Regrettable Substitutes and the Brain: What Animal Models and Human Studies Tell Us about the Neurodevelopmental Effects of Bisphenol, Per- and Polyfluoroalkyl Substances, and Phthalate Replacements
Abstract
:1. Introduction
2. Legacy EDCs and New-Generation Substitutes
2.1. Bisphenols
2.2. Per- and Polyfluoroalkyl Substances
2.3. Phthalates
3. Models of Neurodevelopment
3.1. Zebrafish
3.2. Rodents
3.3. Humans
4. Bisphenol Alternatives and Neurodevelopment
4.1. Bisphenols and Zebrafish
| EDC | Time of Exposure (hpf) | Dose | Findings | Reference |
|---|---|---|---|---|
| BHPF | 4–144 | 300–4500 nM | ↓ Larvae locomotion at 3000, 4500 nM ↓ Larvae recovery to stressful stimuli at 4500 nM in light/dark challenge ↑ CNS neuron differentiation at 300 and 500 nM ↓ CNS neuron differentiation at 750, 1500, 3000, 4500 nM ↑ Expression of tshb, tg, nkx2.1, tshr, dio1, ugt1ab at all doses; tuba 1b at all doses except 1500 nM; crhb in all doses except 750 nM; dio2 at all doses except 750 nM and 1500 nM; cfos at doses above 750 nM; gap43 at doses above 3000 nM (genes related to HPT axis) | Jin et al., 2021 [79] |
| BHPF | 2–120 | 0.1, 10, 1000 nmol/L | ↓ Expression of pax2 expression in the spinal cord and mid-hindbrain at 10 and 1000 nM (genes related to brain morphology) ↑ Wake period during light periods at all doses, decreasing rest time ↓ Motor neuron length at all doses ↑ Expression of hcrt and aanat2 (genes related to circadian rhythm) ↑ Expression of hcrtr at 0.1 and 10 nM; ↓ at 1000 nM (genes related to circadian rhythm) | Mi et al., 2019 [91] |
| BPAF | 8–108 | 0.047, 0.47, 4.7 μM | ↑ Nearest neighbor distance and inter-individual distance at 0.47 μM, indicating alterations in shoaling (social grouping) behavior | Bai et al., 2023 [73] |
| BPAF | 2–144 | 0.1, 1 μM | ↓ Larvae locomotion at 1 μM (0.1 μM not measured) ↑ AroB cells in POA at 1 μM and in NRP at both doses (related to neuroendocrine system) No effect on BrdU (mitosis marker) cells in POA and NRP | Coumailleau et al., 2020 [99] |
| BPAF | 4–144 | 200 μg/L | ↓ Embryo spontaneous movement ↓ Larvae locomotion speed ↓ motor neuron length at 36 and 72 hpf ↓ neurogenesis at 36 and 72 hpf ↓ glutamine, DOPA, dopamine, norepinephrine, tyramine, serotonin, 5-Hydroxy-L-tryptophan, Acetylcholine ↑ 3-methocytyramine, mornetanephrone, 5-hydroxyindoleacetic acid | Gu et al., 2022 [80] |
| BPAF | 2–120 | 1, 100 μg/L | ↓ Larvae locomotion at 100 μg/L ↑ GnRH terminal nerve and hypothalamic neurons with 100 μg/L dose ↑ Expression of ren, pth, gh at 1 μg/L; kiss1, gnrh3, fshβ, lhβ, anp, ren, pth1, gh, prl at 100 μg/L (genes related to reproductive neuroendocrinology) No effect on expression of kiss2 at either dose | Qiu et al., 2021 [81] |
| BPAF | 2–120 | 5, 50, 500 μg/L | ↓ Larvae locomotion at all doses ↑ Oxidative stress at 500 μg/L | Rao et al., 2022 [82] |
| BPAF | 35,186 | 200 μg/L | ↓Larvae locomotion ↓ AChE activity ↓ Neurogenesis ↓ Expression of elavl3, zn5, α-tubulin, syn2a, and mbp (genes related to neurogenesis) ↑ Expression of gap43 (gene related to neurogenesis) ↑ Oxidative stress ↑ Apoptosis in brain | Yang et al., 2023 [83] |
| BPAP | 8–108 | 0.041, 0.41, 4.1 μM | ↑ Nearest neighbor distance at 0.041 μM and inter-individual distance at 0.041 and 0.41 μM, indicating alterations in shoaling (social grouping) behavior | Bai et al., 2023 [73] |
| BPB | 8–108 | 0.1, 1, 10 μM | ↑ Nearest neighbor distance at all doses (no effect on inter-individual distance), indicating alterations in shoaling (social grouping) behavior | Bai et al., 2023 [73] |
| BPB | 2–120 | 1, 100 μg/L | ↓ Larvae locomotion at 100 μg/L ↑ GnRH brain neurons with 100 μg/L ↑ Expression of kiss1, anp, ren, pth1, gh at 100 μg/L (genes related to reproductive neuroendocrinology) No effect on expression of kiss2, gnrh3, fshβ, or lhβ, prl (genes related to reproductive neuroendocrinology) | Qiu et al., 2021 [81] |
| BPB | 0–144 | 10, 100, 1000 μg/L | ↓ Larvae locomotion at 100 and 1000 μg/L ↓ Embryo spontaneous movements at 100 and 1000 μg/L ↑ Oxidative stress at 100 and 1000 μg/L | Wang et al., 2023 [100] |
| BPB | 0–144 | 1–1000 μg/L | ↓ Larvae locomotion at 10–1000 μg/L ↑ Hypothalamus and mesencephalon degeneration at 100 and 1000 μg/L, with no change in other brain regions ↑ T3 hormone and T3/T4 hormone ratio at 10–1000 μg/L ↓ T4 hormone at 1000 μg/L ↑ Expression of dio1, dio2 and trhr1 at 1–1000 μg/L; tg, ttr, thrα at 10–1000 μg/L; thrβ at 100–1000 μg/L (genes related to the HPG axis) ↓ Expression of tshβ and trh at 1–1000 μg/L, (genes related to the HPG axis) ↓ Expression of α1-tubulin, myelin basic protein, syn2a, elavl3, zn5 at 1–1000 μg/L; gap43 at 100–1000 μg/L (genes related to neurodevelopment) | Yang et al., 2021 [85] |
| BPC | 8–108 | 0.049, 0.49, 4.9 μM | ↑ Nearest neighbor distance at all doses and inter-individual distance at 0.49 and 4.9 μM, indicating alterations in shoaling (social grouping) behavior | Bai et al., 2023 [73] |
| BPC | 0–120 | 4.25, 8.5, 17 μg/L | ↑ Larvae locomotion at all doses ↑ Time spent in well center at 8.5 μg/L | Blanc-Legendre et al., 2023 [74] |
| BPF | 8–108 | 0.3, 3, 30 μM | ↑ Embryo spontaneous movement at 3 μM ↑ Nearest neighbor distance at 0.3 and 3 μM and inter-individual distance at 3 μM, indicating alterations in shoaling (social grouping) behavior ↑ Locomotion in the dark period at all doses and ↓ in light period at 0.3 μM at 5 dpf ↓ Locomotion in light-dark exploration test at 3 μM and ↑ time in light at 3 and 30 μM at 10 dpf ↓ Time in social contact at 0.3 and 3 μM, and ↑ number of contacts at 30 μM ↑ Intraocular distance (ID) at 3 and 30 μM, lower jaw length (LJL), and Ceratohyal cartilage length (CCL) at all doses, indicating macrocephaly ↑ Neurogenesis at 3 μM (other doses not measured) ↑ Expression of kctd12, kctd13, kcnab1a, kcnh4a, kcnj6 (K+ ion channel), ryr1a, ryr1b, ryr3 (calcium ion channel) at 3 μM (other doses not measured) ↓ Expression of chrna1 (AChr receptor) and tnnc-2 (calcium ion channel) at 3 μM (other doses not measured) No change in expression of kctd4 (K+ ion channel), scn4bb (sodium ion channel), ryr2, tnnc-1 (calcium ion channel) at 3 μM (other doses not measured) | Bai et al., 2023 [73] |
| BPF | 2–144 | 0.1, 1 μM | No effect on larvae locomotion ↑AroB cells in POA at 1uM and in NRP at both doses (related to neuroendocrine disruption) No effect on BrdU (mitosis marker) cells in POA and NRP | Coumailleau et al., 2020 [99] |
| BPF | 4–144 | 200 μg/L | ↓ Embryo spontaneous movement ↓ Larvae locomotion ↓ Neurogenesis at 36 and 72 hpf ↓ Motor neuron length at 36 hpf ↓ Glutamine, dopamine, norepinephrine, and serotonin ↑ DOPA, normetanephrine, 5-hydroxyindoleacetic acid, and Acetylcholine | Gu et al., 2022 [80] |
| BPF | 0–72/144 | 7, 70, 700 μg/L | ↓ Larvae locomotion at 70 and 700 μg/L administered until 6 dpf (not measured at 3 dpf) ↑ Apoptosis in larvae brain at 70 and 700 μg/L doses until 3 dpf (not measured at 6 dpf) ↓ Larvae expression of α1-tubulin, Syn2a at 700 μg/L, elavl3, mbp, and gfap at 70 and 700 μg/L after administering until 3 dpf. ↓ in all these genes at 70 and 700 μg/L when administered until 6 dpf (genes related to neurodevelopment) ↑ Aberrant brain nuclei arrangement in larvae | Gu et al., 2020 [86] |
| BPF | 0–144 | 2, 20, 200 μg/L | ↓ Embryo spontaneous movement at 20 and 200 μg/L ↓ Larvae locomotion at 2 and 20 μg/L (200 μg/L not) ↓ Expression of Ngn1, Elavl3, mbp, at 20 μg/L and Nrd at both 2 and 20 μg/L (200 μg/L not) (genes important for neuronal differentiation and development) ↑ Expression syn2α, gfap, and gap43 at 2, and 20 μg/L and α1-tubulin at 20 μg/L (200 μg/L not measured) (genes related to neural maturation and regeneration) | Gu et al., 2022 [93] |
| BPF | 4–120 | 5, 10 mg/L | ↓ Larvae locomotion at all doses ↑ Time in center ↑ Delayed response to stimuli at 10 mg/L ↓ Motor neuron length and axonal branching at 10 mg/L ↓ Oligodendrocytes and length of myelin sheath at 10 mg/L ↓ TH, catechol-O-methyltransferase (COMT), dopamine beta-hydroxylase (DBH), allopregnanolone, testosterone at 10 mg/L (5 mg/L not measured) ↑ Progesterone at 10 mg/L (5 mg/L not measured) | Kim et al., 2023 [87] |
| BPF | 2–96 | 0.0005, 0.5, 5.0 mg/L | ↓ Embryo spontaneous movement at 0.5 and 5 mg/L ↓ Motor neuron length at 0.5 and 5 mg/L ↓ Expression of socs3a, fosb, and nlgn2b, at 0.0005 and 0.5 mg/L (5 mg/L not measured) (genes related to neurodevelopment) | Mu et al., 2019 [92] |
| BPF | 2–120 | 1, 100 μg/L | No effect on larvae locomotion at all doses ↑ GnRH hypothalamic neurons with 100 μg/L No effect on GnRH terminal nerve neurons with 100 μg/L ↑ Expression of kiss2, anp, ren, gh at 1 μg/L; lhβ, anp, ren, pth1 at 100 μg/L (genes related to reproductive neuroendocrinology) No effect on expression of kiss1, gnrh3, fshβ, and prl | Qiu et al., 2021 [81] |
| BPF | 2–72 | 0.25, 0.5, 1 μM | ↓ Size of GnRH brain neurons at 2 dpf for 0.25 μM and at 3 dpf for 0.5 and 1 μM | Weiler and Ramakrishnan 2019 [97] |
| BPS | 8–108 | 1, 10, 100 μM | ↑ Nearest neighbor distance at 10 μM and inter-individual distance at 10 and 100 μM, indicating alterations in shoaling (social grouping) behavior ↑ Locomotion in the dark period and light period at all doses at 5 dpf ↓ Locomotion in light-dark exploration test and ↑ time in light at all doses at 10 dpf ↓ Time in social contact and number of contacts at all doses ↑ Intraocular distance (ID), lower jaw length (LJL), and ceratohyal cartilage length (CCL) at 1 and 100 μM, indicating macrocephaly ↑ Neurogenesis at 1 μM (other doses not measured) ↑ Expression of kctd4, kctd12, kctd13, kcnab1a, kcnh4a, kcnj6 (K+ ion channel), ryr2, ryr3 (calcium ion channel), scn4bb (sodium ion channel) at 3 μM (other doses not measured) ↓ Expression of chrna1 (AChr receptor) and tnnc-2 (calcium ion channel) at 1 μM (other doses not measured) No change in expression of ryr1a, ryr1b, tnnc-1 (calcium ion channel) at 3 μM (other doses not measured) | Bai et al., 2023 [73] |
| BPS | 2–144 | 0.03, 0.3, 3.0 mg/L | ↓ Larvae locomotion at all doses No changes in apoptosis at all doses ↓ Expression of α1-tubulin and gap43 at 0.3 and 3 mg/L; elavl3, mbp, syn2a, and gap43 at 3 mg/L, indicating disruption in neurodevelopment ↑ Retinal and optic nerve disruption at all doses | Gu et al., 2019 [95] |
| BPS | 4–144 | 200 μg/L | No effect on larvae locomotion ↓ Neurogenesis at 36 hpf but not 72 hpf ↓ Motor neuron length at 36 hpf but not 72 hpf ↓ Norepinephrine, 5-hydroxy-L-tryptophan ↑ 3-methoxytyramine, tyramine | Gu et al., 2022 [80] |
| BPS | 4–24/48/72/96/120 | 0.01–1 μM | ↑ Larvae locomotion at 1 μM ↑ Expression of elavl3 at 0.03 μM and 1 μM at 120 hpf; ngn1 at all doses at 120 hpf and all but 0.01 μM at 48 hpf; gfap (genes related to neurodevelopment) | Gyimah et al., 2021 [94] |
| BPS | 0–120 | 0.0068 μM | ↑ Larvae locomotion ↑ Hypothalamic neurogenesis | Kinch et al., 2015 [101] |
| BPS | 2–120 | 0.001, 0.01, 0.1 μM | ↑ Time and distance in well peripheral zone at 0.001 μM ↓ Time in “social” zone at 0.1 μM ↓ Exploration of novel object in memory task at 0.01 and 0.1 μM ↑ Expression of esr1, esr2a, it, slc12a5a, slc12a5b, slc12a2 at 0.001 μM (genes related to brain signaling pathways) ↓ Expression of esr2b at 0.01 and 0.1; it and slc12a2 at 0.1 μM (genes related to brain signaling pathways) ↑ Expression of gad1b, slc32a1, slc6a1a, gabra1 at 0.001 μM; slc17a7a, grla1a, grin1a at 0.1 μM (genes associated with GABA and glutamate signaling) ↓ Expression of slc6a1a and gabra1 at 0.1 μM; grabra2 at 0.01 and 0.1 μM (genes related to brain signaling pathways) ↑ Brain Isotocin at 0.01 μM | Naderi et al., 2022 [88] |
