Exploring Acne Treatments: From Pathophysiological Mechanisms to Emerging Therapies
Abstract
:1. Introduction
2. Pathophysiology of Acne
2.1. Genetic and Environmental Factors
2.1.1. Genetic Factors
2.1.2. Environmental Factors
2.2. Hormonal Influences
2.3. Microbiome
2.4. Inflammation
3. Current Treatment Strategies
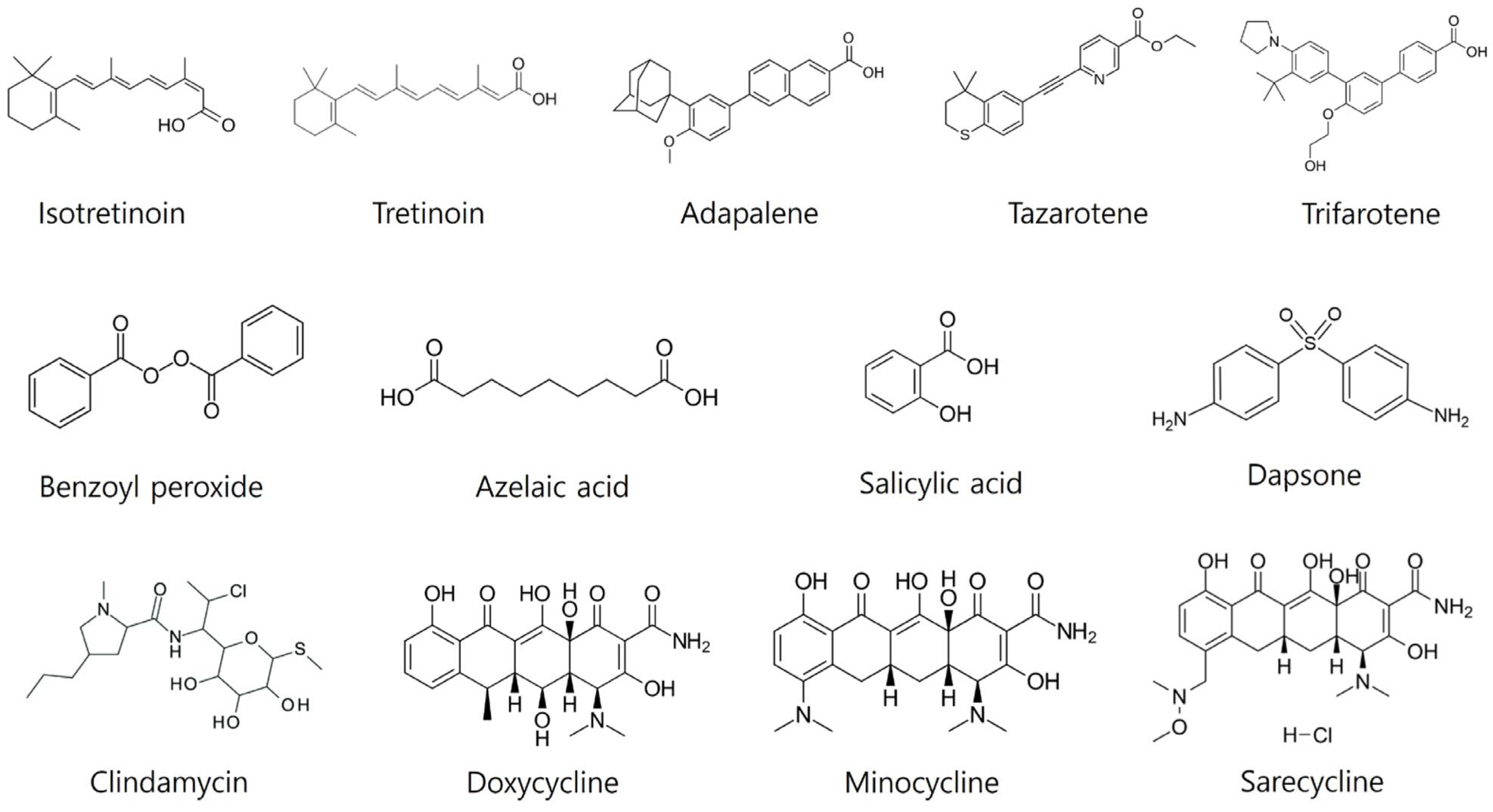
3.1. Topical Treatments
3.1.1. Topical Retinoids
- First-Generation Retinoids
- b.
- Second-Generation Retinoids: There are no available second-generation topical formulations of retinoids.
- c.
- Third-Generation Retinoids
- d.
- Fourth-Generation Retinoids
3.1.2. Topical Benzoyl Peroxide
3.1.3. Topical Antibiotics
- (a)
- (b)
- Erythromycin: A possible alternative to clindamycin. However, concerns exist about higher rates of C. acnes resistance to topical erythromycin compared with clindamycin [126]. Therefore, it should be combined with other agents like BPO (Benzamycin®). Erythromycin is available as a monotherapy (gels, swabs, solutions). It is generally well tolerated, but can cause skin irritation [126].
- (c)
- Minocycline: A tetracycline derivative. Topical minocycline 4% foam (Amzeeq™) demonstrates greater effectiveness than its vehicle alone [140,141]. This lipophilic formulation readily moves into the pilosebaceous unit [142]. While its precise mechanism is not fully understood, its potent antibacterial effects are well documented [142]. Studies indicate effectiveness in improving acne within 12 weeks, with continued improvement observed at 52 weeks [142,143]. Minocycline foam is generally well tolerated in trials, with the most frequent side effects being increased creatinine phosphokinase levels and headaches [140,141]. Topical minocycline 4% foam for acne has received FDA approval but has not yet been approved in the EU.
3.1.4. Topical Azelaic Acid
3.1.5. Topical Salicylic Acid
3.1.6. Topical Dapsone
3.1.7. Topical Sulfur and Sodium Sulfacetamide
- (a)
- Sulfur: Exhibits mild antibacterial and keratolytic properties, helping loosen dead skin cells to prevent clogged pores [162]. Sulfur interacts with the cysteine within skin cells to form hydrogen sulfide, disrupting disulfide bonds for its keratolytic effect [163]. Newer nanoparticle preparations may offer enhanced effectiveness against Staphylococcus bacteria, a contributor to acne pathogenesis [164,165]. Sulfur on its own can treat mild to moderate acne, but its results are improved when combined with sodium sulfacetamide or BPO [166,167,168,169,170].
- (b)
- Sodium sulfacetamide: A bacteriostatic agent that disrupts bacterial DNA synthesis by inhibiting para-aminobenzoic acid [126]. Typically formulated as a topical lotion with 10% sodium sulfacetamide and 5% sulfur, it demonstrates significant acne lesion reduction (50–69% after 8 weeks; 78% after 12 weeks) [166,171].
3.1.8. Topical Clascoterone
| Treatment Examples | Mechanism of Action | Indications | Common Side Effects | References |
|---|---|---|---|---|
| Retinoids (e.g., tretinoin, adapalene) | Normalizes follicular epithelial desquamation; anti-inflammatory | Mild to severe acne, depending on the formulation | Dryness, photosensitivity, initial acne flare-up | [3,103,104,105,106,107,108,109,111,112,113,114,115,116,120,122,123,124,125,126,177,178] |
| Benzoyl peroxide | Kills bacteria; peels out the inner lining of the hair follicle, causing skin peeling | Mild to moderate acne | Dryness, irritation, potential bleaching of clothes | [130,131,132,133,134,135,136,138,146,179,180,181,182,183] |
| Antibiotics (e.g., clindamycin) | Antimicrobial; reduces inflammation | Mild to moderate inflammatory acne | Skin irritation, resistance development | [126,135,139,140,141,142,143,144] |
| Azelaic acid | Kills bacteria; normalizes keratinization; anti-inflammatory | Mild to moderate acne | Pruritus, burning sensation | [106,145,146,147,148] |
| Salicylic acid | Helps break down blackheads and whiteheads; anti-inflammatory | Mild acne, comedonal acne | Skin irritation, dryness | [135,146,149,150,151,152] |
| Sulfur and sodium sulfacetamide | Antibacterial and keratolytic effects | Mild to moderate acne, rosacea | Dryness, skin irritation | [126,162,163,164,165,166,167,168,169,170,171] |
| Clascoterone (topical antiandrogen) | Blocks androgen receptors in the skin; reduces sebum production and inflammation | Moderate to severe acne | Local irritation, erythema | [173,174,175,176] |
| Treatment | Formulation, Dose, Frequency | Common Side Effects | References |
|---|---|---|---|
| Tretinoin/Clindamycin | Gel 0.025%/1.2% daily | Xerosis, irritation, allergic contact dermatitis, erythema | [102] |
| Tretinoin/Benzoyl peroxide | Cream 0.1%/3% once daily | Xerosis, irritation, allergic contact dermatitis, erythema | [127] |
| Adapalene/Benzoyl peroxide | Gel 0.1%/2.5% once daily or 0.3%/2.5% once daily | Xerosis, irritation, allergic contact dermatitis, erythema | [102] |
| Benzoyl peroxide/Clindamycin | Gel 5%/1% once daily or 3.75%/1.2% once daily or 2.5%/1.2% once daily | Xerosis, irritation, allergic contact dermatitis, erythema, bleaching of fabrics | [132,133,134] |
| Benzoyl peroxide/Erythromycin | Gel 5%/3% once daily | Xerosis, irritation, allergic contact dermatitis, erythema, bleaching of fabrics | [138] |
| Clindamycin/Benzoyl peroxide/Adapalene (IDP-126) | Gel 1.2%/3.1%/0.15% | Xerosis, irritation, allergic contact dermatitis, erythema | [128] |
| Novel combined formulation in development: Minocycline/Adapalene (FCD105) | Foam 3%/0.3% | Xerosis, irritation, allergic contact dermatitis, erythema | [129] |
3.2. Systemic Treatments
3.2.1. Oral Antibiotics
3.2.2. Hormonal Therapies
3.2.3. Oral Retinoids
| Treatment Examples | Mechanism of Action | Indications | Common Side Effects | References |
|---|---|---|---|---|
| Oral antibiotics (e.g., tetracycline, doxycycline) | Antimicrobial, reduce inflammation | Moderate to severe acne | Gastrointestinal upset, photosensitivity | [135,144,184,185,186,187,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206] |
| Hormonal therapies (e.g., oral contraceptives, spironolactone) | Regulate hormonal imbalance, reduce sebum production | Hormonally influenced acne, adult women | Mood changes, breast tenderness, thrombosis risk | [135,146,207,208,209,210,211,214,215,217,218] |
| Isotretinoin | Reduces sebaceous gland size and sebum production, anti-inflammatory | Severe scarring acne or acne not responsive to other treatments | Dryness, teratogenicity, elevated liver enzymes | [68,135,184,204,220,221,222,223,224,225,226,227,228,233,234,235,236,237,238,239,240,241,242] |
3.3. Procedural Therapies
| Treatment Type | Mechanism of Action | Indications | Common Side Effects | References |
|---|---|---|---|---|
| Photodynamic therapy | Activates photosensitizing agents that kill bacteria and reduce sebum production | Severe acne, acne resistant to other treatments | Redness, swelling, skin sensitivity to sunlight | [246,248,249,250,251] |
| Blue-light therapy | Kills C. acnes bacteria using blue light without damaging the skin | Mild to moderate acne | Temporary redness and dryness | [252,253,254,255] |
| Chemical peel | Causes the top layer of skin to peel off, helps clear debris from pores | Mild to severe acne, depending on the peel’s strength | Redness, peeling, potential scarring | [126,263,270,271] |
| Intralesional steroid injection | Anti-inflammatory action and decrease in sebum production | Moderate to severe acne | Local atrophy | [277] |
3.4. Diet Modifications
| Supplement Examples | Mechanism of Action | Indications | Common Side Effects | References |
|---|---|---|---|---|
| Omega-3 fatty acids | Anti-inflammatory, modulates sebum production | Various acne types | Fishy aftertaste, gastrointestinal upset | [284,285] |
| Vitamin D | Modulates immune system, may reduce skin inflammation | Various acne types | Rare, potentially increased calcium levels | [288,289,290,291,292,293] |
| Zinc | Reduces inflammation and bacterial growth and modulates the immune system | Various acne types | Nausea, metallic taste | [69,286,294,295] |
4. Emerging Therapies
| Therapy Type | Mechanism of Action | Result | References |
|---|---|---|---|
| Hyaluronic acid | Acts as a major component of the extracellular matrix, downregulating lipid synthesis in a dose-dependent manner through its interaction with cellular receptors. | Significantly decreases sebum production, improving skin hydration and reducing oily skin appearance. | [298] |
| Cannabidiol | Interacts with the skin’s endocannabinoid system, helping to maintain skin health and function, while exerting anti-inflammatory and sebostatic properties. | Reduces inflammation and normalizes sebum production, leading to fewer acne outbreaks. | [299,300,301,302,303,304,305] |
| Biologic treatments (e.g., TNF-α inhibitors like adalimumab; IL-17 and IL-23 inhibitors like secukinumab) | Modulates the immune response by targeting and inhibiting specific cytokines involved in inflammation. | Reduces inflammation and the severity of acne symptoms, improving the overall skin condition. | [85,306,307,308,309,310] |
| Gut microbiome (microbial transplantation) | Transfers a healthy microbiome from a donor to an acne-prone recipient, fostering a microbial environment similar to that of individuals without acne. | The recipient’s skin microbiome adopts characteristics beneficial for preventing acne, reducing lesion formation. | [311] |
| Topical probiotics | Promotes the production of antimicrobial substances from beneficial bacteria and competes with pathogenic microbes on the skin. | Reduces pro-inflammatory cytokine levels and acne severity, promoting a healthier skin barrier. | [312,313,314,315,316,317,318] |
| Oral probiotics | Enhances the systemic immune function and modulates local inflammatory responses through increased levels of anti-inflammatory cytokines like IL-10. | Demonstrates a decrease in acne lesions and an overall improvement in skin clarity. | [319,320,321,322,323] |
| Vaccines | Targets C. acnes and its virulence factors, like CAMP factor 2, to reduce microbial-induced inflammation. | Leads to a significant reduction in acne-related inflammation and lesion count. | [324] |
| Bacteriophages | Specifically targets and kills C. acnes bacteria, thereby directly reducing the bacterial load and associated inflammation. | Early research suggests a potential reduction in acne severity and improved skin condition. | [325,326] |
| Designed antimicrobial peptides (dAMPs) | Provides targeted antimicrobial activity against resistant strains of C. acnes, while also modulating the immune response. | Reduces acne outbreaks and severity through effective bacterial control and reduced inflammation. | [327,328,329,330,331] |
| Phosphodiesterase (PDE) inhibitors | Increases the levels of cyclic adenosine monophosphate (cAMP), which leads to a decreased inflammatory response in the skin. | Shows promising reduction in inflammatory acne lesions, contributing to clearer skin. | [332,333] |
