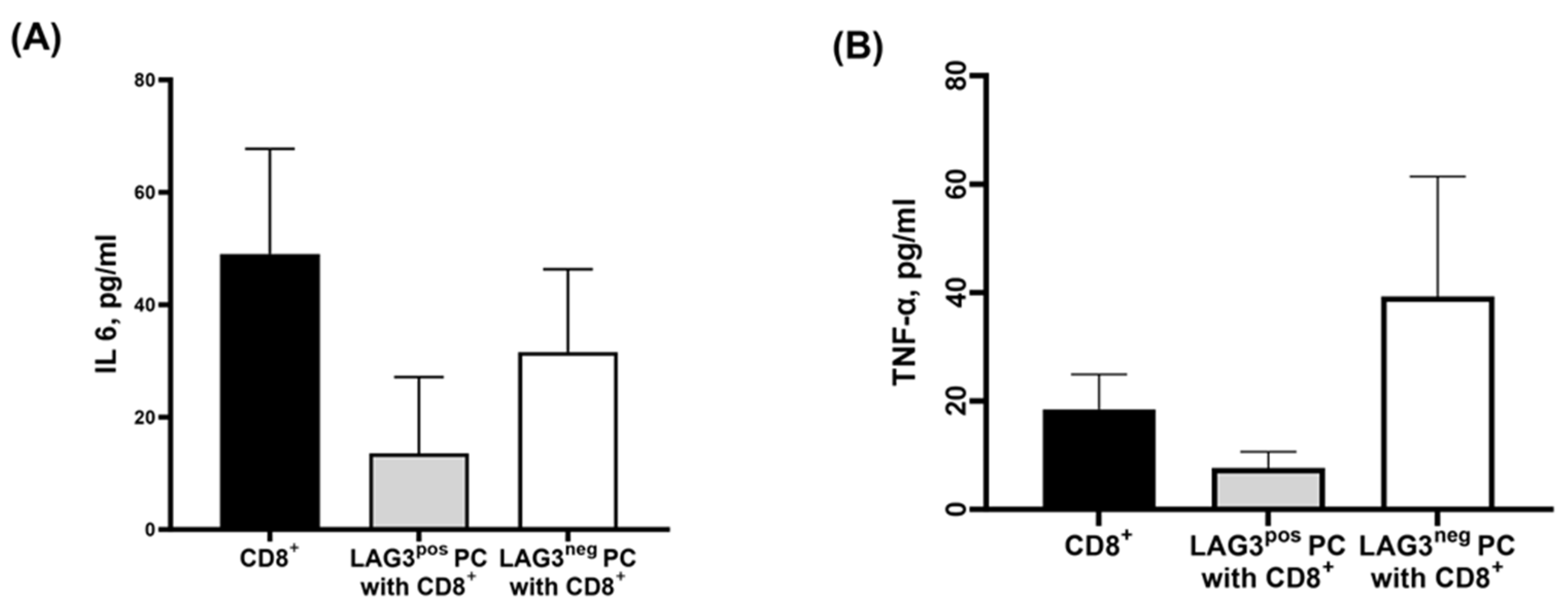
2.4.2. Cytokine Secretion Pattern of CD8+T Cells Cultured with Plasma Cells
2.4.3. CD8+T Cells Effect on Plasma Cells
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Cell Isolation
4.3. Flow Cytometry
4.4. Flow Cytometry Antibodies
4.5. Sorting of Plasma Cells
4.6. Co-Culturing of CD138+ Plasma Cells with Autologous CD8+T Cells
4.7. Luminex Performance Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.-V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Primers 2017, 3, 17046. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 548–567. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A.; Kyle, R.A.; Katzmann, J.A.; Therneau, T.M.; Larson, D.; Benson, J.; Clark, R.J.; Melton, L.J., III; Gertz, M.A.; Kumar, S.K. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood J. Am. Soc. Hematol. 2008, 111, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Persona, E.; Vidriales, M.-B.; Mateo, G.; García-Sanz, R.; Mateos, M.-V.; de Coca, A.G.; Galende, J.; Martín-Nuñez, G.; Alonso, J.M.; de Las Heras, N. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood J. Am. Soc. Hematol. 2007, 110, 2586–2592. [Google Scholar] [CrossRef]
- Gertz, M. Smoldering multiple myeloma: Reviewing the rationale for intervention. Leuk. Lymphoma 2022, 63, 2033–2040. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T. Revised international staging system for multiple myeloma: A report from International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863. [Google Scholar] [CrossRef]
- D’agostino, M.; Cairns, D.A.; Lahuerta, J.J.; Wester, R.; Bertsch, U.; Waage, A.; Zamagni, E.; Mateos, M.-V.; Dall’Olio, D.; van de Donk, N.W. Second Revision of the International Staging System (R2-ISS) for Overall Survival in Multiple Myeloma: A European Myeloma Network (EMN) Report Within the HARMONY Project. J. Clin. Oncol. 2022, 40, 3406–3418. [Google Scholar] [CrossRef]
- Lee, B.-H.; Park, Y.; Kim, J.H.; Kang, K.-W.; Lee, S.J.; Kim, S.J.; Kim, B.S. PD-L1 expression in bone marrow plasma cells as a biomarker to predict multiple myeloma prognosis: Developing a nomogram-based prognostic model. Sci. Rep. 2020, 10, 12641. [Google Scholar] [CrossRef]
- Tremblay-LeMay, R.; Rastgoo, N.; Chang, H. Modulating PD-L1 expression in multiple myeloma: An alternative strategy to target the PD-1/PD-L1 pathway. J. Hematol. Oncol. 2018, 11, 46. [Google Scholar] [CrossRef]
- Moreaux, J.; Hose, D.; Rème, T.; Jourdan, E.; Hundemer, M.; Legouffe, E.; Moine, P.; Bourin, P.; Moos, M.; Corre, J. CD200 is a new prognostic factor in multiple myeloma. Blood 2006, 108, 4194–4197. [Google Scholar] [CrossRef]
- Ramasamy, K.; Gay, F.; Weisel, K.; Zweegman, S.; Mateos, M.V.; Richardson, P. Improving outcomes for patients with relapsed multiple myeloma: Challenges and considerations of current and emerging treatment options. Blood Rev. 2021, 49, 100808. [Google Scholar] [CrossRef] [PubMed]
- Ghandili, S.; Weisel, K.C.; Bokemeyer, C.; Leypoldt, L.B. Current treatment approaches to newly diagnosed multiple myeloma. Oncol. Res. Treat. 2021, 44, 690–699. [Google Scholar] [CrossRef]