| BPS | 2–25/120 | 0.1–1000 μg/L | ↑ HYPO-GnRH3 neurons at 25 hpf with 100 μL/L (other doses not measured) No changes in TN-GnRH3 neurons at 25 hpf with 100 μL/L ↑ Expression of kiss1, gnrh3, er-α at 100 μL/L at 25 hpf with 100 μL/L (genes related to reproductive neuroendocrinology) No effect on kiss2, kiss2r, fshβ, erβ, and sv2 expression at 25 hpf with 100 μL/L | Qiu et al., 2016 [96] |
| BPS | 2–120 | 1, 100 μg/L | No effect on larvae locomotion at either dose ↑ GnRH hypothalamic neurons with 100 μg/L No effect on terminal nerve GnRH neurons at 100 μg/L ↑ Expression of lhβ, anp, prl at 1 μg/L and kiss1, kiss2, lhβ, anp, ren, pth1, gh at 100 μg/L (genes involved in reproductive neuroendocrinology) No effect on gnrh3, fshβ at all doses | Qiu et al., 2021 [81] |
| BPS | 2–120 | 1, 10, 100 μg/L | ↑ Development of optic nerve at 10 μg/L and retinal ganglion cells, hypothalamic neurons, and motor neurons at 10 and 100 μg/L ↓ Cone synapses at 10 and 100 μg/L ↑ Altered mosaic patterning of cones at 10 and 100 μg/L ↑ Oxidative stress-related genes in cones | Qiu et al., 2023 [98] |
| BPS | 2–120 | 1, 10, 100 μg/L | ↓ Yolk lipid supply and LCFA precursors which allow for brain development at all doses ↓ Brain lipid levels at 120 hpf at all doses | Wang et al., 2023 [102] |
| BPS | 2–120 | 4, 400 nM | ↓ Retinal thickness at all layers at all doses ↓ Light-seeking behavior at 400 nM ↓ Phototransduction genes at 400 nM | Wei et al., 2023 [90] |
| BPS-MPE | 0–120 | 185, 570, 1140 μg/L | No effect on larvae locomotion for any of the doses ↓ Time spent in well center, indicating lower anxiety, at 570 and 1140 μg/L | Blanc-Legendre et al., 2023 [74] |
| DM-BPA | 0–120 | 135, 370, 540 μg/L | ↓ Larvae locomotion at 540 μg/L No difference in time spent in well center for any dose | Blanc-Legendre et al., 2023 [74] |
| HPP | 8–108 | 0.061, 0.61, 6.1 μM | ↑ Nearest neighbor distance and inter-individual distance at 0.61 μM, indicating alterations in shoaling (social grouping) behavior | Bai et al., 2023 [73] |
| TMBPF | 4–144 | 0.25–8 mg/L | ↓ Embryo spontaneous movement at all doses ↓ Larvae locomotion at 0.5 mg/L and above ↓ Neurogenesis in larvae brain at 0.5 mg/L and above ↓ Larvae motor-neuron length at 0.5 mg/L and above ↓ Dopamine neuron development at all doses ↓ Expression of syn2a and gafp expression at 0.5 mg/L and above (genes related to neurodevelopment) ↑ Expression of th1 and th2 at 0.24 mg/L and above (genes related to neurodevelopment) | Liang et al., 2023 [26] |
| BPAF, BPB, BPF, BPS, BPS-MAE, TCBPA | 0–120 | 1 | No effect on larvae locomotion or time spent in well center | Blanc-Legendre et al., 2023 [74] |
| BPE, BPP, BPZ | 8–108 | 2 | No effect on shoaling (social grouping) behavior | Bai et al., 2023 [73] |
| BPS, BPAP | 2–144 | 0.1, 1 μM | No effect on larvae locomotion No effect on AroB (neuroendocrine marker) and BrdU (mitosis marker) cells in POA and NRP | Coumailleau et al., 2020 [99] |
4.2. Bisphenols and Rodents
| EDC | Animal | Time of Exposure | Dose | Findings | Reference |
|---|---|---|---|---|---|
| BPAF | Mice | GD 0.5–18.5 | 0.4, 4 mg/kg | ↓ Time in center of open field at all doses in females ↑ Latency to feeding at all doses, total intake at 4 mg/kg, and ↓ total intake at 0.4 mg/kg in females in novelty-suppressed feeding test No effect on the latency to feeding, but ↓ total intake in males at all doses in novelty-suppressed feeding test ↑ Immobility time at all doses but no effect on latency to immobility in females in the tail suspension test ↓ Immobility time at 4 mg/kg and latency to immobility at 0.4 mg/kg in males in tail suspension test ↑ Floating time at 4 mg/kg for females in forced swim test. No effect of floating time in males or latency to floating in males or females. ↓ Sucrose preference for females in 0.4 mg/kg test No effect on short-term or long-term memory in the novel object recognition test | Gong et al., 2022 [108] |
| BPAF | Mice | GD 1–19 | 0.4, 4 mg/kg | ↓ Time in center of open field at both doses in males No effect on locomotion in open field ↑ Latency to feeding at both doses in males and ↓ latency to feeding at 0.4 mg/kg in females in the novelty-suppressed feeding test ↓ Sucrose preference in males at 0.4 mg/kg ↑ Immobility in tail suspension test in males at both doses ↑ Floating time in forced swim test in females at 4 mg/kg ↓ Long-term memory at both doses for males and 0.4 mg/kg for females in novel object recognition test. No change seen in short-term memory. ↓ Freezing time in both long-term and short-term memory in contextual fear conditioning test in males at both doses | Gong et al., 2017 [103] |
| BPAF | Mice | GD 6–P 21 | 0.34, 3.4, 34 mg/kg | ↓ Time spent in target quadrant of MWM in males at all doses, indicating impaired spatial memory ↓ Number of quadrant crossing of MWM in females at 34 mg/kg, indicating impaired spatial memory No effect on hippocampal neuronal damage ↓ Number of intersections in CA1 and DG neurons at all doses in males and only at 34 mg/kg in DG neurons in females ↓ Spinal density in dendrites at 3.4 and 34 mg/kg in males No effect on spinal density in females ↓ PSD-95 at 3.4 and 34 mg/kg and Synapsin-1 at all doses in males; PSD-95 at 34 mg/kg in females ↓ Hippocampal ERα in males at all doses and ERβ in females at all doses No effect on hippocampal ERβ in males and ERα in females ↑ Oxidative stress in male hippocampus (females not tested) No effect on brain weight | Zhang et al., 2021 [113] |
| BPAF | Mice | GD 7–P 0 | 0.4 mg/kg | ↓ Entries/time in center and locomotion in open field for both males and females ↓ Open-arm time and locomotion in EPM for males ↑ Marble burying in MBT for males ↑ Latency to first immobility time and immobility in the tail suspension test for males ↓ Time with novel object in novel object recognition test for males ↓ Sociability for males ↑ Alterations in transcriptome related to synaptic signaling, organization, and structure, neurotransmitters, and neuron development for males (females not tested) | Wu et al., 2023 [104] |
| BPAP | Mice | GD 7–P 21 | 0.4 mg/kg | ↓ Entries and velocity in center for both males and females distance traveled in center for males in the open-field test. No effect on time in center ↓ Time in open arms for females in the elevated plus maze ↑ Number of marbles buried for males and females in marble-burying test ↓ Time spent with novel objects in males but not females. ↓ Preference index for novel object in both males and females in novel object recognition test ↓ Time spent with mouse compared to empty cage and novel mouse compared to familiar mouse in three-chamber test in both males and females ↓ Surviving neurons in male and female CA1 and DG ↑ Alterations in transcriptome related to astrocytes, microglia, neurons, oligodendrocytes, and pathways associated with Parkinson’s and neurodegeneration. ↑ Expression of C1qc, Ctss, and Iba1 (genes related to microglia) ↓ Il1rapl1, Sgk3, Ncam2, Kirrel, Fkbp5 (genes related to neurodevelopment) ↑ Macrophages and activation of dendritic cells | Wu et al., 2023 [105] |
| BPF | Mice | GD 15–P 21 | 2, 200 µg/kg | ↓ Short-term olfactory memory at 200 µg/kg No effect on serum TH levels ↓ Neurogenesis and corpus collosum thickness at 200 µg/kg No effect on oligodendrogenesis, oligodendrocyte differentiation, or myelination ↑ Alterations in transcriptome related to brain development, neuron fate development, neuron differentiation at both doses, in addition to myelination and oligodendrogenesis at 200 µg/kg No alterations in genes related to intracellular TH, important for brain development | Vancamp et al., 2023 [114] |
| BPF | Mice | GD 9.5–P 28 | 50 μg/kg | No effect on sociability ↓ Time spent with novel mouse in three-chamber test ↓ Sniffing of conspecific in open field No effect on anxiety as measured in open field and elevated plus maze No effect on depressive behavior as measured in the tail suspension test and the forced swim test No effect on locomotion and motor learning as measured in rotarod test No effect on cognition and memory as measured in novel object test | Moon et al., 2023 [111] |
| BPF | Rats | GD 12–P 21 | 10 μg/kg | ↓ 5α-reductase type 3 but not type 1 or 2 mRNA ↑ Expression of Cyp2d4, Htr4, Nr4a1 (dopamine and serotonin genes) ↓ Htr1d, Pde4c, Adcy1, Ddc, Dbh, Adcy2, App, Htr1a, Comt, Syn2, Fos, Akt3, Akt1, Tph1, Adcy5, Adrb2, Bdnf (dopamine and serotonin genes) | Castro et al., 2015 [115] |
| BPF | Mice | GD 11.5–18.5 | 10 mg/kg | No effect on locomotion ↓ Time in center of open field for females ↑ Time spent in closed arm of EPM for both females and males ↑ Time immobile in forced swim test for females | Ohtani et al., 2017 [106] |
| BPS | Mice (Female only) | GD 9–P 21 | 2, 200 µg/kg | No effect on the open field ↑ Infanticide, pup neglect, and improper pup care carried out by exposed female offspring at 2 μg/kg ↓ Time spent in nest at both doses ↑ Nest building at 200 μg/kg No effect on time spent grooming, nest size ↓ Latency for pup retrieval No effect on ERα in the MPOA and TH in the VTA | Catanese and Vandenberg, 2016 [110] |
| BPS | Mice | GD 9.5–P 28 | 50 μg/kg | No effect on sociability ↓ Time spent with novel mouse in three-chamber test ↓ Sniffing of conspecific in open field No effect on anxiety as measured in open field and elevated plus maze No effect on depressive behavior as measured in the tail suspension test and the forced swim test No effect on locomotion and motor learning as measured in rotarod test No effect on cognition and memory as measured in novel object test | Moon et al., 2023 [111] |
| BPS | Rats | GD 12–P 21 | 10 μg/kg | ↓ 5α-reductase type 3 but not type 1 or 2 mRNA ↑ Expression of Cyp2d4, Htr4, Nr4a1, Dusp1, and Pde4b (dopamine and serotonin genes) ↓ Adct2, Adrb2, and Tph1 (dopamine and serotonin genes) | Castro et al., 2015 [115] |
| BPS | Rats | GD 0–P 20 | 10, 50 μg/kg | No effect on open field ↓ Time and entries in open arms of males at both doses ↑ High-fat diet consumption at both doses in males and at 10 μg/kg in females No effect on high-sugar diet consumption | Da Silva et al., 2019 [107] |
| BPS | Mice | GD 0–P 28 | 4 μg/kg | ↓ Open-arm time and ↑ locomotion in EPM in females but not males No effect on time spent in center of open field for males and females, ↓ latency to first entry into center for males ↑ Serotonin neurons in DRV and serotonin fractional area in DR and DRV in males and in serotonin fractional area in DRD in females (DR = Dorsal Raphe Nucleus, DRD = dorsal region of DR, DRV = ventral region of DR). No effects in the Median Raphe Nucleus. | Bonaldo et al., 2023 [116] |
| BPS | Mice | GD 9–P 20 | 2, 200 µg/kg | ↓ Litters initiating nursing at 200 μg/kg | LaPlante et al., 2017 [117] |
| BPS | Mice | GD 8–P 21 | 0.2 mg/kg | No effect on open-field test ↓ Time spent with familiar mice and ↑ locomotion in social test | Kim et al., 2015 [112] |
4.3. Bisphenols and Humans
5. PFAS Alternatives and Neurodevelopment
5.1. PFAS and Zebrafish
5.2. PFAS and Rodents
5.3. PFASs and Humans
| EDC | Time EDC Measured | Concentration Measured | Findings | Reference |
|---|---|---|---|---|
| 6:2Cl-PFESA | Birth | Median Serum (Cord): 2.05 μg/L | ↓ Association with communication and gross-motor scores | Zhou et al., 2023 [152] |
| EtFOSAA | Gestation (<22 weeks) and 6.6–10.9 years | Range Plasma (ng/mL): Prenatal: <0.1–44.6 Childhood: NA | ↑ Association with 0.8–1.1 ng/mL prenatal exposure and mid-childhood visual–motor score No association with vocabulary scores, verbal and nonverbal IQ, and visual–spatial perception and memory | Harris et al., 2018 [156] |