5. Optimal Therapeutic Strategies for Varied Acne Severities
5.1. Acne Severity Grading
5.2. Most Effective Treatment Combinations for Each Type
5.2.1. Mild to Moderate Acne
5.2.2. Moderate to Severe Acne
5.2.3. Severe, Nodular, or Treatment-Resistant Acne
6. Discussion and Conclusions
Funding
Conflicts of Interest
References
- Mitchell, B.L.; Saklatvala, J.R.; Dand, N.; Hagenbeek, F.A.; Li, X.; Min, J.L.; Thomas, L.; Bartels, M.; Jan Hottenga, J.; Lupton, M.K.; et al. Genome-wide association meta-analysis identifies 29 new acne susceptibility loci. Nat. Commun. 2022, 13, 702. [Google Scholar] [CrossRef] [PubMed]
- Ip, A.; Muller, I.; Geraghty, A.W.A.; McNiven, A.; Little, P.; Santer, M. Young people’s perceptions of acne and acne treatments: Secondary analysis of qualitative interview data. Br. J. Dermatol. 2020, 183, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Vasam, M.; Korutla, S.; Bohara, R.A. Acne vulgaris: A review of the pathophysiology, treatment, and recent nanotechnology based advances. Biochem. Biophys. Rep. 2023, 36, 101578. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.K.; Haas, K.N.; Sivamani, R.K. Edible plants and their influence on the gut microbiome and acne. Int. J. Mol. Sci. 2017, 18, 1070. [Google Scholar] [CrossRef] [PubMed]
- Cong, T.-X.; Hao, D.; Wen, X.; Li, X.-H.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Plewig, G.; Kligman, A.M. Acne and Rosacea; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Bataille, V.; Snieder, H.; MacGregor, A.; Sasieni, P.; Spector, T. The influence of genetics and environmental factors in the pathogenesis of acne: A twin study of acne in women. J. Investig. Dermatol. 2002, 119, 1317–1322. [Google Scholar] [CrossRef]
- Di Landro, A.; Cazzaniga, S.; Parazzini, F.; Ingordo, V.; Cusano, F.; Atzori, L.; Cutrì, F.T.; Musumeci, M.L.; Zinetti, C.; Pezzarossa, E. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults. J. Am. Acad. Dermatol. 2012, 67, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.; Kirk, K.; Nyholt, D.; Novac, C.; Martin, N. Teenage acne is influenced by genetic factors. Br. J. Dermatol. 2005, 152, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.D. Twin studies of disease heritability based on medical records: Application to acne vulgaris. Acta Genet. Med. Gemellol. Twin Res. 1984, 33, 487–495. [Google Scholar] [CrossRef]
- Ghodsi, S.Z.; Orawa, H.; Zouboulis, C.C. Prevalence, severity, and severity risk factors of acne in high school pupils: A community-based study. J. Investig. Dermatol. 2009, 129, 2136–2141. [Google Scholar] [CrossRef]
- Hecht, H. Hereditary Trends in Acne VulgarisPrevention of Acne. Dermatology 1960, 121, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, R.; Simpson, M.A.; Smith, C.; Barker, J.; Navarini, A.A. Genetic architecture of acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Mina-Vargas, A.; Colodro-Conde, L.; Grasby, K.; Zhu, G.; Gordon, S.; Medland, S.E.; Martin, N.G. Heritability and GWAS analyses of acne in Australian adolescent twins. Twin Res. Hum. Genet. 2017, 20, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.H.; Tan, A.; Barkham, T.; Yan, X.Y.; Zhu, M. Community-based study of acne vulgaris in adolescents in Singapore. Br. J. Dermatol. 2007, 157, 547–551. [Google Scholar] [CrossRef]
- Tan, J.K.; Bhate, K. A global perspective on the epidemiology of acne. Br. J. Dermatol. 2015, 172, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Pang, Y.; Zhu, H.; Qu, L.; Xiao, T.; Wei, H.C.; Chen, H.D.; He, C.D. The epidemiology of adolescent acne in North East China. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, H.; Fan, X.; Sun, L.; Yang, S.; Wang, P.; Xiao, F.; Gao, M.; Cui, Y.; Ren, Y. The familial risk of acne vulgaris in Chinese Hans—A case-control study. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Walton, S.; Wyatt, E.H.; Cunliffe, W.J. Genetic control of sebum excretion and acne—A twin study. Br. J. Dermatol. 1988, 118, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Suggs, A.; Loesch, M.; Ezaldein, H.; Christensen, L.; Dawes, D.; Baron, E. An Acne Survey from the World’s Largest Annual Gathering of Twins. J. Drugs Dermatol. 2018, 17, 380–382. [Google Scholar]
- He, L.; Wu, W.-J.; Yang, J.-K.; Cheng, H.; Zuo, X.-B.; Lai, W.; Gao, T.-W.; Ma, C.-L.; Luo, N.; Huang, J.-Q.; et al. Two new susceptibility loci 1q24.2 and 11p11.2 confer risk to severe acne. Nat. Commun. 2014, 5, 2870. [Google Scholar] [CrossRef]
- Navarini, A.A.; Simpson, M.A.; Weale, M.; Knight, J.; Carlavan, I.; Reiniche, P.; Burden, D.A.; Layton, A.; Bataille, V.; Allen, M.; et al. Genome-wide association study identifies three novel susceptibility loci for severe Acne vulgaris. Nat. Commun. 2014, 5, 4020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qureshi, A.A.; Hunter, D.J.; Han, J. A genome-wide association study of severe teenage acne in European Americans. Hum. Genet. 2014, 133, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Tasli, L.; Turgut, S.; Kaçar, N.; Ayada, C.; Çoban, M.; Akcilar, R.; Ergin, S. Insulin-like growth factor-I gene polymorphism in acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 254–257. [Google Scholar] [CrossRef]
- Yang, J.-K.; Wu, W.-J.; Qi, J.; He, L.; Zhang, Y.-P. TNF-308 G/A polymorphism and risk of acne vulgaris: A meta-analysis. PLoS ONE 2014, 9, e87806. [Google Scholar] [CrossRef] [PubMed]
- Zaenglein, A.L. Making the case for early treatment of acne. Clin. Pediatr. 2010, 49, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, N.R.; Gilliland, K.L.; Zhao, W.; Liu, W.; Thiboutot, D.M. Gene array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. J. Investig. Dermatol. 2006, 126, 1071–1079. [Google Scholar] [CrossRef]
- Smith, R.N.; Mann, N.J.; Braue, A.; Mäkeläinen, H.; Varigos, G.A. The effect of a high-protein, low glycemic–load diet versus a conventional, high glycemic–load diet on biochemical parameters associated with acne vulgaris: A randomized, investigator-masked, controlled trial. J. Am. Acad. Dermatol. 2007, 57, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Mann, N.; Mäkeläinen, H.; Roper, J.; Braue, A.; Varigos, G. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: A nonrandomized, parallel, controlled feeding trial. Mol. Nutr. Food Res. 2008, 52, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Burris, J.; Shikany, J.M.; Rietkerk, W.; Woolf, K. A low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: A short-duration, 2-week randomized controlled trial. J. Acad. Nutr. Diet. 2018, 118, 1874–1885. [Google Scholar] [CrossRef]
- Smith, R.N.; Braue, A.; Varigos, G.A.; Mann, N.J. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J. Dermatol. Sci. 2008, 50, 41–52. [Google Scholar] [CrossRef]
- Çerman, A.A.; Aktaş, E.; Altunay, İ.K.; Arıcı, J.E.; Tulunay, A.; Ozturk, F.Y. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J. Am. Acad. Dermatol. 2016, 75, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Burris, J.; Rietkerk, W.; Woolf, K. Relationships of self-reported dietary factors and perceived acne severity in a cohort of New York young adults. J. Acad. Nutr. Diet. 2014, 114, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Burris, J.; Rietkerk, W.; Shikany, J.M.; Woolf, K. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J. Acad. Nutr. Diet. 2017, 117, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Penso, L.; Touvier, M.; Deschasaux, M.; Hercberg, S.; Ezzedine, K.; Sbidian, E. Association between adult acne and dietary behaviors: Findings from the NutriNet-Santé Prospective Cohort Study. JAMA Dermatol. 2020, 156, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.N.; Mann, N.J.; Braue, A.; Mäkeläinen, H.; Varigos, G.A. A low-glycemic-load diet improves symptoms in acne vulgaris patients: A randomized controlled trial. Am. J. Clin. Nutr. 2007, 86, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, Y.; Adisen, E.; Ilter, N.; Bideci, A.; Gurler, D.; Celik, B. Dietary glycemic index and glucose, insulin, insulin-like growth factor-I, insulin-like growth factor binding protein 3, and leptin levels in patients with acne. J. Am. Acad. Dermatol. 2007, 57, 819–823. [Google Scholar] [CrossRef]
- Silverberg, N.B. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis 2012, 90, 70–72. [Google Scholar] [PubMed]
- Adebamowo, C.A.; Spiegelman, D.; Berkey, C.S.; Danby, F.W.; Rockett, H.H.; Colditz, G.A.; Willett, W.C.; Holmes, M.D. Milk consumption and acne in adolescent girls. Dermatol. Online J. 2006, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Adebamowo, C.A.; Spiegelman, D.; Berkey, C.S.; Danby, F.W.; Rockett, H.H.; Colditz, G.A.; Willett, W.C.; Holmes, M.D. Milk consumption and acne in teenaged boys. J. Am. Acad. Dermatol. 2008, 58, 787–793. [Google Scholar] [CrossRef]
- Okoro, E.O.; Ogunbiyi, A.O.; George, A.O.; Subulade, M.O. Association of diet with acne vulgaris among adolescents in Ibadan, southwest Nigeria. Int. J. Dermatol. 2016, 55, 982–988. [Google Scholar] [CrossRef]
- Grossi, E.; Cazzaniga, S.; Crotti, S.; Naldi, L.; Di Landro, A.; Ingordo, V.; Cusano, F.; Atzori, L.; Tripodi Cutrì, F.; Musumeci, M. The constellation of dietary factors in adolescent acne: A semantic connectivity map approach. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Karadağ, A.S.; Balta, I.; Saricaoğlu, H.; Kilic, S.; Kelekçi, K.H.; Yildirim, M.; Arica, D.A.; Öztürk, S.; Karaman, G.; Cerman, A.A. The effect of personal, familial, and environmental characteristics on acne vulgaris: A prospective, multicenter, case controlled study. G. Ital. Dermatol. Venereol. Organo Uff. Soc. Ital. Dermatol. Sifilogr. 2017, 154, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sardana, K.; Sharma, R.C.; Sarkar, R. Seasonal variation in acne vulgaris—Myth or reality. J. Dermatol. 2002, 29, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; An, S.J.; Liu, X.L.; Ji, A.L.; Cao, Y.; Xiang, Y.; Ma, X.Y.; Hu, Q.; Yuan, Z.Q.; Li, Y.F.; et al. The Association Between Short-Term Air Pollution Exposure and Post-Adolescent Acne: The Evidence from a Time Series Analysis in Xi’an, China. Clin. Cosmet. Investig. Dermatol. 2021, 14, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Isler, M.F.; Coates, S.J.; Boos, M.D. Climate change, the cutaneous microbiome and skin disease: Implications for a warming world. Int. J. Dermatol. 2023, 62, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Jović, A.; Marinović, B.; Kostović, K.; Čeović, R.; Basta-Juzbašić, A.; Bukvić Mokos, Z. The Impact of Pyschological Stress on Acne. Acta Dermatovenerol. Croat. 2017, 25, 1133–1141. [Google Scholar]
- Kurokawa, I.; Danby, F.W.; Ju, Q.; Wang, X.; Xiang, L.F.; Xia, L.; Chen, W.; Nagy, I.; Picardo, M.; Suh, D.H.; et al. New developments in our understanding of acne pathogenesis and treatment. Exp. Dermatol. 2009, 18, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Linking diet to acne metabolomics, inflammation, and comedogenesis: An update. Clin. Cosmet. Investig. Dermatol. 2015, 8, 371–388. [Google Scholar] [CrossRef]