- Sharma, P.; Kanapuru, B.; George, B.; Lin, X.; Xu, Z.; Bryan, W.W.; Pazdur, R.; Theoret, M.R. FDA Approval Summary: Idecabtagene Vicleucel for Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2022, 28, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Gadó, K.; Domján, G.; Hegyesi, H.; Falus, A. Role of interleukin-6 in the pathogenesis of multiple myeloma. Cell Biol. Int. 2000, 24, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Harmer, D.; Falank, C.; Reagan, M.R. Interleukin-6 interweaves the bone marrow microenvironment, bone loss, and multiple myeloma. Front. Endocrinol. 2019, 9, 788. [Google Scholar] [CrossRef] [PubMed]
- Matthes, T.; Manfroi, B.; Huard, B. Revisiting IL-6 antagonism in multiple myeloma. Crit. Rev. Oncol. Hematol. 2016, 105, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, M.; Tarte, K.; Legouffe, E.; Brochier, J.; Rossi, J.-F.; Klein, B. Tumor necrosis factor is a survival and proliferation factor for human myeloma cells. Eur. Cytokine Netw. 1999, 10, 65. [Google Scholar]
- Nachbaur, D.; Herold, M.; Maneschg, A.; Huber, H. Serum levels of interleukin-6 in multiple myeloma and other hematological disorders: Correlation with disease activity and other prognostic parameters. Ann. Hematol. 1991, 62, 54–58. [Google Scholar] [CrossRef]
- Jurišić, V.; Čolović, M. Correlation of sera TNF-α with percentage of bone marrow plasma cells, LDH, β2-microglobulin, and clinical stage in multiple myeloma. Med. Oncol. 2002, 19, 133–139. [Google Scholar] [CrossRef]
- Kumar, S.; Witzig, T.; Timm, M.; Haug, J.; Wellik, L.; Fonseca, R.; Greipp, P.; Rajkumar, S. Expression of VEGF and its receptors by myeloma cells. Leukemia 2003, 17, 2025–2031. [Google Scholar] [CrossRef]
- Ria, R.; Melaccio, A.; Racanelli, V.; Vacca, A. Anti-VEGF drugs in the treatment of multiple myeloma patients. J. Clin. Med. 2020, 9, 1765. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Mateos, M.-V.; San Miguel, J.; Shah, J.; Thompson, S.; Marinello, P.; Jagannath, S. Pembrolizumab in combination with lenalidomide and low-dose dexamethasone in newly diagnosed and treatment-naive multiple myeloma (MM): Randomized, phase 3 KEYNOTE-185 study. Ann. Oncol. 2016, 27, vi324. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Blacklock, H.; Schjesvold, F.; Oriol, A.; Simpson, D.; George, A.; Goldschmidt, H.; Larocca, A.; Chanan-Khan, A.; Sherbenou, D. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e459–e469. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Accardi, F.; Hideshima, T.; Tai, Y.-T.; Prabhala, R.; Shambley, A.; Wen, K.; Rowell, S.; Richardson, P.G.; Munshi, N.C. Targeting LAG3/GAL-3 to overcome immunosuppression and enhance anti-tumor immune responses in multiple myeloma. Leukemia 2022, 36, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Mussetti, A.; Pellegrinelli, A.; Cieri, N.; Garzone, G.; Dominoni, F.; Cabras, A.; Montefusco, V. PD-L1, LAG3, and HLA-DR are increasingly expressed during smoldering myeloma progression. Ann. Hematol. 2019, 98, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Lui, Y.; Davis, S.J. LAG-3: A very singular immune checkpoint. Nat. Immunol. 2018, 19, 1278–1279. [Google Scholar] [CrossRef] [PubMed]