| EtFOSAA | Gestation (<22 weeks) and 6.6–10.9 years | Range plasma (ng/mL): Prenatal: <0.1–33.6 Childhood: NA | ↓ Association with prenatal 0.8–1.1 ng/mL quartile and teacher-rated behavioral regulation and metacognition index problems | Harris et al., 2021 [163] |
| MeFOSAA | Gestation (<22 weeks) and 6.6–10.9 years | Range Plasma (ng/mL): Prenatal: 0.1–29.7 Childhood: NA | ↑ Association with prenatal 1.3–1.9 ng/mL and scores in an assessment of visual–motor abilities and an assessment of vocabulary No association with verbal and nonverbal IQ or visual–spatial perception and memory | Harris et al., 2018 [156] |
| MeFOSAA | Gestation (<22 weeks) and 6.6–10.9 years | Range plasma (ng/mL): Prenatal: 0.1–29.7 Childhood: NA | ↓ Association with prenatal 2–3.1 ng/mL quartile and parent-rated total difficulties and internalizing scores (emotional and peer problems) | Harris et al., 2021 [163] |
| PFBS | Gestation (13–16 weeks) | Range Plasma: 0.01–7 ng/mL | ↑ Association with social–emotional and adaptive scores | Luo et al., 2022 [146] |
| PFBS | Birth | Range Serum (Cord): 0.01–0.98 ng/mL | ↓ Association with gross motor and adaptive skills in boys ↓ Association with social score ↓ Association with TSH and FT4 hormones No association with fine motor and language domain scores | Yao et al., 2022 [151] |
| PFBS | Gestation (9–16 weeks) | Median Serum: 0.05 ng/mL | ↓ Association with IQ | Wang et al., 2023 [145] |
| PFDA | Birth | Median Serum (Cord): 0.24 μg/L | ↓ Association with communication scores | Zhou et al., 2023 [152] |
| PFDA | 2 and 4 years | Range Serum (ng/mL): 2 years: 0.07–1.25 4 years: 0.06–1.27 | ↑ Association with lower exposure at 2 years old and ADHD rating score | Kim et al., 2023 [164] |
| PFDA | Gestation (12–16 weeks) | Mean Plasma: 2.1 ng/mL | ↑ Association with personal–social skills problems in girls No association with gross and fine motor skills and problem-solving skills | Niu et al., 2019 [153] |
| PFDA | Gestation (10–40 weeks) | Range Plasma: 0.02–4.02 ng/mL | ↑ Association with problem behaviors ↑ Association with hyperactivity score | Hoyer et al., 2018 [165] |
| PFDA | Gestation (17 weeks) | Range Plasma: 0.05–1.77 ng/mL | ↑ Association with verbal working memory in boys No association with ADHD symptoms, language skills, or IQ | Skogheim et al., 2021 [166] |
| PFDA | Gestation (32 weeks), 5 and 7 years | Range Serum (ug/L): Gestation: 0.03–0.98 5 years: 0.05–1.2 7 years: 0.07–2.02 | ↑ Association with 5-year exposure and total behavioral development scores, externalizing problems, hyperactivity/inattention, and conduct problems | Oulhote et al., 2016 [167] |
| PFDA | Gestation (<22 weeks) and 6.6–10.9 years | Range Plasma (ng/mL): Prenatal: NA Childhood: <0.1–1.9 | ↑ Association with childhood 0.5–1.9 ng/mL quartile and parent-rated total difficulties, internalizing scores (emotional and peer problems), and externalizing scores (hyperactivity and conduct problems) | Harris et al., 2021 [163] |
| PFDA | Gestation (18 weeks) | Range Plasma: 0.19–0.24 ng/mL | ↓ Association with ADHD No association with ASD | Skogheim et al., 2021 [166] |
| PFDA | Gestation (~37 weeks) | Range Serum: <0.01–5.74 ng/mL | ↓ Association with gross motor function score No association with fine motor function, communication, problem-solving ability, and personal–social skills | Li et al., 2023 [168] |
| PFDA | 9–11 years old | Mean Blood: 0.26 ng/mL | ↓ Association with response inhibition | Gump et al., 2012 [169] |
| PFDeA | Gestation (12–28 weeks) | Mean Serum: 0.08 ng/mL | ↑ Association with attention No association with information processing speed and visual recognition memory | Enright et al., 2023 [159] |
| PFDeA | Gestation (13–16 weeks) | Range Plasma: 0.03–27.8 ng/mL | ↓ Association with cognition, language, and motor scores | Luo et al., 2022 [146] |
| PFDoA | Gestation (12–16 weeks) | Mean Plasma: 0.1 ng/mL | ↑ Association with personal–social skills problems in girls No association with gross and fine motor skills and problem-solving skills | Niu et al., 2019 [153] |
| PFDoA | Gestation (13–16 weeks) | Range Plasma: 0.04–2.9 ng/mL | ↓ Association with cognition and language scores ↑ Association with adaptive scores | Luo et al., 2022 [146] |
| PFDoDA | Gestation (~37 weeks) | Range Serum: <0.01–2.01 ng/mL | ↓ Association with problem-solving ability No association with communication, gross and fine motor function, and personal–social skills | Li et al., 2023 [168] |
| PFHpA | Gestation (10–40 weeks) | Range Plasma: 0.003–0.42 ng/mL | ↑ Association with hyperactivity score No association with problem behaviors | Hoyer et al., 2018 [165] |
| PFHpA | Gestation (13–16 weeks) | Range Plasma: 0.01–2.49 ng/mL | ↓ Association with language and motor scores | Luo et al., 2022 [146] |
| PFHpS | Gestation (18 weeks) | Range Plasma: 0.17–0.23 ng/mL | ↑ Association with ASD in girls No association with ADHD | Skogheim et al., 2021 [166] |
| PFHpS | Gestation (17 weeks) | Range Plasma: 0.05–0.62 ng/mL | ↓ Association with nonverbal working memory No association with ADHD symptoms, language skills, or IQ | Skogheim et al., 2020 [170] |
| PFNA | Birth | Median Serum (Cord): 0.34 μg/L | ↓ Association with communication scores | Zhou et al., 2023 [152] |
| PFNA | Gestation (13–19 weeks), 3 years, and 8 years | Range Serum (ng/mL): Prenatal: 0.1–2.9 3 years: 0.5–41.7 8 years: 0.1–5.2 | ↑ Association with 3- and 8-year-old exposure and completion time of a visual spatial abilities test No association with spatial reference memory, errors of omission (inattention), and reaction time in an attention and impulsivity test | Vuong et al., 2018 [160] |
| PFNA | Gestation | Range Serum: 0.2–1 ng/mL | ↑ Association with ASD | Oh et al., 2021 [154] |
| PFNA | Gestation (11–15 weeks) | Median Plasma: 0.7 ng/ml | ↑ Association with cognitive development ↓ Association with working memory scores | Carrizosa et al., 2021 [147] |
| PFNA | Gestation (10–30 weeks) | Median Serum: 0.9 ng/mL | ↑ Association with DNA methylation sites near genes DPAGT1, SLC6A2, and TMEM56 (related to neuromuscular transmission, ADHD, depression, and bipolar disorder) | Liu et al., 2022 [171] |
| PFNA | Gestation (13–19 weeks, 26 weeks, at delivery) | Mean Serum: 0.90 ng/mL | ↑ Association with externalizing problems, including hyperactivity; Behavior Symptoms Index ↑ Association with ADHD symptoms and criteria | Vuong et al., 2021 [155] |
| PFNA | Gestation (6–26 weeks) | Median Plasma: 0.46 ng/mL | ↑ Association with IQ | Liew et al., 2018 [172] |
| PFNA | 2 and 4 years | Range Serum (ng/mL): 2 years: 0.15–17.4 4 years: 0.13–7.56 | ↑ Association with lower exposure at 2 years old and ADHD rating score | Kim et al., 2023 [164] |
| PFNA | Gestation (12–16 weeks) | Mean Plasma: 1.8 ng/mL | ↑ Association with personal–social skills problems in girls No association with gross and fine motor skills and problem-solving skills | Niu et al., 2019 [153] |
| PFNA | Gestation (13–19 weeks), 3 years, and 8 years | Median Serum (ng/mL): Prenatal: 0.9 3 years: 1.2 8 years: 0.7 | ↑ Association with prenatal exposure and childhood exposure and reading scores | Zhang et al., 2018 [173] |
| PFNA | Gestation (13 weeks–birth), 3 years, 8 years | Mean Serum (ng/mL): Gestation: 0.9 8 years: 0.8 | ↑ Association with prenatal exposure and working memory ↑ Association with prenatal and child exposure and processing speed and verbal comprehension ↑ Association with child exposure and IQ and perceptual reasoning | Vuong et al., 2019 [161] |
| PFNA | Gestation (10–40 weeks) | Range Plasma: 0.14–5.71 ng/mL | ↑ Association with problem behaviors ↑ Association with hyperactivity score | Hoyer et al., 2018 [165] |
| PFNA | Gestation (17 weeks) | Range Plasma: 0.06–5.32 ng/mL | ↑ Association with verbal working memory in boys No association with ADHD symptoms, language skills, or IQ | Skogheim et al., 2020 [170] |
| PFNA | Gestation (<22 weeks) and 6.6–10.9 years | Range Plasma (ng/mL): Prenatal: <0.1–6.0 Childhood: <0.1–25.7 | ↑ Association with 1–6 ng/mL prenatal exposure and visual–spatial perception and memory No association with prenatal exposure and visual–motor scores, vocabulary, and verbal and nonverbal IQ. No association with childhood exposure and visual–motor scores, vocabulary, IQ, and visual–spatial perception and memory. | Harris et al., 2018 [156] |
| PFNA | Gestation (32 weeks), 5 and 7 years | Range Serum (ug/L): Gestation: 0.12–1.93 5 years: 0.39–6.16 7 years: 0.47–9.49 | ↑ Association with 5-year exposure and total behavioral development scores, externalizing problems, hyperactivity/inattention, and conduct problems ↑ Association with 7-year exposure and total behavioral development scores in girls; ↓ association with 7-year exposure and total behavioral development scores in boys | Oulhote et al., 2016 [167] |
| PFNA | Gestation (12–28 weeks) | Mean Serum: 0.28 ng/mL | ↑ Association with attention No association with information processing speed and visual recognition memory | Enright et al., 2023 [159] |
| PFNA | Gestation (<22 weeks) and 6.6–10.9 years | Range plasma (ng/mL): Prenatal: <0.1–6.0 Childhood:<0.1–25.7 | ↑ Association with childhood 2.4–25.7 ng/mL and parent-rated total difficulties, internalizing (emotional and peer problems), and externalizing scores (hyperactivity and conduct problems) ↑ Association with 1.1–1.5 ng/mL childhood quartiles and teacher-rated total difficulties and the 1.6–2.3 ng/mL quartile and externalizing score | Harris et al., 2021 [163] |
| PFNA | Gestation (~16 weeks) | Median Serum: 3 years: 1.9 ng/mL 8 years: 1.2 ng/mL | ↓ Association with 8-year exposure and metacognition scores, including initiation, planning, and organization of materials; association with 8-year exposure; and executive function ↑ Association with risk metacognitive impairments | Vuong et al., 2018 [174] |
| PFNA | Gestation (13–16 weeks) | Range Plasma: 0.05–16.97 ng/mL | ↓ Association with cognition, language, and motor scores | Luo et al., 2022 [146] |
| PFNA | Gestation (8–16 weeks) and 18 months old | Median Serum (ng/mL): Prenatal: 0.65 Childhood: 0.57 | ↓ Association with prenatal exposure and IQ ↑ Association with childhood exposure and IQ | Beck et al., 2023 [175] |
| PFNA | At delivery | Range Cord blood: 0–10.3 ng/mL | ↓ Association with psychomotor development index and verbal IQ | Spratlen et al., 2020 [150] |
| PFNA | Gestation | Mean Serum (ng/mL): 0.48 | ↓ Association with receptive language scores | Oh et al., 2021 [176] |
| PFNA | 9–11 years old | Mean Blood: 0.82 ng/mL | ↓ Association with response inhibition | Gump et al., 2011 [169] |
| PFNA | Gestation (28–40 weeks) | Median Serum: ~1.44–1.58 ng/mL | ↓ Association with verbal, performance, and total IQ | Wang et al., 2015 [149] |
| PFNA | Gestation (~15 weeks) | Median Serum: 0.5 ng/mL | ↓ Association with vocabulary score when mothers were <25 years old ↑ Association with vocabulary score when mothers were >30 years old | Jeddy et al., 2017 [162] |
| PFNA, PFDA, PFUA, Me-FOSAA, PFDOA, Et-FOSAA | Maternal serum collected at 2–5 years to calculate maternal prenatal serum | Range Reconstructed maternal prenatal serum: <0.49–>0.91 ng/mL (see paper for details) | No association with ASD | Shin et al., 2020 [177] |
| PFOSA | 9–11 years old | Mean Blood: 0.75 ng/mL | ↓ Association with response inhibition | Gump et al., 2011 [169] |
| PFTrDA | Gestation (22–15 weeks) and at delivery | Median Serum (ug/mL): Prenatal: 0.24 Birth (Cord Serum): 0.47 | ↓ Association with T3 and T4 No association with TSH | Kim et al., 2011 [178] |
| PFUdA | Gestation (12–16 weeks) | Mean Plasma: 1.6 ng/mL | ↑ Association with personal–social skills problems in girls No association with gross and fine motor skills and problem-solving skills | Niu et al., 2019 [153] |