- Agamia, N.; Abdallah, D.; Sorour, O.; Mourad, B.; Younan, D. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br. J. Dermatol. 2016, 174, 1299–1307. [Google Scholar] [CrossRef]
- Monfrecola, G.; Lembo, S.; Caiazzo, G.; De Vita, V.; Di Caprio, R.; Balato, A.; Fabbrocini, G. Mechanistic target of rapamycin (mTOR) expression is increased in acne patients’ skin. Exp. Dermatol. 2016, 25, 153–155. [Google Scholar] [CrossRef]
- Cottle, D.L.; Kretzschmar, K.; Schweiger, P.J.; Quist, S.R.; Gollnick, H.P.; Natsuga, K.; Aoyagi, S.; Watt, F.M. c-MYC-induced sebaceous gland differentiation is controlled by an androgen receptor/p53 axis. Cell Rep. 2013, 3, 427–441. [Google Scholar] [CrossRef] [PubMed]
- He, T.-C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; Da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, K.; Cottle, D.L.; Schweiger, P.J.; Watt, F.M. The androgen receptor antagonizes Wnt/β-catenin signaling in epidermal stem cells. J. Investig. Dermatol. 2015, 135, 2753–2763. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. FoxO1—The key for the pathogenesis and therapy of acne? JDDG J. Dtsch. Dermatol. Ges. 2010, 8, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, N. Acne vulgaris: New evidence in pathogenesis and future modalities of treatment. J. Dermatol. Treat. 2021, 32, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amitai, D.; Laron, Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, L.R.; Liu, P.; Zhong, J.; Pan, Y.; Angstman, J.; Brand, L.J.; Dehm, S.M.; Huang, H. FOXO1 binds to the TAU5 motif and inhibits constitutively active androgen receptor splice variants. Prostate 2013, 73, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Jeon, S.-M.; Bhaskar, P.T.; Nogueira, V.; Sundararajan, D.; Tonic, I.; Park, Y.; Hay, N. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev. Cell 2010, 18, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yanase, T.; Morinaga, H.; Okabe, T.; Nomura, M.; Daitoku, H.; Fukamizu, A.; Kato, S.; Takayanagi, R.; Nawata, H. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J. Biol. Chem. 2007, 282, 7329–7338. [Google Scholar] [CrossRef]
- Guevara-Aguirre, J.; Balasubramanian, P.; Guevara-Aguirre, M.; Wei, M.; Madia, F.; Cheng, C.-W.; Hwang, D.; Martin-Montalvo, A.; Saavedra, J.; Ingles, S. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 2011, 3, 70ra13. [Google Scholar] [CrossRef]
- Kamei, Y.; Miura, S.; Suganami, T.; Akaike, F.; Kanai, S.; Sugita, S.; Katsumata, A.; Aburatani, H.; Unterman, T.G.; Ezaki, O. Regulation of SREBP1c gene expression in skeletal muscle: Role of retinoid X receptor/liver X receptor and forkhead-O1 transcription factor. Endocrinology 2008, 149, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Klinger, B.; Anin, S.; Silbergeld, A.; Eshet, R.; Laron, Z. Development of hyperandrogenism during treatment with insulin-like growth factor-I (IGF-I) in female patients with Laron syndrome. Clin. Endocrinol. 1998, 48, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Fu, W.; Li, P.; Nicosia, S.V.; Jenster, G.; Zhang, X.; Bai, W. FoxO1 mediates PTEN suppression of androgen receptor N-and C-terminal interactions and coactivator recruitment. Mol. Endocrinol. 2009, 23, 213–225. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Graziene, V.; Fimmel, S.; Zouboulis, C. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br. J. Dermatol. 2009, 160, 345–352. [Google Scholar] [CrossRef]
- Toyoda, M.; Nakamura, M.; Morohashi, M. Neuropeptides and sebaceous glands. Eur. J. Dermatol. 2002, 12, 422–427. [Google Scholar] [PubMed]
- Kutlu, O.; Karadag, A.S.; Wollina, U. Adult acne versus adolescent acne: A narrative review with a focus on epidemiology to treatment. An. Bras. Dermatol. 2023, 98, 75–83. [Google Scholar] [CrossRef]
- Dreno, B.; Bagatin, E.; Blume-Peytavi, U.; Rocha, M.; Gollnick, H. Female type of adult acne: Physiological and psychological considerations and management. J. Dtsch. Dermatol. Ges. 2018, 16, 1185–1194. [Google Scholar] [CrossRef]
- Ramasamy, S.; Barnard, E.; Dawson, T., Jr.; Li, H. The role of the skin microbiota in acne pathophysiology. Br. J. Dermatol. 2019, 181, 691–699. [Google Scholar] [CrossRef]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 5–14. [Google Scholar] [CrossRef]
- Scholz, C.F.; Kilian, M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 4422–4432. [Google Scholar] [CrossRef]
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The skin microbiome: A new actor in inflammatory acne. Am. J. Clin. Dermatol. 2020, 21, 18–24. [Google Scholar] [CrossRef]
- Beylot, C.; Auffret, N.; Poli, F.; Claudel, J.P.; Leccia, M.T.; Del Giudice, P.; Dreno, B. Propionibacterium acnes: An update on its role in the pathogenesis of acne. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, A.R.; Notay, M.; Clark, A.K.; Sivamani, R.K. Skin-gut axis: The relationship between intestinal bacteria and skin health. World J. Dermatol. 2017, 6, 52–58. [Google Scholar] [CrossRef]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential role of the microbiome in acne: A comprehensive review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The gut microbiome as a major regulator of the gut-skin axis. Front. Microbiol. 2018, 9, 382698. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The gut microbiota and healthy aging: A mini-review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.M.; Zhao, H.J.; Guo, D.Y.; Zhu, P.Q.; Zhang, C.L.; Jiang, W. Gut microbiota alterations in moderate to severe acne vulgaris patients. J. Dermatol. 2018, 45, 1166–1171. [Google Scholar] [CrossRef]
- Noureldein, M.H.; Eid, A.A. Gut microbiota and mTOR signaling: Insight on a new pathophysiological interaction. Microb. Pathog. 2018, 118, 98–104. [Google Scholar] [CrossRef]
- Szántó, M.; Dózsa, A.; Antal, D.; Szabó, K.; Kemény, L.; Bai, P. Targeting the gut-skin axis—Probiotics as new tools for skin disorder management? Exp. Dermatol. 2019, 28, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ochoa, M.-T.; Krutzik, S.R.; Takeuchi, O.; Uematsu, S.; Legaspi, A.J.; Brightbill, H.D.; Holland, D.; Cunliffe, W.J.; Akira, S. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J. Immunol. 2002, 169, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, I.; Nakase, K. Recent advances in understanding and managing acne. F1000Research 2020, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Kelhälä, H.-L.; Palatsi, R.; Fyhrquist, N.; Lehtimäki, S.; Väyrynen, J.P.; Kallioinen, M.; Kubin, M.E.; Greco, D.; Tasanen, K.; Alenius, H. IL-17/Th17 pathway is activated in acne lesions. PLoS ONE 2014, 9, e105238. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.; Morris, C.; Cunliffe, W.; Ingham, E. Immunohistochemical investigation of evolving inflammation in lesions of acne vulgaris. Exp. Dermatol. 1998, 7, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Jeremy, A.H.; Holland, D.B.; Roberts, S.G.; Thomson, K.F.; Cunliffe, W.J. Inflammatory events are involved in acne lesion initiation. J. Investig. Dermatol. 2003, 121, 20–27. [Google Scholar] [CrossRef]
- Kistowska, M.; Meier, B.; Proust, T.; Feldmeyer, L.; Cozzio, A.; Kuendig, T.; Contassot, E.; French, L.E. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J. Investig. Dermatol. 2015, 135, 110–118. [Google Scholar] [CrossRef]
- Kang, S.; Cho, S.; Chung, J.H.; Hammerberg, C.; Fisher, G.J.; Voorhees, J.J. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-κB and activator protein-1 in inflammatory acne lesions in vivo. Am. J. Pathol. 2005, 166, 1691–1699. [Google Scholar] [CrossRef]
- Kim, J. Review of the innate immune response in acne vulgaris: Activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology 2005, 211, 193–198. [Google Scholar] [CrossRef]
- Carlavan, I.; Bertino, B.; Rivier, M.; Martel, P.; Bourdes, V.; Motte, M.; Déret, S.; Reiniche, P.; Menigot, C.; Khammari, A. Atrophic scar formation in patients with acne involves long-acting immune responses with plasma cells and alteration of sebaceous glands. Br. J. Dermatol. 2018, 179, 906–917. [Google Scholar] [CrossRef]
- Holland, D.; Jeremy, A.; Roberts, S.; Seukeran, D.; Layton, A.; Cunliffe, W. Inflammation in acne scarring: A comparison of the responses in lesions from patients prone and not prone to scar. Br. J. Dermatol. 2004, 150, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, I.; Layton, A.M.; Ogawa, R. Updated Treatment for Acne: Targeted Therapy Based on Pathogenesis. Dermatol. Ther. 2021, 11, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Im, M.; Kim, S.Y.; Sohn, K.C.; Choi, D.K.; Lee, Y.; Seo, Y.J.; Kim, C.D.; Hwang, Y.L.; Zouboulis, C.C.; Lee, J.H. Epigallocatechin-3-gallate suppresses IGF-I-induced lipogenesis and cytokine expression in SZ95 sebocytes. J. Investig. Dermatol. 2012, 132, 2700–2708. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Moon, S.Y.; Sohn, M.Y.; Lee, W.J. Insulin-like growth factor-1 increases the expression of inflammatory biomarkers and sebum production in cultured sebocytes. Ann. Dermatol. 2017, 29, 20. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.J.; Plymate, S.R.; Rosen, C.J.; Adler, R.A. Testosterone administration increases insulin-like growth factor-I levels in normal men. J. Clin. Endocrinol. Metab. 1993, 77, 776–779. [Google Scholar] [PubMed]
- Mattii, M.; Lovászi, M.; Garzorz, N.; Atenhan, A.; Quaranta, M.; Lauffer, F.; Konstantinow, A.; Küpper, M.; Zouboulis, C.C.; Kemény, L. Sebocytes contribute to skin inflammation by promoting the differentiation of T helper 17 cells. Br. J. Dermatol. 2018, 178, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Aletras, A.J.; Glass, E.; Tsogas, P.; Dionyssopoulos, A.; Adjaye, J.; Fimmel, S.; Gouvousis, P.; Herwig, R.; Lehrach, H. Matrix metalloproteinases of epithelial origin in facial sebum of patients with acne and their regulation by isotretinoin. J. Investig. Dermatol. 2005, 125, 673–684. [Google Scholar] [CrossRef]
- Akamatsu, H.; Horio, T.; Hattori, K. Increased hydrogen peroxide generation by neutrophils from patients with acne inflammation. Int. J. Dermatol. 2003, 42, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Dreno, B.; Kang, S.; Leyden, J.; York, J. Update: Mechanisms of Topical Retinoids in Acne. J. Drugs Dermatol. 2022, 21, 734–740. [Google Scholar] [PubMed]
- Baldwin, H.; Webster, G.; Stein Gold, L.; Callender, V.; Cook-Bolden, F.E.; Guenin, E. 50 Years of Topical Retinoids for Acne: Evolution of Treatment. Am. J. Clin. Dermatol. 2021, 22, 315–327. [Google Scholar] [CrossRef]