- Fillatreau, S. Regulatory functions of B cells and regulatory plasma cells. Biomed. J. 2019, 42, 233–242. [Google Scholar] [CrossRef]
- Lino, A.C.; Lampropoulou, V.; Welle, A.; Joedicke, J.; Pohar, J.; Simon, Q.; Thalmensi, J.; Baures, A.; Flühler, V.; Sakwa, I. LAG-3 inhibitory receptor expression identifies immunosuppressive natural regulatory plasma cells. Immunity 2018, 49, 120–133.e129. [Google Scholar] [CrossRef]
- Yang, M.; Rui, K.; Wang, S.; Lu, L. Regulatory B cells in autoimmune diseases. Cell. Mol. Immunol. 2013, 10, 122–132. [Google Scholar] [CrossRef]
- He, Y.; Qian, H.; Liu, Y.; Duan, L.; Li, Y.; Shi, G. The roles of regulatory B cells in cancer. J. Immunol. Res. 2014, 2014, 215471. [Google Scholar] [CrossRef] [PubMed]
- Fillatreau, S. Natural regulatory plasma cells. Curr. Opin. Immunol. 2018, 55, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Triebel, F. A novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1996, 171, 12. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Rivard, C.J.; Rozeboom, L.; Yu, H.; Ellison, K.; Kowalewski, A.; Zhou, C.; Hirsch, F.R. Lymphocyte-activation gene-3, an important immune checkpoint in cancer. Cancer Sci. 2016, 107, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Keane, C.; Law, S.C.; Gould, C.; Birch, S.; Sabdia, M.B.; Merida de Long, L.; Thillaiyampalam, G.; Abro, E.; Tobin, J.W.; Tan, X. LAG3: A novel immune checkpoint expressed by multiple lymphocyte subsets in diffuse large B-cell lymphoma. Blood Adv. 2020, 4, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Gould, C.; Lickiss, J.; Kankanige, Y.; Yerneni, S.; Lade, S.; Gandhi, M.K.; Chin, C.; Yannakou, C.K.; Villa, D.; Slack, G.W. Characterisation of immune checkpoints in Richter syndrome identifies LAG3 as a potential therapeutic target. Br. J. Haematol. 2021, 195, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Bębnowska, D.; Hrynkiewicz, R.; Grywalska, E.; Pasiarski, M.; Sosnowska-Pasiarska, B.; Smarz-Widelska, I.; Góźdź, S.; Roliński, J.; Niedźwiedzka-Rystwej, P. Immunological prognostic factors in multiple myeloma. Int. J. Mol. Sci. 2021, 22, 3587. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Vescovini, R.; Marchica, V.; Storti, P.; Notarfranchi, L.; Dalla Palma, B.; Toscani, D.; Burroughs-Garcia, J.; Catarozzo, M.T.; Sammarelli, G. PD-L1/PD-1 pattern of expression within the bone marrow immune microenvironment in smoldering myeloma and active multiple myeloma patients. Front. Immunol. 2021, 11, 613007. [Google Scholar] [CrossRef]
- Douds, J.J.; Long, D.J.; Kim, A.S.; Li, S. Diagnostic and prognostic significance of CD200 expression and its stability in plasma cell myeloma. J. Clin. Pathol. 2014, 67, 792–796. [Google Scholar] [CrossRef]