| PFUdA | Gestation (12–28 weeks) | Mean Serum: 0.04 ng/mL | ↑ Association with attention No association with information processing speed and visual recognition memory | Enright et al., 2023 [159] |
| PFUnDA | 2 and 4 years | Range Serum (ng/mL): 2 years: 0.08–1.8 4 years: 0.23–5.98 | ↑ Association with lower exposure at 2 years old and ADHD rating score | Kim et al., 2023 [164] |
| PFUnDA | Gestation (17 weeks) | Range Plasma: 0.05–1.46 ng/mL | ↑ Association with verbal working memory in boys No association with ADHD symptoms, language skills, or IQ | Skogheim et al., 2020 [170] |
| PFUnDA | Gestation (18 weeks) | Range Plasma: 0.23–0.32 ng/mL | ↓ Association with ADHD No association with ASD | Skogheim et al., 2021 [166] |
| PFUnDA | Gestation (13–16 weeks) | Range Plasma: 0.04–24.46 ng/mL | ↓ Association with cognition, language, and motor scores ↑ Association with adaptive scores | Luo et al., 2022 [146] |
| PFUnDA | Gestation (~37 weeks) | Range Serum: <0.01–3.56 ng/mL | ↓ Association with gross motor function score No association with communication, problem-solving ability, fine motor function, and personal–social skills | Li et al., 2023 [168] |
| PFUnDA | Gestation (28–40 weeks) | Median Serum: ~3.13–3.42 ng/mL | ↓ Association with performance IQ | Wang et al., 2015 [149] |
| 6:2 Cl-PFESA, 8:2 Cl-PFESA, PFBA, PFNA, PFTrDA | Gestation (~37 weeks) | Range Serum (ng/mL): <0.01–8.69 (6:2 Cl-PFESA), <0.01–0.23 (8:2 Cl-PFESA), <0.01–15.36 (PFBA), <0.01–2.68 (PFNA), <0.01–3.51 (PFTrDA) | No association with communication, problem-solving ability, gross and fine motor function, and personal–social skills | Li et al., 2023 [168] |
| FOSA | Gestation (<22 weeks) and 6.6–10.9 years | Range Plasma (ng/mL): Prenatal: NA Childhood plasma: <0.1–0.5 | No associations found with childhood exposure and parent and teacher-rated strengths, difficulties, and executive function (including emotional and conduct problems) | Harris et al., 2021 [163] |
| Me-PFOSA-AcOH | Gestation (12–28 weeks) | Mean Serum: 0.04 ng/mL | No association with attention, information processing speed, and visual recognition memory | Enright et al., 2023 [159] |
| PFDA | Birth | Range Serum (Cord): 0.01–0.87 ng/mL | No association with gross and fine motor, adaptive, language, and social domain scores No association with thyroid hormone levels | Yao et al., 2022 [151] |
| PFDA, PFUnDA, PFDoDA, MeFOSAA, EtFOSAA | Gestation | Mean Serum: 0.1–0.19 ng/mL (see paper for details) | No association with scores of cognitive development | Oh et al., 2021 [176] |
| PFDA, Me-PFOSA-AcOH | Gestation (~16 weeks) | Median Serum (ng/mL): 3 years: 0.2–1.2 8 years: 0.2–0.7 (see paper for details) | No association with executive function or risk metacognitive impairments | Vuong et al., 2018 [174] |
| PFDeA | Gestation (<22 weeks) and 6.6–10.9 years | Range Plasma (ng/mL): Prenatal: <0.1–3.0 Childhood: NA | No association with visual–motor scores, vocabulary, verbal and nonverbal IQ, and visual–spatial perception and memory | Harris et al., 2018 [156] |
| PFDoA | Birth | Range Serum (Cord): 0.09–0.76 ng/mL | No association with gross and fine motor, adaptive, language, and social domain scores No association with thyroid hormone levels | Yao et al., 2022 [151] |
| PFHpA | Birth | Range Serum (Cord): 0.02–1.17 ng/mL | No association with gross and fine motor, adaptive, language, and social domain scores No association with thyroid hormone levels | Yao et al., 2022 [151] |
| PFNA | Gestation | Median Plasma (ng/mL): ~0.45–0.49 | No association with Cerebral Palsy | Vilhelmsson et al., 2023 [179] |
| PFTrDA | Gestation (12–16 weeks) | Mean Plasma (ng/mL): 0.1 (PFTrDA) | No association with personal–social skills, gross and fine motor skills, and problem-solving skills | Niu et al., 2019 [153] |
| PFDA | Gestation (8–16 weeks) and 18 months old | Median Serum (ng/mL): Prenatal: 0.29 (PFDA) Childhood: 0.18 (PFDA) | No association with prenatal and childhood exposure and IQ | Beck et al., 2023 [175] |
| PFDeA, PFDoDA | Gestation (28–40 weeks) | Median Serum (ng/mL): 0.44 (PFDeA), ~0.37 (PFDoDA) | No association with verbal, performance, and total IQ | Wang et al., 2015 [149] |
| PFHpS, PFDA, PFOSA | Gestation (6–26 weeks) | Median Plasma: 0.17–2.32 ng/mL (see paper for details) | No association with IQ | Liew et al., 2018 [172] |
| PFHpS, PFNA, PFDA | Gestation (6–12 weeks) | Range Plasma: ~0.11–0.56 (see paper for details) ng/mL | No association with ADHD or ASD | Liew et al., 2015 [180] |
| PFHpS, PFNA, PFDA, PFTeDA, PFUnDA | Gestation (22–15 weeks) and at delivery | Median Serum (ug/mL): Prenatal: 0.09–0.6 Infant (Cord): (PFHxS), 0.06–0.45 (see paper for details) | No association with thyroid hormones | Kim et al., 2011 [178] |
| PFNA, PFDA | Gestation (8–16 weeks) and 18 months | Median Blood (ng/mL): Gestation: 0.29–0.64 18 months: 0.18–0.58 (see paper for details) | No association between prenatal and child exposure and ADHD | Dalsager et al., 2021 [181] |
| PFNA, PFDA | Gestation (8–16 weeks) and 18 months | Median Serum (ng/mL): Gestation: 0.29–0.65 18 months: 0.18–0.58 (see paper for details) | No association between prenatal and child exposure and language development | Beck et al., 2023 [148] |
| PFNA | Birth | Median Serum (Cord): ~0.28–0.31 ng/mL | No association with ADHD | Ode et al., 2014 [182] |
| PFNA | 5–18 years | Range Serum: 0.25–24.1 ng/mL | No association with ADHD | Stein and Savitz, 2011 [183] |
| PFNA | Birth | Range Serum (Cord): 0.08–1.76 ng/mL | No association with gross and fine motor, adaptive, language, and social domain scores No association with thyroid hormone levels | Yao et al., 2022 [151] |
| PFNA, PFDeA | Gestation (~16 weeks–birth) | Range Serum: 0.1–2.9 (PFNA), 0.1–1.3 (PFDeA) ng/mL | No association with behavioral regulation and executive function | Vuong et al., 2016 [184] |
| PFNA, | Gestation (18 weeks) | Range Plasma (ng/mL): 0.39–49 | No association with ADHD or ASD | Skogheim et al., 2021 [166] |
| PFNA, PFDA, PFUA, PFDoA, PFBS, PFHpA | Gestation (9–16 weeks) | Median Serum (ng/mL): 0.05–2.16 (see paper for details) | No association with IQ | Wang et al., 2023 [145] |
| PFNA | Gestation (16–26 weeks) | Median Serum (ug/L):0.9 | No association with social responsiveness scores, a measure of ASD | Braun et al., 2014 [185] |
| PFTrA, PFDoA, PFBA, 8:2Cl-PFESA | Birth | Median Serum (Cord) (ug/L): 0.04–0.25 (see paper for details) | No association with communication scores | Zhou et al., 2023 [152] |
| PFUA | Birth | Range Serum (Cord): 0.03–0.65 ng/mL | No association with gross and fine motor, adaptive, language, and social domain scores No association with thyroid hormone levels | Yao et al., 2022 [151] |
6. Phthalate Alternatives and Neurodevelopment
6.1. Phthalate Alternatives and Zebrafish
6.2. Phthalate Alternatives and Rodents
6.3. Phthalate Alternatives and Humans
7. Discussion
7.1. Bisphenols
7.2. PFASs
7.3. Phthalates
7.4. Bio-Based Alternatives—Are They Any Safer?
7.5. Conclusions and Recommendations for the Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brennan, N.M.; Evans, A.T.; Fritz, M.K.; Peak, S.A.; von Holst, H.E. Trends in the Regulation of Per- and Polyfluoroalkyl Substances (PFAS): A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 10900. [Google Scholar] [CrossRef]
- Warner, G.R.; Flaws, J.A. Bisphenol A and Phthalates: How Environmental Chemicals Are Reshaping Toxicology. Toxicol. Sci. 2018, 166, 246–249. [Google Scholar] [CrossRef]
- Colborn, T.; vom Saal, F.S.; Soto, A.M. Developmental Effects of Endocrine-Disrupting Chemicals in Wildlife and Humans. Environ. Health Perspect. 1993, 101, 378–384. [Google Scholar] [CrossRef]
- National Center for Environmental Health. National Report on Human Exposure to Environmental Chemicals; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022. [Google Scholar] [CrossRef]
- Colorado-Yohar, S.M.; Castillo-González, A.C.; Sánchez-Meca, J.; Rubio-Aparicio, M.; Sánchez-Rodríguez, D.; Salamanca-Fernández, E.; Ardanaz, E.; Amiano, P.; Fernández, M.F.; Mendiola, J.; et al. Concentrations of Bisphenol-A in Adults from the General Population: A Systematic Review and Meta-Analysis. Sci. Total Environ. 2021, 775, 145755. [Google Scholar] [CrossRef] [PubMed]
- Fábelová, L.; Beneito, A.; Casas, M.; Colles, A.; Dalsager, L.; Den Hond, E.; Dereumeaux, C.; Ferguson, K.; Gilles, L.; Govarts, E.; et al. PFAS Levels and Exposure Determinants in Sensitive Population Groups. Chemosphere 2023, 313, 137530. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, D.; Guo, Y.; Mao, W.; Zhao, N.; Zhao, M.; Jin, H. Phthalate Metabolites in Paired Human Serum and Whole Blood. Sci. Total Environ. 2022, 824, 153792. [Google Scholar] [CrossRef]
- Trasande, L. Updating the Toxic Substances Control Act to Protect Human Health. JAMA 2016, 315, 1565–1566. [Google Scholar] [CrossRef]
- Sweetman, A. A Grand Challenge for Environmental Organic Chemistry: How Can We Avoid Regrettable Substitution? Front. Environ. Chem. 2020, 1, 7. [Google Scholar] [CrossRef]
- Hilz, E.N.; Gore, A.C. Endocrine-Disrupting Chemicals: Science and Policy. Policy Insights Behav. Brain Sci. 2023, 10, 142–150. [Google Scholar] [CrossRef]
- McCarthy, M.M. Molecular Aspects of Sexual Differentiation of the Rodent Brain. Psychoneuroendocrinology 1994, 19, 415–427. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Wright, C.L.; Schwarz, J.M. New Tricks by an Old Dogma: Mechanisms of the Organizational/Activational Hypothesis of Steroid-Mediated Sexual Differentiation of Brain and Behavior. Horm. Behav. 2009, 55, 655–665. [Google Scholar] [CrossRef]
- McCARTHY, M.M. Estradiol and the Developing Brain. Physiol. Rev. 2008, 88, 91–134. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the Key Characteristics of Endocrine-Disrupting Chemicals as a Basis for Hazard Identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Combarnous, Y.; Nguyen, T.M.D. Comparative Overview of the Mechanisms of Action of Hormones and Endocrine Disruptor Compounds. Toxics 2019, 7, 5. [Google Scholar] [CrossRef]
- Dickerson, S.M.; Gore, A.C. Estrogenic Environmental Endocrine-Disrupting Chemical Effects on Reproductive Neuroendocrine Function and Dysfunction across the Life Cycle. Rev. Endocr. Metab. Disord. 2007, 8, 143–159. [Google Scholar] [CrossRef]
- Boas, M.; Feldt-Rasmussen, U.; Main, K.M. Thyroid Effects of Endocrine Disrupting Chemicals. Mol. Cell. Endocrinol. 2012, 355, 240–248. [Google Scholar] [CrossRef]
- Schulz, K.M.; Sisk, C.L. The Organizing Actions of Adolescent Gonadal Steroid Hormones on Brain and Behavioral Development. Neurosci. Biobehav. Rev. 2016, 70, 148–158. [Google Scholar] [CrossRef]
- Sisk, C.L.; Zehr, J.L. Pubertal Hormones Organize the Adolescent Brain and Behavior. Front. Neuroendocrinol. 2005, 26, 163–174. [Google Scholar] [CrossRef]
- Levine, S.; Mullins, R.F. Hormonal Influences on Brain Organization in Infant Rats. Science 1966, 152, 1585–1592. [Google Scholar] [CrossRef]
- McEwen, B.S. Actions of Sex Hormones on the Brain: ‘Organization’ and ‘Activation’ in Relation to Functional Teratology. In Progress in Brain Research; Boer, G.J., Feenstra, M.G.P., Mirmiran, M., Swaab, D.F., Van Haaren, F., Eds.; Biochemical Basis of Functional Neuroteratology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 73, pp. 121–134. [Google Scholar]
- Gore, A.C.; Martien, K.M.; Gagnidze, K.; Pfaff, D. Implications of Prenatal Steroid Perturbations for Neurodevelopment, Behavior, and Autism. Endocr. Rev. 2014, 35, 961–991. [Google Scholar] [CrossRef]