- Motamedi, M.; Chehade, A.; Sanghera, R.; Grewal, P. A Clinician’s Guide to Topical Retinoids. J. Cutan. Med. Surg. 2022, 26, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Cosio, T.; Di Prete, M.; Gaziano, R.; Lanna, C.; Orlandi, A.; Di Francesco, P.; Bianchi, L.; Campione, E. Trifarotene: A Current Review and Perspectives in Dermatology. Biomedicines 2021, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Kolli, S.S.; Pecone, D.; Pona, A.; Cline, A.; Feldman, S.R. Topical retinoids in acne vulgaris: A systematic review. Am. J. Clin. Dermatol. 2019, 20, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Adv. Dermatol. Allergol./Postępy Dermatol. Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.; Reyes-Hadsall, S.; Barbieri, J.S.; Mostaghimi, A. New Developments in Topical Acne Therapy. Am. J. Clin. Dermatol. 2022, 23, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; Sugarman, J.L.; Guenin, E.; Harris, S.; Bhatt, V. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris in a preadolescent population. Pediatr. Dermatol. 2019, 36, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Lain, E.; Day, D.; Harper, J.; Guenin, E. Tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: Impact of gender and race on efficacy and safety. J. Drugs Dermatol. JDD 2019, 18, 1128–1138. [Google Scholar] [PubMed]
- Eichenfield, L.F.; Matiz, C.; Funk, A.; Dill, S.W. Study of the efficacy and tolerability of 0.04% tretinoin microsphere gel for preadolescent acne. Pediatrics 2010, 125, e1316–e1323. [Google Scholar] [CrossRef]
- Cunliffe, W.J.; Poncet, M.; Loesche, C.; Verschoore, M. A comparison of the efficacy and tolerability of adapalene 0.1% gel versus tretinoin 0.025% gel in patients with acne vulgaris: A meta-analysis of five randomized trials. Br. J. Dermatol. 1998, 139 (Suppl. S52), 48–56. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ochsendorf, F.R. New developments in acne treatment: Role of combination adapalene–benzoylperoxide. Ther. Clin. Risk Manag. 2016, 12, 1497–1506. [Google Scholar] [CrossRef]
- Tanghetti, E.A.; Werschler, W.P.; Lain, T.; Guenin, E.; Martin, G.; Pillai, R. Tazarotene 0.045% Lotion for Once-Daily Treatment of Moderate-to-Severe Acne Vulgaris: Results from Two Phase 3 Trials. J. Drugs Dermatol. 2020, 19, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Tanghetti, E.A.; Kircik, L.H.; Green, L.J.; Guenin, E.; Harris, S.; Martin, G.; Pillai, R. A Phase 2, Multicenter, Double-Blind, Randomized, Vehicle-Controlled Clinical Study to Compare the Safety and Efficacy of a Novel Tazarotene 0.045% Lotion and Tazarotene 0.1% Cream in the Treatment of Moderate-to-Severe Acne Vulgaris. J. Drugs Dermatol. 2019, 18, 542. [Google Scholar] [PubMed]
- Han, G.; Wu, J.J.; Del Rosso, J.Q. Use of topical tazarotene for the treatment of acne vulgaris in pregnancy: A literature review. J. Clin. Aesthetic Dermatol. 2020, 13, E59. [Google Scholar]
- Gregoriou, S.; Kritsotaki, E.; Katoulis, A.; Rigopoulos, D. Use of tazarotene foam for the treatment of acne vulgaris. Clin. Cosmet. Investig. Dermatol. 2014, 2014, 165–170. [Google Scholar]
- Tamer, F.; Adişen, E. Topical tazarotene use in dermatology: An update. Gazi Med. J. 2020, 31, 86–89. [Google Scholar]
- Webster, G.F.; Berson, D.; Stein, L.F.; Fivenson, D.P.; Tanghetti, E.A.; Ling, M. Efficacy and tolerability of once-daily tazarotene 0.1% gel versus once-daily tretinoin 0.025% gel in the treatment of facial acne vulgaris: A randomized trial. Cutis 2001, 67, 4–9. [Google Scholar] [PubMed]
- Webster, G.F.; Guenther, L.; Poulin, Y.P.; Solomon, B.A.; Loven, K.; Lee, J. A multicenter, double-blind, randomized comparison study of the efficacy and tolerability of once-daily tazarotene 0.1% gel and adapalene 0.1% gel for the treatment of facial acne vulgaris. Cutis 2002, 69, 4–11. [Google Scholar]
- Leyden, J.J.; Shalita, A.; Thiboutot, D.; Washenik, K.; Webster, G. Topical retinoids in inflammatory acne: A retrospective, investigator-blinded, vehicle-controlled, photographic assessment. Clin. Ther. 2005, 27, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Thoreau, E.; Arlabosse, J.M.; Bouix-Peter, C.; Chambon, S.; Chantalat, L.; Daver, S.; Dumais, L.; Duvert, G.; Feret, A.; Ouvry, G.; et al. Structure-based design of Trifarotene (CD5789), a potent and selective RARγ agonist for the treatment of acne. Bioorg Med. Chem. Lett. 2018, 28, 1736–1741. [Google Scholar] [CrossRef]
- Tan, J.; Thiboutot, D.; Popp, G.; Gooderham, M.; Lynde, C.; Del Rosso, J.; Weiss, J.; Blume-Peytavi, U.; Weglovska, J.; Johnson, S.; et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J. Am. Acad. Dermatol. 2019, 80, 1691–1699. [Google Scholar] [CrossRef]
- Tan, J.; Bissonnette, R.; Gratton, D.; Kerrouche, N.; Canosa, J.M. The safety and efficacy of four different fixed combination regimens of adapalene 0.1%/benzoyl peroxide 2.5% gel for the treatment of acne vulgaris: Results from a randomised controlled study. Eur. J. Dermatol. 2018, 28, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Bertolani, M.B.; Rodighiero, E.; Gandolfi, M.; Lotti, T.; Pedrazzi, G.; Puviani, M.; Milani, M.; Feliciani, C.; Satolli, F. Efficacy and tolerability of short contact therapy with tretinoin, clindamycin, and glycolic acid gel in acne: A randomized, controlled, assessor-blinded two-center trial: The MASCOTTE study. Dermatol. Ther. 2021, 34, e14724. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, D.Z.; Sprague, J.; Eichenfield, L.F. Management of Acne Vulgaris: A Review. JAMA 2021, 326, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Tyring, S.K.; Kircik, L.H.; Pariser, D.M.; Guenin, E.; Bhatt, V.; Pillai, R. Novel Tretinoin 0.05% Lotion for the Once-Daily Treatment of Moderate-to-Severe Acne Vulgaris: Assessment of Efficacy and Safety in Patients Aged 9 Years and Older. J. Drugs Dermatol. 2018, 17, 1084–1091. [Google Scholar] [PubMed]
- Mohsin, N.; Hernandez, L.E.; Martin, M.R.; Does, A.V.; Nouri, K. Acne treatment review and future perspectives. Dermatol. Ther. 2022, 35, e15719. [Google Scholar] [CrossRef] [PubMed]
- Kontzias, C.; Zaino, M.; Feldman, S.R. Tretinoin 0.1% and Benzoyl Peroxide 3% Cream for the Treatment of Facial Acne Vulgaris. Ann. Pharmacother. 2023, 57, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Stein Gold, L.; Lain, E.; Del Rosso, J.Q.; Gold, M.; Draelos, Z.D.; Eichenfield, L.F.; Sadick, N.; Werschler, W.P.; Gooderham, M.J.; Lupo, M. Clindamycin phosphate 1.2%/adapalene 0.15%/benzoyl peroxide 3.1% gel for moderate-to-severe acne: Efficacy and safety results from two randomized phase 3 trials. J. Am. Acad. Dermatol. 2023, 89, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Del Rosso, J.Q.; Gold, L.S.; Draelos, Z.; Raoof, T.J.; Hooper, D.; Stuart, I. A prospective, multicenter, randomized, double-blind, vehicle-controlled phase 2 study to evaluate the safety and efficacy of a combination of 3% minocycline and 0.3% adapalene topical foam formulation for the treatment of moderate-to-severe acne. SKIN J. Cutan. Med. 2020, 4, s90. [Google Scholar] [CrossRef]
- Sagransky, M.; Yentzer, B.A.; Feldman, S.R. Benzoyl peroxide: A review of its current use in the treatment of acne vulgaris. Expert. Opin. Pharmacother. 2009, 10, 2555–2562. [Google Scholar] [CrossRef]
- Hegemann, L.; Toso, S.; Kitay, K.; Webster, C. Anti-inflammatory actions of benzoyl peroxide: Effects on the generation of reactive oxygen species by leucocytes and the activity of protein kinase C and calmodulin. Br. J. Dermatol. 1994, 130, 569–575. [Google Scholar] [CrossRef]
- Mohd Nor, N.H.; Aziz, Z. A systematic review of benzoyl peroxide for acne vulgaris. J. Dermatol. Treat. 2013, 24, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.J.; Wortzman, M.; Baldwin, E.K. Antibiotic-resistant Propionibacterium acnes suppressed by a benzoyl peroxide cleanser 6%. Cutis 2008, 82, 417–421. [Google Scholar]
- Kumar, S.; Devi, B.; Goud, V. A comparative study of acne vulgaris with special reference to therapeutic options. IP Indian J. Clin. Exp. Dermatol. 2019, 5, 306–311. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33. [Google Scholar] [CrossRef]
- Thiboutot, D.; Del Rosso, J.Q. Acne Vulgaris and the Epidermal Barrier: Is Acne Vulgaris Associated with Inherent Epidermal Abnormalities that Cause Impairment of Barrier Functions? Do Any Topical Acne Therapies Alter the Structural and/or Functional Integrity of the Epidermal Barrier? J. Clin. Aesthet. Dermatol. 2013, 6, 18–24. [Google Scholar]
- Boonchaya, P.; Rojhirunsakool, S.; Kamanamool, N.; Khunkhet, S.; Yooyongsatit, S.; Udompataikul, M.; Taweechotipatr, M. Minimum contact time of 1.25%, 2.5%, 5%, and 10% benzoyl peroxide for a bactericidal effect against Cutibacterium acnes. Clin. Cosmet. Investig. Dermatol. 2022, 2022, 403–409. [Google Scholar] [CrossRef]
- Leyden, J.J.; Hickman, J.G.; Jarratt, M.T.; Stewart, D.M.; Levy, S.F. The efficacy and safety of a combination benzoyl peroxide/clindamycin topical gel compared with benzoyl peroxide alone and a benzoyl peroxide/erythromycin combination product. J. Cutan. Med. Surg. 2001, 5, 37–42. [Google Scholar] [CrossRef]
- Mosler, E.L.; Leitner, C.; Gouda, M.A.; Carter, B.; Layton, A.M.; KhalafAllah, M.T. Topical antibiotics for acne. Cochrane Database Syst. Rev. 2018, CD012263. [Google Scholar] [CrossRef]
- Raoof, T.J.; Hooper, D.; Moore, A.; Zaiac, M.; Sullivan, T.; Kircik, L.; Lain, E.; Jankicevic, J.; Stuart, I. Efficacy and safety of a novel topical minocycline foam for the treatment of moderate to severe acne vulgaris: A phase 3 study. J. Am. Acad. Dermatol. 2020, 82, 832–837. [Google Scholar] [CrossRef]
- Gold, L.S.; Dhawan, S.; Weiss, J.; Draelos, Z.D.; Ellman, H.; Stuart, I.A. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: Results of 2 randomized, double-blind, phase 3 studies. J. Am. Acad. Dermatol. 2019, 80, 168–177. [Google Scholar] [CrossRef]
- Paik, J. Topical Minocycline Foam 4%: A Review in Acne Vulgaris. Am. J. Clin. Dermatol. 2020, 21, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Stein Gold, L.; Dhawan, S.; Weiss, J.; Draelos, Z.D.; Ellman, H.; Stuart, I. Open-label Extension Study Evaluating Long-term Safety and Efficacy of FMX101 4% Minocycline Foam for Moderate-to-Severe Acne Vulgaris. J. Clin. Aesthet. Dermatol. 2019, 12, 16–23. [Google Scholar]
- Patel, M.; Bowe, W.P.; Heughebaert, C.; Shalita, A.R. The development of antimicrobial resistance due to the antibiotic treatment of acne vulgaris: A review. J. Drugs Dermatol. JDD 2010, 9, 655–664. [Google Scholar] [PubMed]
- Tomić, I.; Miočić, S.; Pepić, I.; Šimić, D.; Filipović-Grčić, J. Efficacy and safety of azelaic acid nanocrystal-loaded in situ hydrogel in the treatment of acne vulgaris. Pharmaceutics 2021, 13, 567. [Google Scholar] [CrossRef] [PubMed]
- Zaenglein, A.L. Acne Vulgaris. N. Engl. J. Med. 2018, 379, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Webster, G. Combination azelaic acid therapy for acne vulgaris. J. Am. Acad. Dermatol. 2000, 43, S47–S50. [Google Scholar] [CrossRef]
- Hashim, P.W.; Chen, T.; Harper, J.C.; Kircik, L.H. The Efficacy and Safety of Azelaic Acid 15% Foam in the Treatment of Facial Acne Vulgaris. J. Drugs Dermatol. JDD 2018, 17, 641–645. [Google Scholar] [PubMed]