- Shi, Q.; Wu, C.; Han, W.; Zhao, S.; Wu, Y.; Jin, Y.; Qu, X.; Li, J.; Zhang, R.; Chen, L. Clinical significance of CD200 expression in newly diagnosed multiple myeloma patients and dynamic changing during treatment. Leuk. Lymphoma 2021, 62, 709–715. [Google Scholar] [CrossRef]
- Sze, D.M.; Brown, R.D.; Yuen, E.; Gibson, J.; Ho, J.; Raitakari, M.; Basten, A.; Joshua, D.E.; Fazekas de St Groth, B. Clonal cytotoxic T cells in myeloma. Leuk. Lymphoma 2003, 44, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Crispin, J.C.; Tsokos, G.C. Cancer immunosurveillance by CD8 T cells. F1000Research 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8+ T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Zelle-Rieser, C.; Thangavadivel, S.; Biedermann, R.; Brunner, A.; Stoitzner, P.; Willenbacher, E.; Greil, R.; Jöhrer, K. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J. Hematol. Oncol. 2016, 9, 116. [Google Scholar] [CrossRef]
- Tian, T.; Wang, M.; Ma, D. TNF-α, a good or bad factor in hematological diseases? Stem Cell Investig. 2014, 1, 12. [Google Scholar]
- Reinders, M.E.; Sho, M.; Izawa, A.; Wang, P.; Mukhopadhyay, D.; Koss, K.E.; Geehan, C.S.; Luster, A.D.; Sayegh, M.H.; Briscoe, D.M. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J. Clin. Investig. 2003, 112, 1655–1665. [Google Scholar] [CrossRef]
- Basu, A.; Hoerning, A.; Datta, D.; Edelbauer, M.; Stack, M.P.; Calzadilla, K.; Pal, S.; Briscoe, D.M. Cutting edge: Vascular endothelial growth factor-mediated signaling in human CD45RO+ CD4+ T cells promotes Akt and ERK activation and costimulates IFN-γ production. J. Immunol. 2010, 184, 545–549. [Google Scholar] [CrossRef]
| MGUS n = 17 | SMM n = 20 | NDMM n = 27 | RRMM n = 5 | |
|---|---|---|---|---|
| Age, mean ± SD (Year) | 69 ± 9 | 71 ± 10 | 71 ± 9 | 63 ± 6 |
| Sex, M/F (n) | 10/7 | 11/9 | 14/13 | 2/3 |
| Fish high risk n, % | 1 | 4 | 6 | 2 |
| Monoclonal IgG, IgA, IgM, FLC (n) | 11,3,2,0 | 9,6,1,4 | 12,5,0,6 | 2,1,0,2 |
| Immunoparesis n, % | 3 | 6 | 20 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreiniz, N.; Eiza, N.; Tadmor, T.; Levy Yurkovski, I.; Matarasso Greenfeld, S.; Sabag, A.; Mubariki, R.; Suriu, C.; Votinov, E.; Toubi, E.; et al. The Involvement of LAG-3positive Plasma Cells in the Development of Multiple Myeloma. Int. J. Mol. Sci. 2024, 25, 549. https://doi.org/10.3390/ijms25010549
Kreiniz N, Eiza N, Tadmor T, Levy Yurkovski I, Matarasso Greenfeld S, Sabag A, Mubariki R, Suriu C, Votinov E, Toubi E, et al. The Involvement of LAG-3positive Plasma Cells in the Development of Multiple Myeloma. International Journal of Molecular Sciences. 2024; 25(1):549. https://doi.org/10.3390/ijms25010549
Chicago/Turabian StyleKreiniz, Natalia, Nasren Eiza, Tamar Tadmor, Ilana Levy Yurkovski, Sarah Matarasso Greenfeld, Adi Sabag, Raeda Mubariki, Celia Suriu, Ekaterina Votinov, Elias Toubi, and et al. 2024. "The Involvement of LAG-3positive Plasma Cells in the Development of Multiple Myeloma" International Journal of Molecular Sciences 25, no. 1: 549. https://doi.org/10.3390/ijms25010549