- Hilz, E.N.; Gore, A.C. Sex-Specific Effects of Endocrine-Disrupting Chemicals on Brain Monoamines and Cognitive Behavior. Endocrinology 2022, 163, bqac128. [Google Scholar] [CrossRef]
- Hernandez Scudder, M.E.; Young, R.L.; Thompson, L.M.; Kore, P.; Crews, D.; Hofmann, H.A.; Gore, A.C. EDCs Reorganize Brain-Behavior Phenotypic Relationships in Rats. J. Endocr. Soc. 2021, 5, bvab021. [Google Scholar] [CrossRef]
- Liang, M.; Deng, J.; Gu, J.; Yang, J.; Ge, F.; Huang, C.; Wu, W. TMBPF-Induced Neurotoxicity and Oxidative Stress in Zebrafish Larvae: Impacts on Central Nervous System Development and Dopamine Neurons. Ecotoxicol. Environ. Saf. 2023, 268, 115710. [Google Scholar] [CrossRef]
- Liu, D.; Yan, S.; Liu, Y.; Chen, Q.; Ren, S. Association of Prenatal Exposure to Perfluorinated and Polyfluoroalkyl Substances with Childhood Neurodevelopment: A Systematic Review and Meta-Analysis. Ecotoxicol. Environ. Saf. 2024, 271, 115939. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Henao-Mejia, J.; Simmons, R.A. Immune System: An Emerging Player in Mediating Effects of Endocrine Disruptors on Metabolic Health. Endocrinology 2018, 159, 32–45. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Vandenberg, L.N.; Demeneix, B.A.; Porta, M.; Slama, R.; Trasande, L. Endocrine-Disrupting Chemicals: Economic, Regulatory, and Policy Implications. Lancet Diabetes Endocrinol. 2020, 8, 719–730. [Google Scholar] [CrossRef]
- Trasande, L.; Attina, T.M. Association of Exposure to Di-2-Ethylhexylphthalate Replacements With Increased Blood Pressure in Children and Adolescents. Hypertension 2015, 66, 301–308. [Google Scholar] [CrossRef]
- Duh-Leong, C.; Maffini, M.V.; Kassotis, C.D.; Vandenberg, L.N.; Trasande, L. The Regulation of Endocrine-Disrupting Chemicals to Minimize Their Impact on Health. Nat. Rev. Endocrinol. 2023, 19, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.A. The Politics of Plastics: The Making and Unmaking of Bisphenol A “Safety”. Am. J. Public. Health 2009, 99, S559–S566. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Asai, D.; Toita, R. Bisphenol A (BPA) and Cardiovascular or Cardiometabolic Diseases. J. Xenobiot. 2023, 13, 775–810. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Yazid, M.D.; Bahari, H.; Keong, Y.Y.; Rajandram, R.; Embong, H.; Teoh, S.H.; Halim, S.; Othman, F. Bisphenol A (BPA) Leading to Obesity and Cardiovascular Complications: A Compilation of Current In Vivo Study. Int. J. Mol. Sci. 2022, 23, 2969. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and Human Health: A Review of the Literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.L.; Fasano, S.; Guida, M.; Plunk, E.; Viggiano, A.; Pierantoni, R.; et al. Neuro-Toxic and Reproductive Effects of BPA. Curr. Neuropharmacol. 2019, 17, 1109–1132. [Google Scholar] [CrossRef]
- Gore, A.C.; Krishnan, K.; Reilly, M.P. Endocrine-Disrupting Chemicals: Effects on Neuroendocrine Systems and the Neurobiology of Social Behavior. Horm. Behav. 2019, 111, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Pelch, K.; Wignall, J.A.; Goldstone, A.E.; Ross, P.K.; Blain, R.B.; Shapiro, A.J.; Holmgren, S.D.; Hsieh, J.-H.; Svoboda, D.; Auerbach, S.S.; et al. A Scoping Review of the Health and Toxicological Activity of Bisphenol A (BPA) Structural Analogues and Functional Alternatives. Toxicology 2019, 424, 152235. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.-L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Oliviero, F.; Marmugi, A.; Viguié, C.; Gayrard, V.; Picard-Hagen, N.; Mselli-Lakhal, L. Are BPA Substitutes as Obesogenic as BPA? Int. J. Mol. Sci. 2022, 23, 4238. [Google Scholar] [CrossRef] [PubMed]
- Edaes, F.S.; de Souza, C.B. BPS and BPF Are as Carcinogenic as BPA and Are Not Viable Alternatives for Its Replacement. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, S.; Tokunaga, T.; Liu, X.; Okada, H.; Matsushima, A.; Shimohigashi, Y. Endocrine Disruptor Bisphenol A Strongly Binds to Human Estrogen-Related Receptor Gamma (ERRgamma) with High Constitutive Activity. Toxicol. Lett. 2006, 167, 95–105. [Google Scholar] [CrossRef]
- Bonefeld-Jørgensen, E.C.; Long, M.; Hofmeister, M.V.; Vinggaard, A.M. Endocrine-Disrupting Potential of Bisphenol A, Bisphenol A Dimethacrylate, 4-n-Nonylphenol, and 4-n-Octylphenol In Vitro: New Data and a Brief Review. Environ. Health Perspect. 2007, 115 (Suppl. S1), 69–76. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid Hormone Action Is Disrupted by Bisphenol A as an Antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, G.K.; Divya, L.M.; Sadasivan, C. Bisphenol-A Can Bind to Human Glucocorticoid Receptor as an Agonist: An In Silico Study. J. Appl. Toxicol. 2010, 30, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Zhang, D.-H.; Jiang, L.-D.; Qi, Y.; Guo, L.-H. Binding and Activity of Bisphenol Analogues to Human Peroxisome Proliferator-Activated Receptor β/δ. Ecotoxicol. Environ. Saf. 2021, 226, 112849. [Google Scholar] [CrossRef]
- Schug, T.T.; Blawas, A.M.; Gray, K.; Heindel, J.J.; Lawler, C.P. Elucidating the Links between Endocrine Disruptors and Neurodevelopment. Endocrinology 2015, 156, 1941–1951. [Google Scholar] [CrossRef]
- McPartland, M.; Stevens, S.; Bartosova, Z.; Vardeberg, I.G.; Völker, J.; Wagner, M. Beyond the Nucleus: Plastic Chemicals Activate G Protein-Coupled Receptors. Environ. Sci. Technol. 2024, 58, 4872–4883. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Buha Djordjevic, A.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10, 44. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An Overview of the Uses of Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- O’Hagan, D. Understanding Organofluorine Chemistry. An Introduction to the C–F Bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef]
- Brunn, H.; Arnold, G.; Körner, W.; Rippen, G.; Steinhäuser, K.G.; Valentin, I. PFAS: Forever Chemicals—Persistent, Bioaccumulative and Mobile. Reviewing the Status and the Need for Their Phase out and Remediation of Contaminated Sites. Environ. Sci. Eur. 2023, 35, 20. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Steenland, K.; Winquist, A. PFAS and Cancer, a Scoping Review of the Epidemiologic Evidence. Environ. Res. 2021, 194, 110690. [Google Scholar] [CrossRef]
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per- and Poly-Fluoroalkyl Substances (PFAS) and Female Reproductive Outcomes: PFAS Elimination, Endocrine-Mediated Effects, and Disease. Toxicology 2022, 465, 153031. [Google Scholar] [CrossRef]
- Jane L Espartero, L.; Yamada, M.; Ford, J.; Owens, G.; Prow, T.; Juhasz, A. Health-Related Toxicity of Emerging per- and Polyfluoroalkyl Substances: Comparison to Legacy PFOS and PFOA. Environ. Res. 2022, 212, 113431. [Google Scholar] [CrossRef]
- Stockholm Convention on Persistent Organic Pollutants (2022) Guidance for the Inventory of Perfluorooctane Sulfonic Acid (PFOS) and Related Chemicals Listed under the Stockholm Convention on Persistent Organic Pollutants. Available online: https://chm.pops.int/Implementation/IndustrialPOPs/PFAS/Guidance/tabid/5225/Default.aspx (accessed on 11 March 2024).
- Conder, J.M.; Hoke, R.A.; de Wolf, W.; Russell, M.H.; Buck, R.C. Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophilic Compounds. Environ. Sci. Technol. 2008, 42, 995–1003. [Google Scholar] [CrossRef]
- Frömel, T.; Knepper, T.P. Biodegradation of Fluorinated Alkyl Substances. In Reviews of Environmental Contamination and Toxicology Volume 208: Perfluorinated Alkylated Substances; De Voogt, P., Ed.; Springer: New York, NY, USA, 2010; pp. 161–177. ISBN 978-1-4419-6880-7. [Google Scholar]
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Hungerbühler, K. Fluorinated Alternatives to Long-Chain Perfluoroalkyl Carboxylic Acids (PFCAs), Perfluoroalkane Sulfonic Acids (PFSAs) and Their Potential Precursors. Environ. Int. 2013, 60, 242–248. [Google Scholar] [CrossRef]
- Brase, R.A.; Mullin, E.J.; Spink, D.C. Legacy and Emerging Per- and Polyfluoroalkyl Substances: Analytical Techniques, Environmental Fate, and Health Effects. Int. J. Mol. Sci. 2021, 22, 995. [Google Scholar] [CrossRef]
- Ahrens, L.; Rakovic, J.; Ekdahl, S.; Kallenborn, R. Environmental Distribution of Per- and Polyfluoroalkyl Substances (PFAS) on Svalbard: Local Sources and Long-Range Transport to the Arctic. Chemosphere 2023, 345, 140463. [Google Scholar] [CrossRef]
- Eales, J.; Bethel, A.; Galloway, T.; Hopkinson, P.; Morrissey, K.; Short, R.E.; Garside, R. Human Health Impacts of Exposure to Phthalate Plasticizers: An Overview of Reviews. Environ. Int. 2022, 158, 106903. [Google Scholar] [CrossRef]
- Bernard, L.; Bouattour, Y.; Masse, M.; Boeuf, B.; Decaudin, B.; Genay, S.; Lambert, C.; Moreau, E.; Pereira, B.; Pinguet, J.; et al. Association between Urinary Metabolites and the Exposure of Intensive Care Newborns to Plasticizers of Medical Devices Used for Their Care Management. Metabolites 2021, 11, 252. [Google Scholar] [CrossRef]
- Consumer Product Safety Commission. Prohibition of Children’s Toys and Child Care Articles Containing Specified Phthalates. Fed. Regist. 2018, 82, 49938–49982. [Google Scholar]
- Food and Drug Administration Requests FDA Remove Its Prior Sanction of Five Ortho-Phthalates and Ban Eight Ortho-Phthalates. 2023. Available online: https://www.regulations.gov/docket/FDA-2016-P-1171 (accessed on 20 June 2024).
- Krithivasan, R.; Miller, G.Z.; Belliveau, M.; Gearhart, J.; Krishnamoorthi, V.; Lee, S.; Kannan, K. Analysis of Ortho-Phthalates and Other Plasticizers in Select Organic and Conventional Foods in the United States. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 778–786. [Google Scholar] [CrossRef]
- Moche, H.; Chentouf, A.; Neves, S.; Corpart, J.-M.; Nesslany, F. Comparison of In Vitro Endocrine Activity of Phthalates and Alternative Plasticizers. J. Toxicol. 2021, 2021, 8815202. [Google Scholar] [CrossRef]
- Engel, A.; Buhrke, T.; Kasper, S.; Behr, A.-C.; Braeuning, A.; Jessel, S.; Seidel, A.; Völkel, W.; Lampen, A. The Urinary Metabolites of DINCH® Have an Impact on the Activities of the Human Nuclear Receptors ERα, ERβ, AR, PPARα and PPARγ. Toxicol. Lett. 2018, 287, 83–91. [Google Scholar] [CrossRef]
- Schaffert, A.; Arnold, J.; Karkossa, I.; Blüher, M.; von Bergen, M.; Schubert, K. The Emerging Plasticizer Alternative DINCH and Its Metabolite MINCH Induce Oxidative Stress and Enhance Inflammatory Responses in Human THP-1 Macrophages. Cells 2021, 10, 2367. [Google Scholar] [CrossRef]
- Schaffert, A.; Karkossa, I.; Ueberham, E.; Schlichting, R.; Walter, K.; Arnold, J.; Blüher, M.; Heiker, J.T.; Lehmann, J.; Wabitsch, M.; et al. Di-(2-Ethylhexyl) Phthalate Substitutes Accelerate Human Adipogenesis through PPARγ Activation and Cause Oxidative Stress and Impaired Metabolic Homeostasis in Mature Adipocytes. Environ. Int. 2022, 164, 107279. [Google Scholar] [CrossRef]
- d’Amora, M.; Giordani, S. The Utility of Zebrafish as a Model for Screening Developmental Neurotoxicity. Front. Neurosci. 2018, 12, 976. [Google Scholar] [CrossRef]
- Bai, C.; Zheng, Y.; Tian, L.; Lin, J.; Song, Y.; Huang, C.; Dong, Q.; Chen, J. Structure-Based Developmental Toxicity and ASD-Phenotypes of Bisphenol A Analogues in Embryonic Zebrafish. Ecotoxicol. Environ. Saf. 2023, 253, 114643. [Google Scholar] [CrossRef]
- Blanc-Legendre, M.; Sire, S.; Christophe, A.; Brion, F.; Bégout, M.-L.; Cousin, X. Embryonic Exposures to Chemicals Acting on Brain Aromatase Lead to Different Locomotor Effects in Zebrafish Larvae. Environ. Toxicol. Pharmacol. 2023, 102, 104221. [Google Scholar] [CrossRef]
- Ngoc Hieu, B.T.; Ngoc Anh, N.T.; Audira, G.; Juniardi, S.; Liman, R.A.D.; Villaflores, O.B.; Lai, Y.-H.; Chen, J.-R.; Liang, S.-T.; Huang, J.-C.; et al. Development of a Modified Three-Day T-Maze Protocol for Evaluating Learning and Memory Capacity of Adult Zebrafish. Int. J. Mol. Sci. 2020, 21, 1464. [Google Scholar] [CrossRef]