- Chauhan, P.N.; Sharma, A.; Rasheed, H.; Mathur, H.; Sharma, P. Treatment Opportunities and Technological Progress Prospective for Acne Vulgaris. Curr. Drug Deliv. 2023, 20, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.; Lewis, J.; McHugh, L.; Pellegrino, A.; Popescu, L. Novel retinoid ester in combination with salicylic acid for the treatment of acne. J. Cosmet. Dermatol. 2016, 15, 36–42. [Google Scholar] [CrossRef]
- Calvisi, L. Efficacy of a combined chemical peel and topical salicylic acid-based gel combination in the treatment of active acne. J. Cosmet. Dermatol. 2021, 20, 2–6. [Google Scholar] [CrossRef]
- Bilal, H.; Xiao, Y.; Khan, M.N.; Chen, J.; Wang, Q.; Zeng, Y.; Lin, X. Stabilization of acne vulgaris-associated microbial dysbiosis with 2% supramolecular salicylic acid. Pharmaceuticals 2023, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Stotland, M.; Shalita, A.R.; Kissling, R.F. Dapsone 5% gel: A review of its efficacy and safety in the treatment of acne vulgaris. Am. J. Clin. Dermatol. 2009, 10, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Beheshti-Mall, L.; Shafaroodi, H.; Jafariazar, Z.; Afshar, M. A novel hydrogel-thickened microemulsion of dapsone for acne treatment: Development, characterization, physicochemical stability and ex vivo permeation studies. Marmara Pharm. J. 2018, 22, 267–276. [Google Scholar] [CrossRef]
- Al Sabaa, H.; Mady, F.M.; Hussein, A.K.; Abdel-Wahab, H.M.; Ragaie, M.H. Dapsone in topical niosomes for treatment of acne vulgaris. Afr. J. Pharm. Pharmacol. 2018, 12, 221–230. [Google Scholar]
- Mohamed, G.N.; Gharib, K.M.; Samir, M.A. Emerging role of dapsone in the management of acne vulgaris. Egypt. J. Hosp. Med. 2022, 87, 1204–1207. [Google Scholar] [CrossRef]
- Lucky, A.W.; Maloney, J.M.; Roberts, J.; Taylor, S.; Jones, T.; Ling, M.; Garrett, S. Dapsone gel 5% for the treatment of acne vulgaris: Safety and efficacy of long-term (1 year) treatment. J. Drugs Dermatol. 2007, 6, 981–987. [Google Scholar] [PubMed]
- Lynde, C.W.; Andriessen, A. Cohort study on the treatment with dapsone 5% gel of mild to moderate inflammatory acne of the face in women. Skinmed 2014, 12, 15–21. [Google Scholar] [PubMed]
- Alexis, A.F.; Burgess, C.; Callender, V.D.; Herzog, J.L.; Roberts, W.E.; Schweiger, E.S.; Stockton, T.C.; Gallagher, C.J. The Efficacy and Safety of Topical Dapsone Gel, 5% for the Treatment of Acne Vulgaris in Adult Females With Skin of Color. J. Drugs Dermatol. 2016, 15, 197–204. [Google Scholar] [PubMed]
- Piette, W.W.; Taylor, S.; Pariser, D.; Jarratt, M.; Sheth, P.; Wilson, D. Hematologic safety of dapsone gel, 5%, for topical treatment of acne vulgaris. Arch. Dermatol. 2008, 144, 1564–1570. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Carter, E.; Maloney, J.M.; Elewski, B.; Poulin, Y.; Lynde, C.; Garrett, S.; United States/Canada Dapsone Gel Study Group. Two randomized studies demonstrate the efficacy and safety of dapsone gel, 5% for the treatment of acne vulgaris. J. Am. Acad. Dermatol. 2007, 56, 439.e1–439.e10. [Google Scholar] [CrossRef]
- Akhavan, A.; Bershad, S. Topical acne drugs: Review of clinical properties, systemic exposure, and safety. Am. J. Clin. Dermatol. 2003, 4, 473–492. [Google Scholar] [CrossRef]
- Pace, W.E. A Benzoyl Peroxide-Sulfur Cream for acne vulgaris. Can. Med. Assoc. J. 1965, 93, 252–254. [Google Scholar]
- Hashem, N.M.; Hosny, A.; Abdelrahman, A.A.; Zakeer, S. Antimicrobial activities encountered by sulfur nanoparticles combating Staphylococcal species harboring sccmecA recovered from acne vulgaris. AIMS Microbiol. 2021, 7, 481–498. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Leyden, J.J.; Thiboutot, D.; Webster, G.F. Antibiotic use in acne vulgaris and rosacea: Clinical considerations and resistance issues of significance to dermatologists. Cutis 2008, 82, 5–12. [Google Scholar]
- Breneman, D.L.; Ariano, M.C. Successful treatment of acne vulgaris in women with a new topical sodium sulfacetamide/sulfur lotion. Int. J. Dermatol. 1993, 32, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, R.D.; Adam, J.E.; Murray, J.J.; Craig, G.E. Benzoyl peroxide and sulfur: Foundation for acne management. Can. Med. Assoc. J. 1966, 95, 28–29. [Google Scholar]
- Gupta, A.K.; Nicol, K. The use of sulfur in dermatology. J. Drugs Dermatol. 2004, 3, 427–431. [Google Scholar] [PubMed]
- Danto, J.L.; Maddin, W.S.; Stewart, W.D.; Nelson, A.J. A controlled trial of benzoyl peroxide and precipitated sulfur cream in acne vulgaris. Appl. Ther. 1966, 8, 624–625. [Google Scholar] [PubMed]
- Del Rosso, J.Q. The use of sodium sulfacetamide 10%-sulfur 5% emollient foam in the treatment of acne vulgaris. J. Clin. Aesthet. Dermatol. 2009, 2, 26–29. [Google Scholar]
- Draelos, Z.D. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J. Drugs Dermatol. 2010, 9, 234–236. [Google Scholar]
- Auffret, N.; Claudel, J.P.; Leccia, M.T.; Ballanger, F.; Dreno, B. Novel and emerging treatment options for acne vulgaris. Eur. J. Dermatol. 2022, 32, 451–458. [Google Scholar] [PubMed]
- Rosette, C.; Agan, F.J.; Mazzetti, A.; Moro, L.; Gerloni, M. Cortexolone 17α-propionate (Clascoterone) Is a Novel Androgen Receptor Antagonist that Inhibits Production of Lipids and Inflammatory Cytokines from Sebocytes In Vitro. J. Drugs Dermatol. 2019, 18, 412–418. [Google Scholar] [PubMed]
- Hebert, A.; Thiboutot, D.; Stein Gold, L.; Cartwright, M.; Gerloni, M.; Fragasso, E.; Mazzetti, A. Efficacy and Safety of Topical Clascoterone Cream, 1%, for Treatment in Patients With Facial Acne: Two Phase 3 Randomized Clinical Trials. JAMA Dermatol. 2020, 156, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, J.S. A New Class of Topical Acne Treatment Addressing the Hormonal Pathogenesis of Acne. JAMA Dermatol. 2020, 156, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Trifu, V.; Tiplica, G.S.; Naumescu, E.; Zalupca, L.; Moro, L.; Celasco, G. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. A pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br. J. Dermatol. 2011, 165, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Zaenglein, A.L.; Thiboutot, D.M. Expert committee recommendations for acne management. Pediatrics 2006, 118, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.; Mamun, S.; Uddin, M.; Khan, R.M.; Hoque, M.; Shikder, M. Oral azithromycin pulse therapy and daily topical adapalene in the treatment of acne vulgaris: An open randomized noncomparative study. Med. Today 2017, 10, 52–56. [Google Scholar] [CrossRef]
- Krakowski, A.C.; Stendardo, S.; Eichenfield, L.F. Practical considerations in acne treatment and the clinical impact of topical combination therapy. Pediatr. Dermatol. 2008, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S.; Tsunoda, T. Twenty cases of allergic contact dermatitis due to benzoyl peroxide in acne patients in Japan. J. Cutan. Immunol. Allergy 2019, 2, 108–112. [Google Scholar] [CrossRef]
- Garg, A.K.; Maddiboyina, B.; Alqarni, M.H.S.; Alam, A.; Aldawsari, H.M.; Rawat, P.; Singh, S.; Kesharwani, P. Solubility enhancement, formulation development and antifungal activity of luliconazole niosomal gel-based system. J. Biomater. Sci. Polym. Ed. 2021, 32, 1009–1023. [Google Scholar] [CrossRef]
- Cebrián, R.; Arévalo, S.; Rubiño, S.; Arias-Santiago, S.; Rojo, M.D.; Montalbán-López, M.; Martínez-Bueno, M.; Valdivia, E.; Maqueda, M. Control of Propionibacterium acnes by natural antimicrobial substances: Role of the bacteriocin AS-48 and lysozyme. Sci. Rep. 2018, 8, 11766. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yin, S.; Xia, Y.; Chen, J.; Ye, C.; Zeng, Q.; Lai, W. Efficacy and safety of 2% supramolecular salicylic acid compared with 5% benzoyl peroxide/0.1% adapalene in the acne treatment: A randomized, split-face, open-label, single-center study. Cutan. Ocul. Toxicol. 2019, 38, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Tzellos, T.; Zampeli, V.; Makrantonaki, E.; Zouboulis, C.C. Treating acne with antibiotic-resistant bacterial colonization. Expert. Opin. Pharmacother. 2011, 12, 1233–1247. [Google Scholar] [CrossRef]
- Poulin, Y.; Sanchez, N.; Bucko, A.; Fowler, J.; Jarratt, M.; Kempers, S.; Kerrouche, N.; Dhuin, J.C.; Kunynetz, R. A 6-month maintenance therapy with adapalene-benzoyl peroxide gel prevents relapse and continuously improves efficacy among patients with severe acne vulgaris: Results of a randomized controlled trial. Br. J. Dermatol. 2011, 164, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, D.M.; Shalita, A.R.; Yamauchi, P.S.; Dawson, C.; Kerrouche, N.; Arsonnaud, S.; Kang, S. Adapalene gel, 0.1%, as maintenance therapy for acne vulgaris: A randomized, controlled, investigator-blind follow-up of a recent combination study. Arch. Dermatol. 2006, 142, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Santer, M.; Burden-Teh, E.; Ravenscroft, J. Managing acne vulgaris: An update. Drug Ther. Bull. 2023, 62, 6–10. [Google Scholar] [CrossRef]
- Dessinioti, C.; Katsambas, A. Antibiotics and Antimicrobial Resistance in Acne: Epidemiological Trends and Clinical Practice Considerations. Yale J. Biol. Med. 2022, 95, 429–443. [Google Scholar] [PubMed]
- Zhanel, G.; Critchley, I.; Lin, L.-Y.; Alvandi, N. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.J.; Del Rosso, J.Q. Oral antibiotic therapy for acne vulgaris: Pharmacokinetic and pharmacodynamic perspectives. J. Clin. Aesthetic Dermatol. 2011, 4, 40. [Google Scholar]
- Leyden, J.J.; Kaidbey, K.; Gans, E. The antimicrobial effects in vivo of minocycline, doxycycline and tetracycline in humans. J. Dermatol. Treat. 1996, 7, 223–225. [Google Scholar] [CrossRef]
- Deeks, E.D. Sarecycline: First Global Approval. Drugs 2019, 79, 325–329. [Google Scholar] [CrossRef]
- Marson, J.W.; Baldwin, H.E. An Overview of Acne Therapy, Part 1: Topical therapy, Oral Antibiotics, Laser and Light Therapy, and Dietary Interventions. Dermatol. Clin. 2019, 37, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.Y.; Charles, J.E.M.; Moore, S. Sarecycline: A narrow spectrum tetracycline for the treatment of moderate-to-severe acne vulgaris. Future Microbiol. 2019, 14, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Green, L.J.; Bruce, S.; Sadick, N.; Tschen, E.; Werschler, P.; Cook-Bolden, F.E.; Dhawan, S.S.; Forsha, D.; Gold, M.H.; et al. Once-Daily Oral Sarecycline 1.5 mg/kg/day Is Effective for Moderate to Severe Acne Vulgaris: Results from Two Identically Designed, Phase 3, Randomized, Double-Blind Clinical Trials. J. Drugs Dermatol. 2018, 17, 987–996. [Google Scholar] [CrossRef]
- Kadayifci, A.; Gulsen, M.; Koruk, M.; Savas, M. Doxycycline-induced pill esophagitis. Dis. Esophagus 2004, 17, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Bjellerup, M.; Ljunggren, B. Double blind cross-over studies on phototoxicity to three tetracycline derivatives in human volunteers. Photo-dermatology 1987, 4, 281–287. [Google Scholar]