- Robbins, T.W. From Rodent Behavioral Models to Human Disorders. In Translational Neuroscience: Toward New Therapies; Nikolich, K., Hyman, S.E., Eds.; MIT Press: Cambridge, MA, USA, 2015; ISBN 978-0-262-02986-5. [Google Scholar]
- Regan, S.L.; Williams, M.T.; Vorhees, C.V. Review of Rodent Models of Attention Deficit Hyperactivity Disorder. Neurosci. Biobehav. Rev. 2022, 132, 621–637. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T.; Hawkey, A.B.; Levin, E.D. Translating Neurobehavioral Toxicity Across Species from Zebrafish to Rats to Humans: Implications for Risk Assessment. Front. Toxicol. 2021, 3, 629229. [Google Scholar] [CrossRef]
- Jin, M.; Dang, J.; Paudel, Y.N.; Wang, X.; Wang, B.; Wang, L.; Li, P.; Sun, C.; Liu, K. The Possible Hormetic Effects of Fluorene-9-Bisphenol on Regulating Hypothalamic-Pituitary-Thyroid Axis in Zebrafish. Sci. Total Environ. 2021, 776, 145963. [Google Scholar] [CrossRef]
- Gu, J.; Guo, M.; Yin, X.; Huang, C.; Qian, L.; Zhou, L.; Wang, Z.; Wang, L.; Shi, L.; Ji, G. A Systematic Comparison of Neurotoxicity of Bisphenol A and Its Derivatives in Zebrafish. Sci. Total Environ. 2022, 805, 150210. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, S.; Chen, H.; Luo, S.; Xiong, Y.; Wang, X.; Xu, B.; Zheng, C.; Wang, K.-J. The Comparative Toxicities of BPA, BPB, BPS, BPF, and BPAF on the Reproductive Neuroendocrine System of Zebrafish Embryos and Its Mechanisms. J. Hazard. Mater. 2021, 406, 124303. [Google Scholar] [CrossRef]
- Rao, C.; Cao, X.; Li, L.; Zhou, J.; Sun, D.; Li, B.; Guo, S.; Yuan, R.; Cui, H.; Chen, J. Bisphenol AF Induces Multiple Behavioral and Biochemical Changes in Zebrafish (Danio rerio) at Different Life Stages. Aquat. Toxicol. 2022, 253, 106345. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, J.; Ding, J.; Liu, J. Neurodevelopmental Toxicity of Bisphenol AF in Zebrafish Larvae and the Protective Effects of Curcumin. J. Appl. Toxicol. 2023, 43, 1806–1818. [Google Scholar] [CrossRef]
- Wang, J.; Wu, C.; Zhang, X.; Song, Y.; Wang, B.; Zhang, K.; Sun, M. Developmental Neurotoxic Effects of Bisphenol A and Its Derivatives in Drosophila Melanogaster. Ecotoxicol. Environ. Saf. 2023, 260, 115098. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, Z.; Liu, Q.; Chen, L. Adverse Effects of Bisphenol B Exposure on the Thyroid and Nervous System in Early Life Stages of Zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 250, 109167. [Google Scholar] [CrossRef]
- Gu, J.; Wu, J.; Xu, S.; Zhang, L.; Fan, D.; Shi, L.; Wang, J.; Ji, G. Bisphenol F Exposure Impairs Neurodevelopment in Zebrafish Larvae (Danio rerio). Ecotoxicol. Environ. Saf. 2020, 188, 109870. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.S.; Park, B.H.; Hwang, K.-S.; Bae, M.A.; Cho, S.-H.; Kim, S.; Park, H.-C. Mechanism of Bisphenol F Affecting Motor System and Motor Activity in Zebrafish. Toxics 2023, 11, 477. [Google Scholar] [CrossRef]
- Naderi, M.; Puar, P.; JavadiEsfahani, R.; Kwong, R.W.M. Early Developmental Exposure to Bisphenol A and Bisphenol S Disrupts Socio-Cognitive Function, Isotocin Equilibrium, and Excitation-Inhibition Balance in Developing Zebrafish. NeuroToxicology 2022, 88, 144–154. [Google Scholar] [CrossRef]
- Gu, J.; Zhu, Y.; Guo, M.; Yin, X.; Liang, M.; Lou, X.; Chen, J.; Zhou, L.; Fan, D.; Shi, L.; et al. The Potential Mechanism of BPF-Induced Neurotoxicity in Adult Zebrafish: Correlation between Untargeted Metabolomics and Gut Microbiota. Sci. Total Environ. 2022, 839, 156221. [Google Scholar] [CrossRef]
- Wei, S.; Qiu, L.; Ru, S.; Yang, Y.; Wang, J.; Zhang, X. Bisphenol S Disrupts Opsins Gene Expression and Impairs the Light-Sensing Function via Antagonizing TH-TRβ Signaling Pathway in Zebrafish Larvae. Food Chem. Toxicol. 2023, 172, 113588. [Google Scholar] [CrossRef]
- Mi, P.; Zhang, Q.-P.; Li, S.-B.; Liu, X.-Y.; Zhang, S.-H.; Li, M.; Chen, D.-Y.; Zhao, X.; Feng, D.-F.; Feng, X.-Z. Melatonin Protects Embryonic Development and Maintains Sleep/Wake Behaviors from the Deleterious Effects of Fluorene-9-Bisphenol in Zebrafish (Danio rerio). J. Pineal Res. 2019, 66, e12530. [Google Scholar] [CrossRef]
- Mu, X.; Liu, J.; Yuan, L.; Yang, K.; Huang, Y.; Wang, C.; Yang, W.; Shen, G.; Li, Y. The Mechanisms Underlying the Developmental Effects of Bisphenol F on Zebrafish. Sci. Total Environ. 2019, 687, 877–884. [Google Scholar] [CrossRef]
- Gu, J.; Li, L.; Yin, X.; Liang, M.; Zhu, Y.; Guo, M.; Zhou, L.; Fan, D.; Shi, L.; Ji, G. Long-Term Exposure of Zebrafish to Bisphenol F: Adverse Effects on Parental Reproduction and Offspring Neurodevelopment. Aquat. Toxicol. 2022, 248, 106190. [Google Scholar] [CrossRef]
- Gyimah, E.; Xu, H.; Dong, X.; Qiu, X.; Zhang, Z.; Bu, Y.; Akoto, O. Developmental Neurotoxicity of Low Concentrations of Bisphenol A and S Exposure in Zebrafish. Chemosphere 2021, 262, 128045. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, J.; Chen, Y.; Wang, H.; Guo, M.; Wang, L.; Wang, Z.; Wu, S.; Shi, L.; Gu, A.; et al. Neurobehavioral Effects of Bisphenol S Exposure in Early Life Stages of Zebrafish Larvae (Danio rerio). Chemosphere 2019, 217, 629–635. [Google Scholar] [CrossRef]
- Qiu, W.; Zhao, Y.; Yang, M.; Farajzadeh, M.; Pan, C.; Wayne, N.L. Actions of Bisphenol A and Bisphenol S on the Reproductive Neuroendocrine System During Early Development in Zebrafish. Endocrinology 2016, 157, 636–647. [Google Scholar] [CrossRef]
- Weiler, K.; Ramakrishnan, S. Bisphenol F Causes Disruption of Gonadotropin-Releasing Hormone Neural Development in Zebrafish via an Estrogenic Mechanism. Neurotoxicology 2019, 71, 31–38. [Google Scholar] [CrossRef]
- Qiu, L.; Wei, S.; Yang, Y.; Zhang, R.; Ru, S.; Zhang, X. Mechanism of Bisphenol S Exposure on Color Sensitivity of Zebrafish Larvae. Environ. Pollut. 2023, 316, 120670. [Google Scholar] [CrossRef]
- Coumailleau, P.; Trempont, S.; Pellegrini, E.; Charlier, T.D. Impacts of Bisphenol A Analogues on Zebrafish Post-Embryonic Brain. J. Neuroendocrinol. 2020, 32, e12879. [Google Scholar] [CrossRef]
- Wang, F.; Ma, X.; Sun, Q.; Zhang, Y.; Liu, Y.; Gu, J.; Wang, L. Bisphenol B Induces Developmental Toxicity in Zebrafish via Oxidative Stress. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef]
- Kinch, C.D.; Ibhazehiebo, K.; Jeong, J.-H.; Habibi, H.R.; Kurrasch, D.M. Low-Dose Exposure to Bisphenol A and Replacement Bisphenol S Induces Precocious Hypothalamic Neurogenesis in Embryonic Zebrafish. Proc. Natl. Acad. Sci. USA 2015, 112, 1475–1480. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Zhang, X.; Zhang, J.; Ru, S. Bisphenol S Impairs Behaviors through Disturbing Endoplasmic Reticulum Function and Reducing Lipid Levels in the Brain of Zebrafish. Environ. Sci. Technol. 2023, 57, 582–594. [Google Scholar] [CrossRef]
- Gong, M.; Huai, Z.; Song, H.; Cui, L.; Guo, Q.; Shao, J.; Gao, Y.; Shi, H. Effects of Maternal Exposure to Bisphenol AF on Emotional Behaviors in Adolescent Mice Offspring. Chemosphere 2017, 187, 140–146. [Google Scholar] [CrossRef]
- Wu, X.; Li, S.; Ni, Y.; Qi, C.; Bai, S.; Xu, Q.; Fan, Y.; Ma, X.; Lu, C.; Du, G.; et al. Maternal BPAF Exposure Impaired Synaptic Development and Caused Behavior Abnormality in Offspring. Ecotoxicol. Environ. Saf. 2023, 256, 114859. [Google Scholar] [CrossRef]
- Wu, X.; Li, S.; Zhang, M.; Bai, S.; Ni, Y.; Xu, Q.; Fan, Y.; Lu, C.; Xu, Z.; Ji, C.; et al. Early-Life Bisphenol AP Exposure Impacted Neurobehaviors in Adulthood through Microglial Activation in Mice. Chemosphere 2023, 317, 137935. [Google Scholar] [CrossRef]
- Ohtani, N.; Iwano, H.; Suda, K.; Tsuji, E.; Tanemura, K.; Inoue, H.; Yokota, H. Adverse Effects of Maternal Exposure to Bisphenol F on the Anxiety- and Depression-like Behavior of Offspring. J. Vet. Med. Sci. 2017, 79, 432–439. [Google Scholar] [CrossRef]
- da Silva, B.S.; Pietrobon, C.B.; Bertasso, I.M.; Lopes, B.P.; Carvalho, J.C.; Peixoto-Silva, N.; Santos, T.R.; Claudio-Neto, S.; Manhães, A.C.; Oliveira, E.; et al. Short and Long-Term Effects of Bisphenol S (BPS) Exposure during Pregnancy and Lactation on Plasma Lipids, Hormones, and Behavior in Rats. Environ. Pollut. 2019, 250, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Song, H.; Dong, Y.; Huai, Z.; Fu, Y.; Yu, P.; Huang, B.; Yang, R.; Guo, Y.; Meng, Q.; et al. Sex-Dependent and Long-Lasting Effects of Bisphenol AF Exposure on Emotional Behaviors in Mice. Physiol. Behav. 2022, 249, 113747. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, B.; Gioiosa, L.; Panzica, G.; Marraudino, M. Exposure to Either Bisphenol A or S Represents a Risk for Crucial Behaviors for Pup Survival, Such as Spontaneous Maternal Behavior in Mice. Neuroendocrinology 2022, 113, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Catanese, M.C.; Vandenberg, L.N. Bisphenol S (BPS) Alters Maternal Behavior and Brain in Mice Exposed during Pregnancy/Lactation and Their Daughters. Endocrinology 2016, 158, 516–530. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Shin, H.S.; Lee, S.H.; Hong, E.-J.; Ahn, C.; Yoo, Y.-M.; Jeung, E.-B.; Lee, G.-S.; An, B.-S.; Jung, E.-M. Effects of Prenatal Bisphenol S and Bisphenol F Exposure on Behavior of Offspring Mice. Anim. Cells Syst. 2023, 27, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Colon, E.; Chawla, S.; Vandenberg, L.N.; Suvorov, A. Endocrine Disruptors Alter Social Behaviors and Indirectly Influence Social Hierarchies via Changes in Body Weight. Environ. Health 2015, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, X.-C.; Li, S.; Dou, L.-J.; Zhou, L.; Wang, F.-H.; Ma, K.; Huang, D.; Pan, Y.; Gu, J.-J.; et al. Perinatal Low-Dose Bisphenol AF Exposure Impairs Synaptic Plasticity and Cognitive Function of Adult Offspring in a Sex-Dependent Manner. Sci. Total Environ. 2021, 788, 147918. [Google Scholar] [CrossRef] [PubMed]
- Vancamp, P.; Butruille, L.; Herranen, A.; Boelen, A.; Fini, J.-B.; Demeneix, B.A.; Remaud, S. Transient Developmental Exposure to Low Doses of Bisphenol F Negatively Affects Neurogliogenesis and Olfactory Behaviour in Adult Mice. Environ. Int. 2023, 172, 107770. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.; Sánchez, P.; Torres, J.M.; Ortega, E. Bisphenol A, Bisphenol F and Bisphenol S Affect Differently 5α-Reductase Expression and Dopamine-Serotonin Systems in the Prefrontal Cortex of Juvenile Female Rats. Environ. Res. 2015, 142, 281–287. [Google Scholar] [CrossRef]
- Bonaldo, B.; Casile, A.; Ostuni, M.T.; Bettarelli, M.; Nasini, S.; Marraudino, M.; Panzica, G.; Gotti, S. Perinatal Exposure to Bisphenol A or S: Effects on Anxiety-Related Behaviors and Serotonergic System. Chemosphere 2024, 349, 140827. [Google Scholar] [CrossRef]