- Pariser, D.M.; Green, L.J.; Lain, E.L.; Schmitz, C.; Chinigo, A.S.; McNamee, B.; Berk, D.R. Safety and tolerability of sarecycline for the treatment of acne vulgaris: Results from a phase III, multicenter, open-label study and a phase I phototoxicity study. J. Clin. Aesthetic Dermatol. 2019, 12, E53. [Google Scholar] [CrossRef]
- Nast, A.; Dréno, B.; Bettoli, V.; Bukvic Mokos, Z.; Degitz, K.; Dressler, C.; Finlay, A.Y.; Haedersdal, M.; Lambert, J.; Layton, A. European evidence-based (S3) guideline for the treatment of acne–update 2016–short version. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1261–1268. [Google Scholar] [CrossRef]
- Asai, Y.; Baibergenova, A.; Dutil, M.; Humphrey, S.; Hull, P.; Lynde, C.; Poulin, Y.; Shear, N.H.; Tan, J.; Toole, J. Management of acne: Canadian clinical practice guideline. Cmaj 2016, 188, 118–126. [Google Scholar] [CrossRef]
- Xu, J.; Mavranezouli, I.; Kuznetsov, L.; Murphy, M.S.; Healy, E. Management of acne vulgaris: Summary of NICE guidance. BMJ 2021, 374, n1800. [Google Scholar] [CrossRef]
- Fox, L.; Csongradi, C.; Aucamp, M.; Du Plessis, J.; Gerber, M. Treatment modalities for acne. Molecules 2016, 21, 1063. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, L.; Muller, I.; Layton, A.M.; Rucinski, G.; Venkatess, V.; Sufraz, A.; Dove, S.; Lown, M.; Stuart, B.; Francis, N.; et al. Systematic review of clinical practice guidelines for acne vulgaris published between January 2017 and July 2021. Skin Health Dis. 2023, 3, e240. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, D.M.; Dréno, B.; Abanmi, A.; Alexis, A.F.; Araviiskaia, E.; Cabal, M.I.B.; Bettoli, V.; Casintahan, F.; Chow, S.; da Costa, A. Practical management of acne for clinicians: An international consensus from the Global Alliance to Improve Outcomes in Acne. J. Am. Acad. Dermatol. 2018, 78, S1–S23.e21. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Efthimiou, J.; Dréno, B. Systematic review of antibiotic resistance in acne: An increasing topical and oral threat. Lancet Infect. Dis. 2016, 16, e23–e33. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Bienenfeld, A.; Orlow, S.J.; Nagler, A.R. The use of hormonal antiandrogen therapy in female patients with acne: A 10-year retrospective study. Am. J. Clin. Dermatol. 2018, 19, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Elsaie, M.L. Hormonal treatment of acne vulgaris: An update. Clin. Cosmet. Investig. Dermatol. 2016, 9, 241–248. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Krakowski, A.C.; Piggott, C.; Del Rosso, J.; Baldwin, H.; Friedlander, S.F.; Levy, M.; Lucky, A.; Mancini, A.J.; Orlow, S.J. Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics 2013, 131, S163–S186. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, Y.; Eijkemans, M.; Coelingh Bennink, H.; Blankenstein, M.; Fauser, B. The effect of combined oral contraception on testosterone levels in healthy women: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 76–105. [Google Scholar] [CrossRef] [PubMed]
- Koo, E.B.; Petersen, T.D.; Kimball, A.B. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J. Am. Acad. Dermatol. 2014, 71, 450–459. [Google Scholar] [CrossRef]
- Gabbard, R.D.; Hoopes, R.R.; Kemp, M.G. Spironolactone and XPB: An old drug with a new molecular target. Biomolecules 2020, 10, 756. [Google Scholar] [CrossRef]
- Kim, G.K.; Del Rosso, J.Q. Oral Spironolactone in Post-teenage Female Patients with Acne Vulgaris: Practical Considerations for the Clinician Based on Current Data and Clinical Experience. J. Clin. Aesthet. Dermatol. 2012, 5, 37–50. [Google Scholar] [PubMed]
- Charny, J.W.; Choi, J.K.; James, W.D. Spironolactone for the treatment of acne in women, a retrospective study of 110 patients. Int. J. Women’s Dermatol. 2017, 3, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Plovanich, M.; Weng, Q.Y.; Mostaghimi, A. Low Usefulness of Potassium Monitoring Among Healthy Young Women Taking Spironolactone for Acne. JAMA Dermatol. 2015, 151, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Liszewski, W.; Boull, C. Lack of evidence for feminization of males exposed to spironolactone in utero: A systematic review. J. Am. Acad. Dermatol. 2019, 80, 1147–1148. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.M.; Eady, E.A.; Whitehouse, H.; Del Rosso, J.Q.; Fedorowicz, Z.; van Zuuren, E.J. Oral Spironolactone for Acne Vulgaris in Adult Females: A Hybrid Systematic Review. Am. J. Clin. Dermatol. 2017, 18, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Ebede, T.L.; Arch, E.L.; Berson, D. Hormonal treatment of acne in women. J. Clin. Aesthetic Dermatol. 2009, 2, 16. [Google Scholar]
- Dagnelie, M.A.; Poinas, A.; Dréno, B. What is new in adult acne for the last 2 years: Focus on acne pathophysiology and treatments. Int. J. Dermatol. 2022, 61, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Scott-Emuakpor, R.; Vuthaluru, K.; Nagre, A.; Jawed, I.; Patel, P.A.; Sidhu, H.K. Role of Oral Retinoids in Treatment of Acne Vulgaris With a Bioinformatics-Based Perspective of Personalized Medicine. Cureus 2023, 15, e38019. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, R.; Meng, T.; Zhang, F.; Li, J.; Jin, Y.; Lee, J.; Zhu, M.; Jiang, J. Stem cell membrane-coated isotretinoin for acne treatment. J. Nanobiotechnol. 2020, 18, 106. [Google Scholar] [CrossRef]
- Landis, M.N. Optimizing isotretinoin treatment of acne: Update on current recommendations for monitoring, dosing, safety, adverse effects, compliance, and outcomes. Am. J. Clin. Dermatol. 2020, 21, 411–419. [Google Scholar] [CrossRef]
- Bagatin, E.; Costa, C.S. The use of isotretinoin for acne–an update on optimal dosing, surveillance, and adverse effects. Expert. Rev. Clin. Pharmacol. 2020, 13, 885–897. [Google Scholar] [CrossRef]
- Tan, J.; Knezevic, S.; Boyal, S.; Waterman, B.; Janik, T. Evaluation of evidence for acne remission with oral isotretinoin cumulative dosing of 120–150 mg/kg. J. Cutan. Med. Surg. 2016, 20, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, D.; Dréno, B.; Sanders, V.; Rueda, M.J.; Gollnick, H. Changes in the management of acne: 2009–2019. J. Am. Acad. Dermatol. 2020, 82, 1268–1269. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, M. Making sense of the effects of the cumulative dose of isotretinoin in acne vulgaris. Int. J. Dermatol. 2016, 55, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Fallah, H.; Rademaker, M. Isotretinoin in the management of acne vulgaris: Practical prescribing. Int. J. Dermatol. 2021, 60, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Sladden, M.J.; Harman, K.E. What is the chance of a normal pregnancy in a woman whose fetus has been exposed to isotretinoin? Arch. Dermatol. 2007, 143, 1187–1188. [Google Scholar] [CrossRef] [PubMed]
- Hodgkiss-Harlow, C.J.; Eichenfield, L.F.; Dohil, M.A. Effective monitoring of isotretinoin safety in a pediatric dermatology population: A novel “patient symptom survey” approach. J. Am. Acad. Dermatol. 2011, 65, 517–524. [Google Scholar] [CrossRef]
- Kaymak, Y. Creatine phosphokinase values during isotretinoin treatment for acne. Int. J. Dermatol. 2008, 47, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, M.; Bettoli, V.; Forconi, R.; Pacetti, L.; Farnetani, F.; Corazza, M.; Pellacani, G. Creatine Phosphokinase Values during Low Starting Dose Isotretinoin Therapy. Skin Appendage Disord. 2020, 6, 142–146. [Google Scholar] [CrossRef]
- Busetti, B.M.; Azulay, D.R.; Aguinaga, F.; Cordova, E.O. Evaluation of CPK levels during acne treatment with oral isotretinoin. An. Bras. Dermatol. 2021, 96, 626–627. [Google Scholar] [CrossRef]
- Rivillas, J.A.; Santos Andrade, V.A.; Hormaza-Jaramillo, A.A. Myositis Induced by Isotretinoin: A Case Report and Literature Review. Am. J. Case Rep. 2020, 21, e917801. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.J.; Lucking, S.; Miller, J.J.; Kirby, J.S.; Thiboutot, D.M.; Zaenglein, A.L. Standardized laboratory monitoring with use of isotretinoin in acne. J. Am. Acad. Dermatol. 2016, 75, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Zane, L.T.; Leyden, W.A.; Marqueling, A.L.; Manos, M.M. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch. Dermatol. 2006, 142, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Opel, D.; Kramer, O.; Chevalier, M.; Bigby, M.; Albrecht, J. Not every patient needs a triglyceride check, but all can get pancreatitis: A systematic review and clinical characterization of isotretinoin-associated pancreatitis. Br. J. Dermatol. 2017, 177, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, J.S.; Shin, D.B.; Wang, S.; Margolis, D.J.; Takeshita, J. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J. Am. Acad. Dermatol. 2020, 82, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.; Tkachenko, E.; Sharma, P.; Barbieri, J.S.; Mostaghimi, A. Psychiatric Adverse Events in Patients Taking Isotretinoin as Reported in a Food and Drug Administration Database From 1997 to 2017. JAMA Dermatol. 2019, 155, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Droitcourt, C.; Nowak, E.; Rault, C.; Happe, A.; Le Nautout, B.; Kerbrat, S.; Balusson, F.; Poizeau, F.; Travers, D.; Sapori, J.M.; et al. Risk of suicide attempt associated with isotretinoin: A nationwide cohort and nested case-time-control study. Int. J. Epidemiol. 2019, 48, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Etminan, M.; Bird, S.T.; Delaney, J.A.; Bressler, B.; Brophy, J.M. Isotretinoin and risk for inflammatory bowel disease: A nested case-control study and meta-analysis of published and unpublished data. JAMA Dermatol. 2013, 149, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jamal, M.M.; Nguyen, E.T.; Bechtold, M.L.; Nguyen, D.L. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 210–216. [Google Scholar] [CrossRef]
- Margolis, D.J.; Fanelli, M.; Hoffstad, O.; Lewis, J.D. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am. J. Gastroenterol. 2010, 105, 2610–2616. [Google Scholar] [CrossRef]
- Barbieri, J.S. Isotretinoin and risk of inflammatory bowel disease: More data to support lack of meaningful risk. J. Am. Acad. Dermatol. 2021, 84, 228–229. [Google Scholar] [CrossRef] [PubMed]
- Scheman, A.J. Nodulocystic acne and hidradenitis suppurativa treated with acitretin: A case report. Cutis 2002, 69, 287–288. [Google Scholar] [PubMed]
- Pilkington, T.; Brogden, R.N. Acitretin. A review of its pharmacology and therapeutic use. Drugs 1992, 43, 597–627. [Google Scholar] [CrossRef] [PubMed]
- Handler, M.Z.; Bloom, B.S.; Goldberg, D.J. Energy-based devices in treatment of acne vulgaris. Dermatol. Surg. 2016, 42, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, C.; Ge, S.; Chen, Z.; Lu, L. Efficacy of photodynamic therapy for the treatment of inflammatory acne vulgaris: A systematic review and meta-analysis. J. Cosmet. Dermatol. 2020, 19, 10–21. [Google Scholar] [CrossRef]
- Sadick, N.S.; Cardona, A. Laser treatment for facial acne scars: A review. J. Cosmet. Laser Ther. 2018, 20, 424–435. [Google Scholar] [CrossRef]
- Posadzki, P.; Car, J. Light therapies for acne. JAMA Dermatol. 2018, 154, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Mavranezouli, I.; Daly, C.H.; Welton, N.J.; Deshpande, S.; Berg, L.; Bromham, N.; Arnold, S.; Phillippo, D.M.; Wilcock, J.; Xu, J. A systematic review and network meta-analysis of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Br. J. Dermatol. 2022, 187, 639–649. [Google Scholar] [CrossRef]
- Choi, Y.; Suh, H.; Yoon, M.; Min, S.; Lee, D.; Suh, D. Intense pulsed light vs. pulsed-dye laser in the treatment of facial acne: A randomized split-face trial. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 773–780. [Google Scholar] [CrossRef]
- Seaton, E.; Charakida, A.; Mouser, P.; Grace, I.; Clement, R.; Chu, A. Pulsed-dye laser treatment for inflammatory acne vulgaris: Randomised controlled trial. Lancet 2003, 362, 1347–1352. [Google Scholar] [CrossRef]
- Papageorgiou, P.; Katsambas, A.; Chu, A. Phototherapy with blue (415 nm) and red (660 nm) light in the treatment of acne vulgaris. Br. J. Dermatol. 2000, 142, 973–978. [Google Scholar] [CrossRef]
- Lim, W.; Lee, S.; Kim, I.; Chung, M.; Kim, M.; Lim, H.; Park, J.; Kim, O.; Choi, H. The anti-inflammatory mechanism of 635 nm light-emitting-diode irradiation compared with existing COX inhibitors. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 2007, 39, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Sami, N.A.; Attia, A.T.; Badawi, A.M. Phototherapy in the treatment of acne vulgaris. J. Drugs Dermatol. JDD 2008, 7, 627–632. [Google Scholar] [PubMed]
- Li, J.; Li, J.; Zhang, L.; Liu, X.; Cao, Y.; Wang, P.; Wang, X. Comparison of red light and blue light therapies for mild-to-moderate acne vulgaris: A randomized controlled clinical study. Photodermatol. Photoimmunol. Photomed. 2022, 38, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.E.; Ahn, S.J.; Rhee, D.Y.; Choi, J.H.; Moon, K.C.; Suh, H.S.; Soyun-Cho, M. Treatment of facial acne papules and pustules in Korean patients using an intense pulsed light device equipped with a 530-to 750-nm filter. Dermatol. Surg. 2007, 33, 676–679. [Google Scholar]
- Barakat, M.T.; Moftah, N.H.; El Khayyat, M.A.; Abdelhakim, Z.A. Significant reduction of inflammation and sebaceous glands size in acne vulgaris lesions after intense pulsed light treatment. Dermatol. Ther. 2017, 30, e12418. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Porter, R.; Gonzalez, M. Intense pulsed light may improve inflammatory acne through TNF-α down-regulation. J. Cosmet. Laser Ther. 2014, 16, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Uebelhoer, N.S.; Bogle, M.A.; Dover, J.S.; Arndt, K.A.; Rohrer, T.E. Comparison of stacked pulses versus double-pass treatments of facial acne with a 1450-nm laser. Dermatol. Surg. 2007, 33, 552–559. [Google Scholar] [PubMed]
- Kwon, H.H.; Choi, S.C.; Jung, J.Y.; Bae, Y.I.; Park, G.H. Comparison of novel dual mode vs conventional single pass of a 1450-nm diode laser in the treatment of acne vulgaris for Korean patients: A 20-week prospective, randomized, split-face study. J. Cosmet. Dermatol. 2018, 17, 1063–1068. [Google Scholar] [CrossRef]
- De Vries, F.; Meulendijks, A.; Driessen, R.; van Dooren, A.; Tjin, E.; van de Kerkhof, P. The efficacy and safety of non-pharmacological therapies for the treatment of acne vulgaris: A systematic review and best-evidence synthesis. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1195–1203. [Google Scholar] [CrossRef]
- Scopelliti, M.G.; Kothare, A.; Karavitis, M. A novel 1726-nm laser system for safe and effective treatment of acne vulgaris. Lasers Med. Sci. 2022, 37, 3639–3647. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.; Vecerek, N.; Elbuluk, N. Targeting Inflammation in Acne: Current Treatments and Future Prospects. Am. J. Clin. Dermatol. 2023, 24, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Paithankar, D.Y.; Sakamoto, F.H.; Farinelli, W.A.; Kositratna, G.; Blomgren, R.D.; Meyer, T.J.; Faupel, L.J.; Kauvar, A.N.; Lloyd, J.R.; Cheung, W.L. Acne treatment based on selective photothermolysis of sebaceous follicles with topically delivered light-absorbing gold microparticles. J. Investig. Dermatol. 2015, 135, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Han, H.S.; Hong, J.Y.; Seo, S.J.; Lee, S.J. Gold nanoshell-mediated photothermal therapy for acne vulgaris. Dermatol. Ther. 2020, 33, e13189. [Google Scholar] [CrossRef] [PubMed]
- Artounian, K.; Bundogji, N.; Hoss, E.; Boen, M. Applications of Gold and Silver Nanoparticles in the Treatment of Acne Vulgaris: A Systematic Review. J. Drugs Dermatol. JDD 2021, 20, 666–670. [Google Scholar] [PubMed]
- Fuchs, C.S.K.; Bay, C.; Adatto, M.; Lomholt, H.; Haedersdal, M. Acne treatment with light absorbing gold microparticles and optical pulses: An open-label European multi-centered study in moderate to moderately severe acne vulgaris patients. Lasers Surg. Med. 2019, 51, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Park, T.J.; Jeong, J.Y.; Kim, K.E.; Park, J.H.; Lee, S.J.; Kim, H.J.; Ryu, H.J. Photothermal therapy using gold nanoparticles for acne in Asian patients: A preliminary study. Dermatol. Ther. 2021, 34, e14918. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lee, S.J.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Zouboulis, C.C.; Han, S.-H.; Ma, K.-H.; Jang, J.-K.; Lee, J.Y. Repurposing auranofin, an anti-rheumatic gold compound, to treat acne vulgaris by targeting the NLRP3 inflammasome. Biomol. Ther. 2020, 28, 437. [Google Scholar] [CrossRef]
- Kempiak, S.J.; Uebelhoer, N. Superficial chemical peels and microdermabrasion for acne vulgaris. Semin. Cutan. Med. Surg. 2008, 27, 212–220. [Google Scholar] [CrossRef]
- Kessler, E.; Flanagan, K.; Chia, C.; Rogers, C.; Glaser, D.A. Comparison of alpha- and beta-hydroxy acid chemical peels in the treatment of mild to moderately severe facial acne vulgaris. Dermatol. Surg. 2008, 34, 45–50; discussion 51. [Google Scholar] [CrossRef]
- Lee, S.J.; Goo, J.W.; Shin, J.; Chung, W.S.; Kang, J.M.; Kim, Y.K.; Cho, S.B. Use of fractionated microneedle radiofrequency for the treatment of inflammatory acne vulgaris in 18 Korean patients. Dermatol. Surg. 2012, 38, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Lee, K.H.; Sim, H.J.; Suh, K.S.; Jang, M.S. Treatment of acne vulgaris with fractional radiofrequency microneedling. J. Dermatol. 2014, 41, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.I.; Kim, J.; Jung, J.Y.; Lee, W.J.; Lee, J.H. Safety of combined fractional microneedle radiofrequency and CO2 as an early intervention for inflammatory acne and scarring treated with concomitant isotretinoin. Dermatol. Surg. 2020, 46, e71–e77. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, M.; Sun, Y.; Jin, Y.; Lu, C.; Pan, X.; Quan, G.; Wu, C. Microneedle-mediated transdermal drug delivery for treating diverse skin diseases. Acta Biomater. 2021, 121, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jongudomsombat, T.; Lee, Y.I.; Kim, J.; Oh, S.H.; Hong, J.W.; Lee, J.H. Combined use of energy-based interventions with low-dose isotretinoin for the treatment of inflammatory acne: An retrospective cohort analysis. J. Cosmet. Dermatol. 2022, 21, 4383–4391. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.M.; Rasmussen, J.E. Intralesional corticosteroids in the treatment of nodulocystic acne. Arch. Dermatol. 1983, 119, 480–481. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; John, S.M.; Schmitz, G. Over-stimulation of insulin/IGF-1 signaling by western diet may promote diseases of civilization: Lessons learnt from laron syndrome. Nutr. Metab. 2011, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, C.; Mølgaard, C.; Dalum, C.; Vaag, A.; Michaelsen, K.F. Differential effects of casein versus whey on fasting plasma levels of insulin, IGF-1 and IGF-1/IGFBP-3: Results from a randomized 7-day supplementation study in prepubertal boys. Eur. J. Clin. Nutr. 2009, 63, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Berra, B.; Rizzo, A.M. Glycemic index, glycemic load: New evidence for a link with acne. J. Am. Coll. Nutr. 2009, 28, 450S–454S. [Google Scholar] [CrossRef]
- Hoyt, G.; Hickey, M.S.; Cordain, L. Dissociation of the glycaemic and insulinaemic responses to whole and skimmed milk. Br. J. Nutr. 2005, 93, 175–177. [Google Scholar] [CrossRef]
- Melnik, B. Dietary intervention in acne: Attenuation of increased mTORC1 signaling promoted by Western diet. Derm.-Endocrinol. 2012, 4, 20–32. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, H.; Zhou, J.; Mou, Y.; Wang, G.; Xiong, X. Patients with acne vulgaris have a distinct gut microbiota in comparison with healthy controls. Acta Derm. Venereol. 2018, 98, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Kwon, H.H.; Hong, J.S.; Yoon, J.Y.; Park, M.S.; Jang, M.Y.; Suh, D.H. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: A randomised, double-blind, controlled trial. Acta Derm. Venereol. 2014, 94, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Makhlouf, Z.; Khan, N.A. The increasing importance of the gut microbiome in acne vulgaris. Folia Microbiol. 2022, 67, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.; Szmurło, A.; Sińska, B. Significance of diet in treated and untreated acne vulgaris. Adv. Dermatol. Allergol. Postępy Dermatol. Alergol. 2016, 33, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Rani, K.; Datt, C. Molecular link between dietary fibre, gut microbiota and health. Mol. Biol. Rep. 2020, 47, 6229–6237. [Google Scholar] [CrossRef] [PubMed]
- Yildizgören, M.T.; Togral, A.K. Preliminary evidence for vitamin D deficiency in nodulocystic acne. Derm.-Endocrinol. 2014, 6, e983687. [Google Scholar] [CrossRef] [PubMed]
- Alhetheli, G.; Elneam, A.I.A.; Alsenaid, A.; Al-Dhubaibi, M. Vitamin D levels in patients with and without acne and its relation to acne severity: A case-control study. Clin. Cosmet. Investig. Dermatol. 2020, 2020, 759–765. [Google Scholar] [CrossRef]
- Lee, W.J.; Choi, Y.H.; Sohn, M.Y.; Lee, S.-J. Expression of inflammatory biomarkers from cultured sebocytes was influenced by treatment with vitamin D. Indian J. Dermatol. 2013, 58, 327. [Google Scholar] [CrossRef]
- Al-Taiar, A.; AlKhabbaz, M.; Rahman, A.; Al-Sabah, R.; Shaban, L.; Akhtar, S. Plasma 25-hydroxy vitamin D is not associated with acne vulgaris. Nutrients 2018, 10, 1525. [Google Scholar] [CrossRef]