- LaPlante, C.D.; Catanese, M.C.; Bansal, R.; Vandenberg, L.N. Bisphenol S Alters the Lactating Mammary Gland and Nursing Behaviors in Mice Exposed during Pregnancy and Lactation. Endocrinology 2017, 158, 3448–3461. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, A.; Philips, E.M.; Ghassabian, A.; Santos, S.; Asimakopoulos, A.G.; Kannan, K.; Kortenkamp, A.; Jaddoe, V.W.V.; Trasande, L.; Peeters, R.P.; et al. Association of Urinary Bisphenols during Pregnancy with Maternal, Cord Blood and Childhood Thyroid Function. Environ. Int. 2021, 146, 106160. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Lv, C.; Zhang, Y.; Shi, R.; Lu, Q.; Tian, Y.; Lei, X.; Gao, Y. Associations of Exposure to Bisphenol A and Its Substitutes with Neurodevelopmental Outcomes among Infants at 12 Months of Age: A Cross-Sectional Study. Chemosphere 2023, 341, 139973. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Miao, M.; Wang, Z.; Ji, H.; Zhou, Y.; Liang, H.; He, G.; Yuan, W. Prenatal Bisphenol Exposure and Intelligence Quotient in Children at Six Years of Age: A Prospective Cohort Study. Chemosphere 2023, 334, 139023. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.M.; Hallerbäck, M.U.; Wikström, S.; Lindh, C.; Kiviranta, H.; Gennings, C.; Bornehag, C.-G. Early Prenatal Exposure to Suspected Endocrine Disruptor Mixtures Is Associated with Lower IQ at Age Seven. Environ. Int. 2020, 134, 105185. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kim, J.L.; Hwang, K.-S.; Park, H.-C.; Bae, M.A.; Kim, K.-T.; Cho, S.-H. Mechanism of Action and Neurotoxic Effects of Chronic Exposure to Bisphenol F in Adult Zebrafish. Sci. Total Environ. 2022, 851, 158258. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, J.; Xu, S.; Zhou, Y.; Zhao, H.; Li, Y.; Xiong, C.; Sun, X.; Liu, H.; Liu, W.; et al. Prenatal Exposure to Bisphenol A and Its Alternatives and Child Neurodevelopment at 2 Years. J. Hazard. Mater. 2020, 388, 121774. [Google Scholar] [CrossRef] [PubMed]
- van den Dries, M.A.; Guxens, M.; Spaan, S.; Ferguson, K.K.; Philips, E.; Santos, S.; Jaddoe, V.W.V.; Ghassabian, A.; Trasande, L.; Tiemeier, H.; et al. Phthalate and Bisphenol Exposure during Pregnancy and Offspring Nonverbal IQ. Environ. Health Perspect. 2020, 128, 077009. [Google Scholar] [CrossRef]
- Geiger, S.D.; Musaad, S.; Hill, J.; Aguiar, A.; Schantz, S. Sex-Specific Associations between Urinary Bisphenols Concentrations during Pregnancy and Problematic Child Behaviors at Age 2 Years. Neurotoxicol Teratol. 2023, 96, 107152. [Google Scholar] [CrossRef]
- Liu, J.; Martin, L.J.; Dinu, I.; Field, C.J.; Dewey, D.; Martin, J.W. Interaction of Prenatal Bisphenols, Maternal Nutrients, and Toxic Metal Exposures on Neurodevelopment of 2-Year-Olds in the APrON Cohort. Environ. Int. 2021, 155, 106601. [Google Scholar] [CrossRef]
- Bornehag, C.-G.; Engdahl, E.; Unenge Hallerbäck, M.; Wikström, S.; Lindh, C.; Rüegg, J.; Tanner, E.; Gennings, C. Prenatal Exposure to Bisphenols and Cognitive Function in Children at 7 Years of Age in the Swedish SELMA Study. Environ. Int. 2021, 150, 106433. [Google Scholar] [CrossRef]
- Kim, J.I.; Lee, Y.A.; Shin, C.H.; Hong, Y.-C.; Kim, B.-N.; Lim, Y.-H. Association of Bisphenol A, Bisphenol F, and Bisphenol S with ADHD Symptoms in Children. Environ. Int. 2022, 161, 107093. [Google Scholar] [CrossRef]
- Ivantsova, E.; Lopez-Scarim, V.; Sultan, A.; English, C.; Biju, A.; Souders, C.L.; Padillo-Anthemides, N.E.; Konig, I.; Martyniuk, C.J. Evidence for Neurotoxicity and Oxidative Stress in Zebrafish Embryos/Larvae Treated with HFPO-DA Ammonium Salt (GenX). Environ. Toxicol. Pharmacol. 2023, 104, 104315. [Google Scholar] [CrossRef] [PubMed]
- Wasel, O.; King, H.; Choi, Y.J.; Lee, L.S.; Freeman, J.L. Differential Developmental Neurotoxicity and Tissue Uptake of the Per- and Polyfluoroalkyl Substance Alternatives, GenX and PFBS. Environ. Sci. Technol. 2023, 57, 19274–19284. [Google Scholar] [CrossRef]
- Rericha, Y.; Cao, D.; Truong, L.; Simonich, M.; Field, J.A.; Tanguay, R.L. Behavior Effects of Structurally Diverse Per- and Polyfluoroalkyl Substances (PFAS) in Zebrafish. Chem. Res. Toxicol. 2021, 34, 1409–1416. [Google Scholar] [CrossRef]
- Wasel, O.; Thompson, K.M.; Freeman, J.L. Assessment of Unique Behavioral, Morphological, and Molecular Alterations in the Comparative Developmental Toxicity Profiles of PFOA, PFHxA, and PFBA Using the Zebrafish Model System. Environ. Int. 2022, 170, 107642. [Google Scholar] [CrossRef]
- Ulhaq, M.; Örn, S.; Carlsson, G.; Morrison, D.A.; Norrgren, L. Locomotor Behavior in Zebrafish (Danio rerio) Larvae Exposed to Perfluoroalkyl Acids. Aquat. Toxicol. 2013, 144–145, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Menger, F.; Pohl, J.; Ahrens, L.; Carlsson, G.; Örn, S. Behavioural Effects and Bioconcentration of Per- and Polyfluoroalkyl Substances (PFASs) in Zebrafish (Danio rerio) Embryos. Chemosphere 2020, 245, 125573. [Google Scholar] [CrossRef]
- Truong, L.; Rericha, Y.; Thunga, P.; Marvel, S.; Wallis, D.; Simonich, M.T.; Field, J.A.; Cao, D.; Reif, D.M.; Tanguay, R.L. Systematic Developmental Toxicity Assessment of a Structurally Diverse Library of PFAS in Zebrafish. J. Hazard. Mater. 2022, 431, 128615. [Google Scholar] [CrossRef]
- Annunziato, K.M.; Jantzen, C.E.; Gronske, M.C.; Cooper, K.R. Subtle Morphometric, Behavioral and Gene Expression Effects in Larval Zebrafish Exposed to PFHxA, PFHxS and 6:2 FTOH. Aquat. Toxicol. 2019, 208, 126–137. [Google Scholar] [CrossRef]
- Kim, S.; Stroski, K.M.; Killeen, G.; Smitherman, C.; Simcik, M.F.; Brooks, B.W. 8:8 Perfluoroalkyl Phosphinic Acid Affects Neurobehavioral Development, Thyroid Disruption, and DNA Methylation in Developing Zebrafish. Sci. Total Environ. 2020, 736, 139600. [Google Scholar] [CrossRef]
- Gebreab, K.Y.; Eeza, M.N.H.; Bai, T.; Zuberi, Z.; Matysik, J.; O’Shea, K.E.; Alia, A.; Berry, J.P. Comparative Toxicometabolomics of Perfluorooctanoic Acid (PFOA) and next-Generation Perfluoroalkyl Substances. Environ. Pollut. 2020, 265, 114928. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Lu, S.; Zheng, B.; Xie, P.; Chen, J.; Li, G.; Liu, C.; Wu, Q.; Cheng, H.; et al. Perfluorododecanoic Acid Exposure Induced Developmental Neurotoxicity in Zebrafish Embryos. Environ. Pollut. 2018, 241, 1018–1026. [Google Scholar] [CrossRef]
- Gaballah, S.; Swank, A.; Sobus, J.R.; Howey, X.M.; Schmid, J.; Catron, T.; McCord, J.; Hines, E.; Strynar, M.; Tal, T. Evaluation of Developmental Toxicity, Developmental Neurotoxicity, and Tissue Dose in Zebrafish Exposed to GenX and Other PFAS. Environ. Health Perspect. 2020, 128, 047005. [Google Scholar] [CrossRef]
- Jantzen, C.E.; Annunziato, K.A.; Bugel, S.M.; Cooper, K.R. PFOS, PFNA, and PFOA Sub-Lethal Exposure to Embryonic Zebrafish Have Different Toxicity Profiles in Terms of Morphometrics, Behavior and Gene Expression. Aquat. Toxicol. 2016, 175, 160–170. [Google Scholar] [CrossRef]
- Satbhai, K.; Vogs, C.; Crago, J. Comparative Toxicokinetics and Toxicity of PFOA and Its Replacement GenX in the Early Stages of Zebrafish. Chemosphere 2022, 308, 136131. [Google Scholar] [CrossRef]
- Conley, J.M.; Lambright, C.S.; Evans, N.; Strynar, M.J.; McCord, J.; McIntyre, B.S.; Travlos, G.S.; Cardon, M.C.; Medlock-Kakaley, E.; Hartig, P.C.; et al. Adverse Maternal, Fetal, and Postnatal Effects of Hexafluoropropylene Oxide Dimer Acid (GenX) from Oral Gestational Exposure in Sprague-Dawley Rats. Environ. Health Perspect. 2019, 127, 037008. [Google Scholar] [CrossRef]
- Blake, B.E.; Cope, H.A.; Hall, S.M.; Keys, R.D.; Mahler, B.W.; McCord, J.; Scott, B.; Stapleton, H.M.; Strynar, M.J.; Elmore, S.A.; et al. Evaluation of Maternal, Embryo, and Placental Effects in CD-1 Mice Following Gestational Exposure to Perfluorooctanoic Acid (PFOA) or Hexafluoropropylene Oxide Dimer Acid (HFPO-DA or GenX). Environ. Health Perspect. 2020, 128, 27006. [Google Scholar] [CrossRef]
- Wang, H.; Luo, F.; Zhang, Y.; Yang, X.; Zhang, S.; Zhang, J.; Tian, Y.; Zheng, L. Prenatal Exposure to Perfluoroalkyl Substances and Child Intelligence Quotient: Evidence from the Shanghai Birth Cohort. Environ. Int. 2023, 174, 107912. [Google Scholar] [CrossRef]
- Luo, F.; Chen, Q.; Yu, G.; Huo, X.; Wang, H.; Nian, M.; Tian, Y.; Xu, J.; Zhang, J.; Zhang, J. Exposure to Perfluoroalkyl Substances and Neurodevelopment in 2-Year-Old Children: A Prospective Cohort Study. Environ. Int. 2022, 166, 107384. [Google Scholar] [CrossRef]
- Carrizosa, C.; Murcia, M.; Ballesteros, V.; Costa, O.; Manzano-Salgado, C.B.; Ibarluzea, J.; Iñiguez, C.; Casas, M.; Andiarena, A.; Llop, S.; et al. Prenatal Perfluoroalkyl Substance Exposure and Neuropsychological Development throughout Childhood: The INMA Project. J. Hazard. Mater. 2021, 416, 125185. [Google Scholar] [CrossRef] [PubMed]
- Beck, I.H.; Bilenberg, N.; Andersen, H.R.; Trecca, F.; Bleses, D.; Jensen, T.K. Association between Prenatal or Early Postnatal Exposure to Perfluoroalkyl Substances and Language Development in 18 to 36-Month-Old Children from the Odense Child Cohort. Environ. Health 2023, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rogan, W.J.; Chen, H.-Y.; Chen, P.-C.; Su, P.-H.; Chen, H.-Y.; Wang, S.-L. Prenatal Exposure to Perfluroalkyl Substances and Children’s IQ: The Taiwan Maternal and Infant Cohort Study. Int. J. Hyg. Environ. Health 2015, 218, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Spratlen, M.J.; Perera, F.P.; Lederman, S.A.; Rauh, V.A.; Robinson, M.; Kannan, K.; Trasande, L.; Herbstman, J. The Association between Prenatal Exposure to Perfluoroalkyl Substances and Childhood Neurodevelopment. Environ. Pollut. 2020, 263, 114444. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Vinturache, A.; Lei, X.; Wang, Z.; Pan, C.; Shi, R.; Yuan, T.; Gao, Y.; Tian, Y. Prenatal Exposure to Per- and Polyfluoroalkyl Substances, Fetal Thyroid Hormones, and Infant Neurodevelopment. Environ. Res. 2022, 206, 112561. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Q.; Wang, P.; Li, J.; Zhao, W.; Zhang, L.; Wang, H.; Cheng, Y.; Shi, H.; Li, J.; et al. Associations of Prenatal PFAS Exposure and Early Childhood Neurodevelopment: Evidence from the Shanghai Maternal-Child Pairs Cohort. Environ. Int. 2023, 173, 107850. [Google Scholar] [CrossRef]
- Niu, J.; Liang, H.; Tian, Y.; Yuan, W.; Xiao, H.; Hu, H.; Sun, X.; Song, X.; Wen, S.; Yang, L.; et al. Prenatal Plasma Concentrations of Perfluoroalkyl and Polyfluoroalkyl Substances and Neuropsychological Development in Children at Four Years of Age. Environ. Health 2019, 18, 53. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Bennett, D.H.; Calafat, A.M.; Tancredi, D.; Roa, D.L.; Schmidt, R.J.; Hertz-Picciotto, I.; Shin, H.-M. Prenatal Exposure to Per- and Polyfluoroalkyl Substances in Association with Autism Spectrum Disorder in the MARBLES Study. Environ. Int. 2021, 147, 106328. [Google Scholar] [CrossRef] [PubMed]
- Vuong, A.M.; Webster, G.M.; Yolton, K.; Calafat, A.M.; Muckle, G.; Lanphear, B.P.; Chen, A. Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFAS) and Neurobehavior in US Children through 8 Years of Age: The HOME Study. Environ. Res. 2021, 195, 110825. [Google Scholar] [CrossRef]
- Harris, M.H.; Oken, E.; Rifas-Shiman, S.L.; Calafat, A.M.; Ye, X.; Bellinger, D.C.; Webster, T.F.; White, R.F.; Sagiv, S.K. Prenatal and Childhood Exposure to Per- and Polyfluoroalkyl Substances (PFASs) and Child Cognition. Environ. Int. 2018, 115, 358–369. [Google Scholar] [CrossRef]
- Kapadia, R.; Yi, J.-H.; Vemuganti, R. Mechanisms of Anti-Inflammatory and Neuroprotective Actions of PPAR-Gamma Agonists. Front. Biosci. 2008, 13, 1813–1826. [Google Scholar] [CrossRef]
- Vanden Heuvel, J.P.; Thompson, J.T.; Frame, S.R.; Gillies, P.J. Differential Activation of Nuclear Receptors by Perfluorinated Fatty Acid Analogs and Natural Fatty Acids: A Comparison of Human, Mouse, and Rat Peroxisome Proliferator-Activated Receptor-Alpha, -Beta, and -Gamma, Liver X Receptor-Beta, and Retinoid X Receptor-Alpha. Toxicol. Sci. 2006, 92, 476–489. [Google Scholar] [CrossRef]
- Enright, E.A.; Eick, S.M.; Morello-Frosch, R.; Aguiar, A.; Woodbury, M.L.; Sprowles, J.L.N.; Geiger, S.D.; Trowbridge, J.; Andrade, A.; Smith, S.; et al. Associations of Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFAS) with Measures of Cognition in 7.5-Month-Old Infants: An Exploratory Study. Neurotoxicol. Teratol. 2023, 98, 107182. [Google Scholar] [CrossRef]
- Vuong, A.M.; Braun, J.M.; Yolton, K.; Wang, Z.; Xie, C.; Webster, G.M.; Ye, X.; Calafat, A.M.; Dietrich, K.N.; Lanphear, B.P.; et al. Prenatal and Childhood Exposure to Perfluoroalkyl Substances (PFAS) and Measures of Attention, Impulse Control, and Visual Spatial Abilities. Environ. Int. 2018, 119, 413–420. [Google Scholar] [CrossRef]