- Stewart, T.J.; Bazergy, C. Hormonal and dietary factors in acne vulgaris versus controls. Derm.-Endocrinol. 2018, 10, e1442160. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.; Prietl, B.; Tauschmann, M.; Mautner, S.I.; Kump, P.K.; Treiber, G.; Wurm, P.; Gorkiewicz, G.; Högenauer, C.; Pieber, T.R. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur. J. Nutr. 2016, 55, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Rostami Mogaddam, M.; Safavi Ardabili, N.; Maleki, N.; Soflaee, M. Correlation between the severity and type of acne lesions with serum zinc levels in patients with acne vulgaris. BioMed Res. Int. 2014, 2014, 474108. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, J.; Eber, A.E.; Perper, M.; Nascimento, V.M.; Nouri, K.; Keri, J.E. The role of zinc in the treatment of acne: A review of the literature. Dermatol. Ther. 2018, 31, e12576. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Hadsall, S.; Ju, T.; Keri, J.E. Use of Oral Supplements and Topical Adjuvants for Isotretinoin-Associated Side Effects: A Narrative Review. Skin Appendage Disord. 2023, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aksac, S.E.; Bilgili, S.G.; Yavuz, G.O.; Yavuz, I.H.; Aksac, M.; Karadag, A.S. Evaluation of biophysical skin parameters and hair changes in patients with acne vulgaris treated with isotretinoin, and the effect of biotin use on these parameters. Int. J. Dermatol. 2021, 60, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Ewurum, A.; Alur, A.A.; Glenn, M.; Schnepf, A.; Borchman, D. Hyaluronic acid-lipid binding. BMC Chem. 2021, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The endocannabinoid system: A potential target for the treatment of various diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid Signaling in the Skin: Therapeutic Potential of the “C (ut) annabinoid” System. Molecules 2019, 24, 918. [Google Scholar] [CrossRef]
- Oláh, A.; Tóth, B.I.; Borbíró, I.; Sugawara, K.; Szöllõsi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J. Clin. Investig. 2014, 124, 3713–3724. [Google Scholar] [CrossRef]
- Mounessa, J.S.; Siegel, J.A.; Dunnick, C.A.; Dellavalle, R.P. The role of cannabinoids in dermatology. J. Am. Acad. Dermatol. 2017, 77, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.H.; Friedman, A. The therapeutic potential of cannabinoids in dermatology. Skin Ther. Lett. 2018, 23, 1–5. [Google Scholar]
- Eagleston, L.R.; Kalani, N.K.; Patel, R.R.; Flaten, H.K.; Dunnick, C.A.; Dellavalle, R.P. Cannabinoids in dermatology: A scoping review. Dermatol. Online J. 2018, 24, 1. [Google Scholar] [CrossRef]
- Sheriff, T.; Lin, M.J.; Dubin, D.; Khorasani, H. The potential role of cannabinoids in dermatology. J. Dermatol. Treat. 2020, 31, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Sand, F.L.; Thomsen, S.F. Adalimumab for the treatment of refractory acne conglobata. JAMA Dermatol. 2013, 149, 1306–1307. [Google Scholar] [CrossRef] [PubMed]
- Yiu, Z.; Madan, V.; Griffiths, C. Acne conglobata and adalimumab: Use of tumour necrosis factor-α antagonists in treatment-resistant acne conglobata, and review of the literature. Clin. Exp. Dermatol. 2015, 40, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Flora, A.; Holland, R.; Smith, A.; Frew, J.W. Rapid and sustained remission of synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome with IL-23p19 antagonist (risankizumab). JAAD Case Rep. 2021, 14, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhuo, N.; Li, J. Off-label Use of Secukinumab: A Potential Therapeutic Option for SAPHO Syndrome. J. Rheumatol. 2022, 49, 656. [Google Scholar] [CrossRef]
- Thiboutot, D.M.; Craft, N.; Rissmann, R.; Gatlik, E.; Souquières, M.; Jones, J.; Loesche, C. Anti-IL-17A blockade did not significantly reduce inflammatory lesions in a placebo-controlled pilot study in adult patients with moderate to severe acne. J. Dermatol. Treat. 2023, 34, 2138691. [Google Scholar] [CrossRef]
- Paetzold, B.; Willis, J.R.; Pereira de Lima, J.; Knödlseder, N.; Brüggemann, H.; Quist, S.R.; Gabaldón, T.; Güell, M. Skin microbiome modulation induced by probiotic solutions. Microbiome 2019, 7, 95. [Google Scholar] [CrossRef]
- Goodarzi, A.; Mozafarpoor, S.; Bodaghabadi, M.; Mohamadi, M. The potential of probiotics for treating acne vulgaris: A review of literature on acne and microbiota. Dermatol. Ther. 2020, 33, e13279. [Google Scholar] [CrossRef] [PubMed]
- Gueniche, A.; Benyacoub, J.; Philippe, D.; Bastien, P.; Kusy, N.; Breton, L.; Blum, S.; Castiel-Higounenc, I. Lactobacillus paracasei CNCM I-2116 (ST11) inhibits substance P-induced skin inflammation and accelerates skin barrier function recovery in vitro. Eur. J. Dermatol. 2010, 20, 731–737. [Google Scholar] [PubMed]
- Cosseau, C.; Devine, D.A.; Dullaghan, E.; Gardy, J.L.; Chikatamarla, A.; Gellatly, S.; Yu, L.L.; Pistolic, J.; Falsafi, R.; Tagg, J.; et al. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect. Immun. 2008, 76, 4163–4175. [Google Scholar] [CrossRef] [PubMed]
- Bowe, W.P.; Filip, J.C.; DiRienzo, J.M.; Volgina, A.; Margolis, D.J. Inhibition of propionibacterium acnes by bacteriocin-like inhibitory substances (BLIS) produced by Streptococcus salivarius. J. Drugs Dermatol. 2006, 5, 868–870. [Google Scholar] [PubMed]
- Oh, S.; Kim, S.H.; Ko, Y.; Sim, J.H.; Kim, K.S.; Lee, S.H.; Park, S.; Kim, Y.J. Effect of bacteriocin produced by Lactococcus sp. HY 449 on skin-inflammatory bacteria. Food Chem. Toxicol. 2006, 44, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.S.; Seo, J.G.; Lee, G.S.; Kim, J.H.; Kim, S.Y.; Han, Y.W.; Kang, H.; Kim, H.O.; Rhee, J.H.; Chung, M.J.; et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J. Microbiol. 2009, 47, 101–109. [Google Scholar] [CrossRef]
- Muizzuddin, N.; Maher, W.; Sullivan, M.; Schnittger, S.; Mammone, T. Physiological effect of a probiotic on skin. J. Cosmet. Sci. 2012, 63, 385–395. [Google Scholar]
- Rahmayani, T.; Putra, I.B.; Jusuf, N.K. The Effect of Oral Probiotic on the Interleukin-10 Serum Levels of Acne Vulgaris. Open Access Maced. J. Med. Sci. 2019, 7, 3249–3252. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.W.; Tse, J.E.; Guiha, I.; Rao, J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J. Cutan. Med. Surg. 2013, 17, 114–122. [Google Scholar] [CrossRef]
- Kim, J.; Ko, Y.; Park, Y.K.; Kim, N.I.; Ha, W.K.; Cho, Y. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition 2010, 26, 902–909. [Google Scholar] [CrossRef]
- Rinaldi, F.; Marotta, L.; Mascolo, A.; Amoruso, A.; Pane, M.; Giuliani, G.; Pinto, D. Facial Acne: A Randomized, Double-Blind, Placebo-Controlled Study on the Clinical Efficacy of a Symbiotic Dietary Supplement. Dermatol. Ther. 2022, 12, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Fabbrocini, G.; Bertona, M.; Picazo, Ó.; Pareja-Galeano, H.; Monfrecola, G.; Emanuele, E. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef. Microbes 2016, 7, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.F.; Nakatsuji, T.; Zhu, W.; Gallo, R.L.; Huang, C.M. Passive immunoprotection targeting a secreted CAMP factor of Propionibacterium acnes as a novel immunotherapeutic for acne vulgaris. Vaccine 2011, 29, 3230–3238. [Google Scholar] [CrossRef]
- Lam, H.Y.P.; Lai, M.J.; Chen, T.Y.; Wu, W.J.; Peng, S.Y.; Chang, K.C. Therapeutic Effect of a Newly Isolated Lytic Bacteriophage against Multi-Drug-Resistant Cutibacterium acnes Infection in Mice. Int. J. Mol. Sci. 2021, 22, 7031. [Google Scholar] [CrossRef] [PubMed]
- Van Steensel, M.A.M.; Goh, B.C. Cutibacterium acnes: Much ado about maybe nothing much. Exp. Dermatol. 2021, 30, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Clemens, L.E.; Jaynes, J.; Lim, E.; Kolar, S.S.; Reins, R.Y.; Baidouri, H.; Hanlon, S.; McDermott, A.M.; Woodburn, K.W. Designed Host Defense Peptides for the Treatment of Bacterial Keratitis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6273–6281. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, K.W.; Jaynes, J.M.; Clemens, L.E. Evaluation of the Antimicrobial Peptide, RP557, for the Broad-Spectrum Treatment of Wound Pathogens and Biofilm. Front. Microbiol. 2019, 10, 1688. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, K.W.; Clemens, L.E.; Jaynes, J.; Joubert, L.M.; Botha, A.; Nazik, H.; Stevens, D.A. Designed Antimicrobial Peptides for Recurrent Vulvovaginal Candidiasis Treatment. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef]
- Woodburn, K.W.; Jaynes, J.; Clemens, L.E. Designed Antimicrobial Peptides for Topical Treatment of Antibiotic Resistant Acne Vulgaris. Antibiotics 2020, 9, 23. [Google Scholar] [CrossRef]
- Adamo, S.; Nilsson, J.; Krebs, A.; Steiner, U.; Cozzio, A.; French, L.E.; Kolios, A.G.A. Successful treatment of SAPHO syndrome with apremilast. Br. J. Dermatol. 2018, 179, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Velázquez, A.; Falkenhain-López, D.; Arroyo-Andrés, J.; Montero-Menárguez, J.; García-Donoso, C.; Postigo-Llorente, C. Apremilast: A novel adjuvant treatment for refractory isotretinoin-induced acne fulminans. Dermatol. Ther. 2022, 35, e15637. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. Are therapeutic effects of antiacne agents mediated by activation of F ox O 1 and inhibition of m TORC 1? Exp. Dermatol. 2013, 22, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Boateng, S.T.; Uddin, M.B.; Banang-Mbeumi, S.; Yadav, R.K.; Bock, C.R.; Folahan, J.T.; Siwe-Noundou, X.; Walker, A.L.; King, J.A.; et al. The PI3K-Akt-mTOR and Associated Signaling Pathways as Molecular Drivers of Immune-Mediated Inflammatory Skin Diseases: Update on Therapeutic Strategy Using Natural and Synthetic Compounds. Cells 2023, 12, 1671. [Google Scholar] [CrossRef] [PubMed]
- Fabbrocini, G.; Staibano, S.; De Rosa, G.; Battimiello, V.; Fardella, N.; Ilardi, G.; La Rotonda, M.I.; Longobardi, A.; Mazzella, M.; Siano, M. Resveratrol-containing gel for the treatment of acne vulgaris: A single-blind, vehicle-controlled, pilot study. Am. J. Clin. Dermatol. 2011, 12, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.V.; Yeung, H.; Cheng, C.E.; Cook-Bolden, F.; Desai, S.R.; Druby, K.M.; Freeman, E.E.; Keri, J.E.; Stein Gold, L.F.; Tan, J.K.L.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2024, 90, 1006.e1–1006.e30. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.I.; Yang, J.H.; Suh, D.H. Analysis of trends and status of physician-based evaluation methods in acne vulgaris from 2000 to 2019. J. Dermatol. 2021, 48, 42–48. [Google Scholar] [CrossRef]
- Bae, I.H.; Kwak, J.H.; Na, C.H.; Kim, M.S.; Shin, B.S.; Choi, H. A Comprehensive Review of the Acne Grading Scale in 2023. Ann. Dermatol. 2024, 36, 65–73. [Google Scholar] [CrossRef]
- US_FDA Guidance for Industry Acne Vulgaris: Developing Drugs for Treatment. Available online: https://downloads.regulations.gov/FDA-1975-N-0012-0317/attachment_250.pdf (accessed on 6 May 2024).
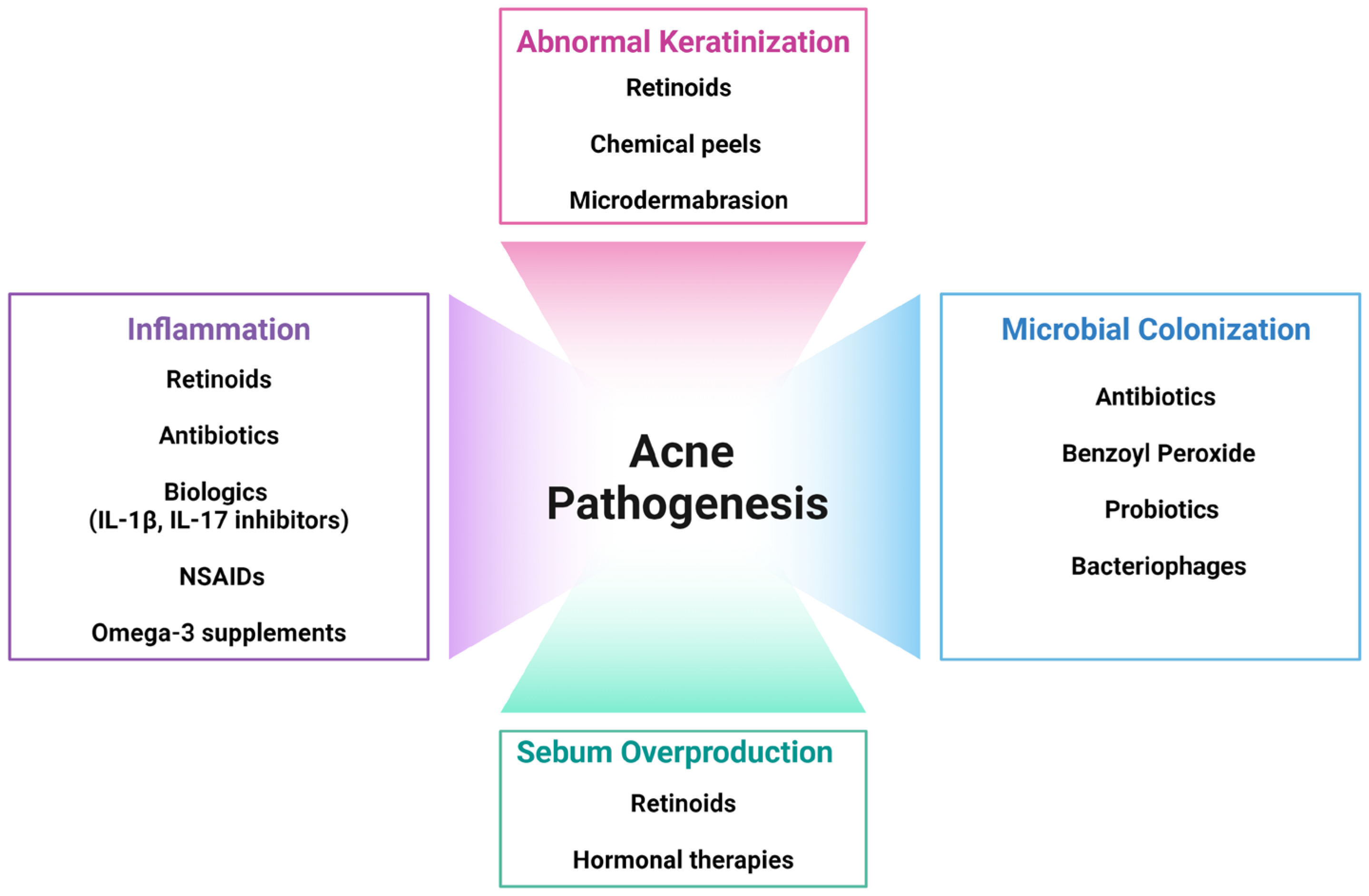
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Kim, Y.H. Exploring Acne Treatments: From Pathophysiological Mechanisms to Emerging Therapies. Int. J. Mol. Sci. 2024, 25, 5302. https://doi.org/10.3390/ijms25105302
Kim HJ, Kim YH. Exploring Acne Treatments: From Pathophysiological Mechanisms to Emerging Therapies. International Journal of Molecular Sciences. 2024; 25(10):5302. https://doi.org/10.3390/ijms25105302
Chicago/Turabian StyleKim, Hyun Jee, and Yeong Ho Kim. 2024. "Exploring Acne Treatments: From Pathophysiological Mechanisms to Emerging Therapies" International Journal of Molecular Sciences 25, no. 10: 5302. https://doi.org/10.3390/ijms25105302