- Vuong, A.M.; Yolton, K.; Xie, C.; Dietrich, K.N.; Braun, J.M.; Webster, G.M.; Calafat, A.M.; Lanphear, B.P.; Chen, A. Prenatal and Childhood Exposure to Poly- and Perfluoroalkyl Substances (PFAS) and Cognitive Development in Children at Age 8 Years. Environ. Res. 2019, 172, 242–248. [Google Scholar] [CrossRef]
- Jeddy, Z.; Hartman, T.J.; Taylor, E.V.; Poteete, C.; Kordas, K. Prenatal Concentrations of Perfluoroalkyl Substances and Early Communication Development in British Girls. Early Hum. Dev. 2017, 109, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.H.; Oken, E.; Rifas-Shiman, S.L.; Calafat, A.M.; Bellinger, D.C.; Webster, T.F.; White, R.F.; Sagiv, S.K. Prenatal and Childhood Exposure to Per- and Polyfluoroalkyl Substances (PFAS) and Child Executive Function and Behavioral Problems. Environ. Res. 2021, 202, 111621. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, B.-N.; Lee, Y.A.; Shin, C.H.; Hong, Y.-C.; Døssing, L.D.; Hildebrandt, G.; Lim, Y.-H. Association between Early-Childhood Exposure to Perfluoroalkyl Substances and ADHD Symptoms: A Prospective Cohort Study. Sci. Total Environ. 2023, 879, 163081. [Google Scholar] [CrossRef]
- Høyer, B.B.; Bonde, J.P.; Tøttenborg, S.S.; Ramlau-Hansen, C.H.; Lindh, C.; Pedersen, H.S.; Toft, G. Exposure to Perfluoroalkyl Substances during Pregnancy and Child Behaviour at 5 to 9years of Age. Horm. Behav. 2018, 101, 105–112. [Google Scholar] [CrossRef]
- Skogheim, T.S.; Weyde, K.V.F.; Aase, H.; Engel, S.M.; Surén, P.; Øie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Brantsæter, A.L.; Haug, L.S.; et al. Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFAS) and Associations with Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Children. Environ. Res. 2021, 202, 111692. [Google Scholar] [CrossRef]
- Oulhote, Y.; Steuerwald, U.; Debes, F.; Weihe, P.; Grandjean, P. Behavioral Difficulties in 7-Year Old Children in Relation to Developmental Exposure to Perfluorinated Alkyl Substances. Environ. Int. 2016, 97, 237–245. [Google Scholar] [CrossRef]
- Li, Q.-Q.; Huang, J.; Cai, D.; Chou, W.-C.; Zeeshan, M.; Chu, C.; Zhou, Y.; Lin, L.; Ma, H.-M.; Tang, C.; et al. Prenatal Exposure to Legacy and Alternative Per- and Polyfluoroalkyl Substances and Neuropsychological Development Trajectories over the First 3 Years of Life. Environ. Sci. Technol. 2023, 57, 3746–3757. [Google Scholar] [CrossRef]
- Gump, B.B.; Wu, Q.; Dumas, A.K.; Kannan, K. Perfluorochemical (PFC) Exposure in Children: Associations with Impaired Response Inhibition. Environ. Sci. Technol. 2011, 45, 8151–8159. [Google Scholar] [CrossRef]
- Skogheim, T.S.; Villanger, G.D.; Weyde, K.V.F.; Engel, S.M.; Surén, P.; Øie, M.G.; Skogan, A.H.; Biele, G.; Zeiner, P.; Øvergaard, K.R.; et al. Prenatal Exposure to Perfluoroalkyl Substances and Associations with Symptoms of Attention-Deficit/Hyperactivity Disorder and Cognitive Functions in Preschool Children. Int. J. Hyg. Environ. Health 2020, 223, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Eliot, M.N.; Papandonatos, G.D.; Kelsey, K.T.; Fore, R.; Langevin, S.; Buckley, J.; Chen, A.; Lanphear, B.P.; Cecil, K.M.; et al. Gestational Perfluoroalkyl Substance Exposure and DNA Methylation at Birth and 12 Years of Age: A Longitudinal Epigenome-Wide Association Study. Environ. Health Perspect. 2022, 130, 037005. [Google Scholar] [CrossRef]
- Liew, Z.; Ritz, B.; Bach, C.C.; Asarnow, R.F.; Bech, B.H.; Nohr, E.A.; Bossi, R.; Henriksen, T.B.; Bonefeld-Jørgensen, E.C.; Olsen, J. Prenatal Exposure to Perfluoroalkyl Substances and IQ Scores at Age 5; a Study in the Danish National Birth Cohort. Environ. Health Perspect. 2018, 126, 067004. [Google Scholar] [CrossRef]
- Zhang, H.; Yolton, K.; Webster, G.M.; Ye, X.; Calafat, A.M.; Dietrich, K.N.; Xu, Y.; Xie, C.; Braun, J.M.; Lanphear, B.P.; et al. Prenatal and Childhood Perfluoroalkyl Substances Exposures and Children’s Reading Skills at Ages 5 and 8 Years. Environ. Int. 2018, 111, 224–231. [Google Scholar] [CrossRef]
- Vuong, A.M.; Yolton, K.; Wang, Z.; Xie, C.; Webster, G.M.; Ye, X.; Calafat, A.M.; Braun, J.M.; Dietrich, K.N.; Lanphear, B.P.; et al. Childhood Perfluoroalkyl Substance Exposure and Executive Function in Children at 8 years. Environ. Int. 2018, 119, 212–219. [Google Scholar] [CrossRef]
- Beck, I.H.; Bilenberg, N.; Möller, S.; Nielsen, F.; Grandjean, P.; Højsager, F.D.; Halldorsson, T.I.; Nielsen, C.; Jensen, T.K. Association between Prenatal and Early Postnatal Exposure to Perfluoroalkyl Substances and IQ Score in 7-Year-Old Children from the Odense Child Cohort. Am. J. Epidemiol. 2023, 192, 1522–1535. [Google Scholar] [CrossRef]
- Oh, J.; Schmidt, R.J.; Tancredi, D.; Calafat, A.M.; Roa, D.L.; Hertz-Picciotto, I.; Shin, H.-M. Prenatal Exposure to Per- and Polyfluoroalkyl Substances and Cognitive Development in Infancy and Toddlerhood. Environ. Res. 2021, 196, 110939. [Google Scholar] [CrossRef]
- Shin, H.-M.; Bennett, D.H.; Calafat, A.M.; Tancredi, D.; Hertz-Picciotto, I. Modeled Prenatal Exposure to Per- and Polyfluoroalkyl Substances in Association with Child Autism Spectrum Disorder: A Case-Control Study. Environ. Res. 2020, 186, 109514. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, K.; Ji, K.; Seo, J.; Kho, Y.; Park, J.; Kim, S.; Park, S.; Hwang, I.; Jeon, J.; et al. Trans-Placental Transfer of Thirteen Perfluorinated Compounds and Relations with Fetal Thyroid Hormones. Environ. Sci. Technol. 2011, 45, 7465–7472. [Google Scholar] [CrossRef] [PubMed]
- Vilhelmsson, A.; Rylander, L.; Jöud, A.; Lindh, C.H.; Mattsson, K.; Liew, Z.; Guo, P.; Ritz, B.; Källén, K.; Thacher, J.D. Exposure to per- and Polyfluoroalkyl Substances in Early Pregnancy and Risk of Cerebral Palsy in Children. Sci. Total Environ. 2023, 899, 165622. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Ritz, B.; von Ehrenstein, O.S.; Bech, B.H.; Nohr, E.A.; Fei, C.; Bossi, R.; Henriksen, T.B.; Bonefeld-Jørgensen, E.C.; Olsen, J. Attention Deficit/Hyperactivity Disorder and Childhood Autism in Association with Prenatal Exposure to Perfluoroalkyl Substances: A Nested Case–Control Study in the Danish National Birth Cohort. Environ. Health Perspect. 2015, 123, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Dalsager, L.; Jensen, T.K.; Nielsen, F.; Grandjean, P.; Bilenberg, N.; Andersen, H.R. No Association between Maternal and Child PFAS Concentrations and Repeated Measures of ADHD Symptoms at Age 2½ and 5 Years in Children from the Odense Child Cohort. Neurotoxicol. Teratol. 2021, 88, 107031. [Google Scholar] [CrossRef] [PubMed]
- Ode, A.; Källén, K.; Gustafsson, P.; Rylander, L.; Jönsson, B.A.G.; Olofsson, P.; Ivarsson, S.A.; Lindh, C.H.; Rignell-Hydbom, A. Fetal Exposure to Perfluorinated Compounds and Attention Deficit Hyperactivity Disorder in Childhood. PLoS ONE 2014, 9, e95891. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.R.; Savitz, D.A. Serum Perfluorinated Compound Concentration and Attention Deficit/Hyperactivity Disorder in Children 5-18 Years of Age. Environ. Health Perspect. 2011, 119, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Vuong, A.M.; Yolton, K.; Webster, G.M.; Sjödin, A.; Calafat, A.M.; Braun, J.M.; Dietrich, K.N.; Lanphear, B.P.; Chen, A. Prenatal Polybrominated Diphenyl Ether and Perfluoroalkyl Substance Exposures and Executive Function in School-Age Children. Environ. Res. 2016, 147, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Kalkbrenner, A.E.; Just, A.C.; Yolton, K.; Calafat, A.M.; Sjödin, A.; Hauser, R.; Webster, G.M.; Chen, A.; Lanphear, B.P. Gestational Exposure to Endocrine-Disrupting Chemicals and Reciprocal Social, Repetitive, and Stereotypic Behaviors in 4- and 5-Year-Old Children: The HOME Study. Environ. Health Perspect. 2014, 122, 513–520. [Google Scholar] [CrossRef]
- Yun, K.; Jeon, H.; Lee, J.; Kho, Y.; Ji, K. Effects of Two Alternative Plasticizers on the Growth Hormone-Related Endocrine System, Neurodevelopment, and Oxidative Stress of Zebrafish Larvae. Environ. Pollut. 2024, 341, 122947. [Google Scholar] [CrossRef]
- Saad, N.; Bereketoglu, C.; Pradhan, A. Di(Isononyl) Cyclohexane-1,2-Dicarboxylate (DINCH) Alters Transcriptional Profiles, Lipid Metabolism and Behavior in Zebrafish Larvae. Heliyon 2021, 7, e07951. [Google Scholar] [CrossRef]
- Horie, Y.; Yap, C.K.; Okamura, H. Developmental Toxicity and Thyroid Hormone-Disrupting Effects of Acetyl Tributyl Citrate in Zebrafish and Japanese Medaka. J. Hazard. Mater. Adv. 2022, 8, 100199. [Google Scholar] [CrossRef]
- Tan, H.; Gao, P.; Luo, Y.; Gou, X.; Xia, P.; Wang, P.; Yan, L.; Zhang, S.; Guo, J.; Zhang, X.; et al. Are New Phthalate Ester Substitutes Safer than Traditional DBP and DiBP? Comparative Endocrine-Disrupting Analyses on Zebrafish Using In Vivo, Transcriptome, and In Silico Approaches. Environ. Sci. Technol. 2023, 57, 13744–13756. [Google Scholar] [CrossRef]
- Lee, H.-C.; Yamanouchi, K.; Nishihara, M. Effects of Perinatal Exposure to Phthalate/Adipate Esters on Hypothalamic Gene Expression and Sexual Behavior in Rats. J. Reprod. Dev. 2006, 52, 343–352. [Google Scholar] [CrossRef]
- Park, S.; Zimmerman, E.; Huerta-Montañez, G.; Rosario-Pabón, Z.; Vélez-Vega, C.M.; Cordero, J.F.; Alshwabekah, A.; Meeker, J.D.; Watkins, D.J. Gestational Exposure to Phthalates and Phthalate Replacements in Relation to Neurodevelopmental Delays in Early Childhood. Toxics 2023, 11, 65. [Google Scholar] [CrossRef]
- Rosolen, V.; Giordani, E.; Mariuz, M.; Parpinel, M.; Ronfani, L.; Vecchi Brumatti, L.; Bin, M.; Calamandrei, G.; Mustieles, V.; Gilles, L.; et al. Concurrent Assessment of Phthalates/HEXAMOLL® DINCH Exposure and Wechsler Intelligence Scale for Children Performance in Three European Cohorts of the HBM4EU Aligned Studies. Toxics 2022, 10, 538. [Google Scholar] [CrossRef]
- Cathey, A.L.; Watkins, D.; Rosario, Z.Y.; Vélez, C.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Associations of Phthalates and Phthalate Replacements With CRH and Other Hormones among Pregnant Women in Puerto Rico. J. Endocr. Soc. 2019, 3, 1127–1149. [Google Scholar] [CrossRef]
- Derakhshan, A.; Shu, H.; Broeren, M.A.C.; Lindh, C.H.; Peeters, R.P.; Kortenkamp, A.; Demeneix, B.; Bornehag, C.-G.; Korevaar, T.I.M. Association of Phthalate Exposure with Thyroid Function during Pregnancy. Environ. Int. 2021, 157, 106795. [Google Scholar] [CrossRef]
- Mu, X.; Huang, Y.; Li, X.; Lei, Y.; Teng, M.; Li, X.; Wang, C.; Li, Y. Developmental Effects and Estrogenicity of Bisphenol A Alternatives in a Zebrafish Embryo Model. Environ. Sci. Technol. 2018, 52, 3222–3231. [Google Scholar] [CrossRef]
- Moreman, J.; Lee, O.; Trznadel, M.; David, A.; Kudoh, T.; Tyler, C.R. Acute Toxicity, Teratogenic, and Estrogenic Effects of Bisphenol A and Its Alternative Replacements Bisphenol S, Bisphenol F, and Bisphenol AF in Zebrafish Embryo-Larvae. Environ. Sci. Technol. 2017, 51, 12796–12805. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are Bioplastics and Plant-Based Materials Safer than Conventional Plastics? In Vitro Toxicity and Chemical Composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.P.J.; Estrela, F.N.; Rodrigues, A.S.d.L.; Guimarães, A.T.B.; Rocha, T.L.; Malafaia, G. Behavioral and Biochemical Consequences of Danio Rerio Larvae Exposure to Polylactic Acid Bioplastic. J. Hazard. Mater. 2021, 404, 124152. [Google Scholar] [CrossRef]
- Patisaul, H.B. Endocrine Disruption by Dietary Phyto-Oestrogens: Impact on Dimorphic Sexual Systems and Behaviours. Proc. Nutr. Soc. 2017, 76, 130–144. [Google Scholar] [CrossRef]

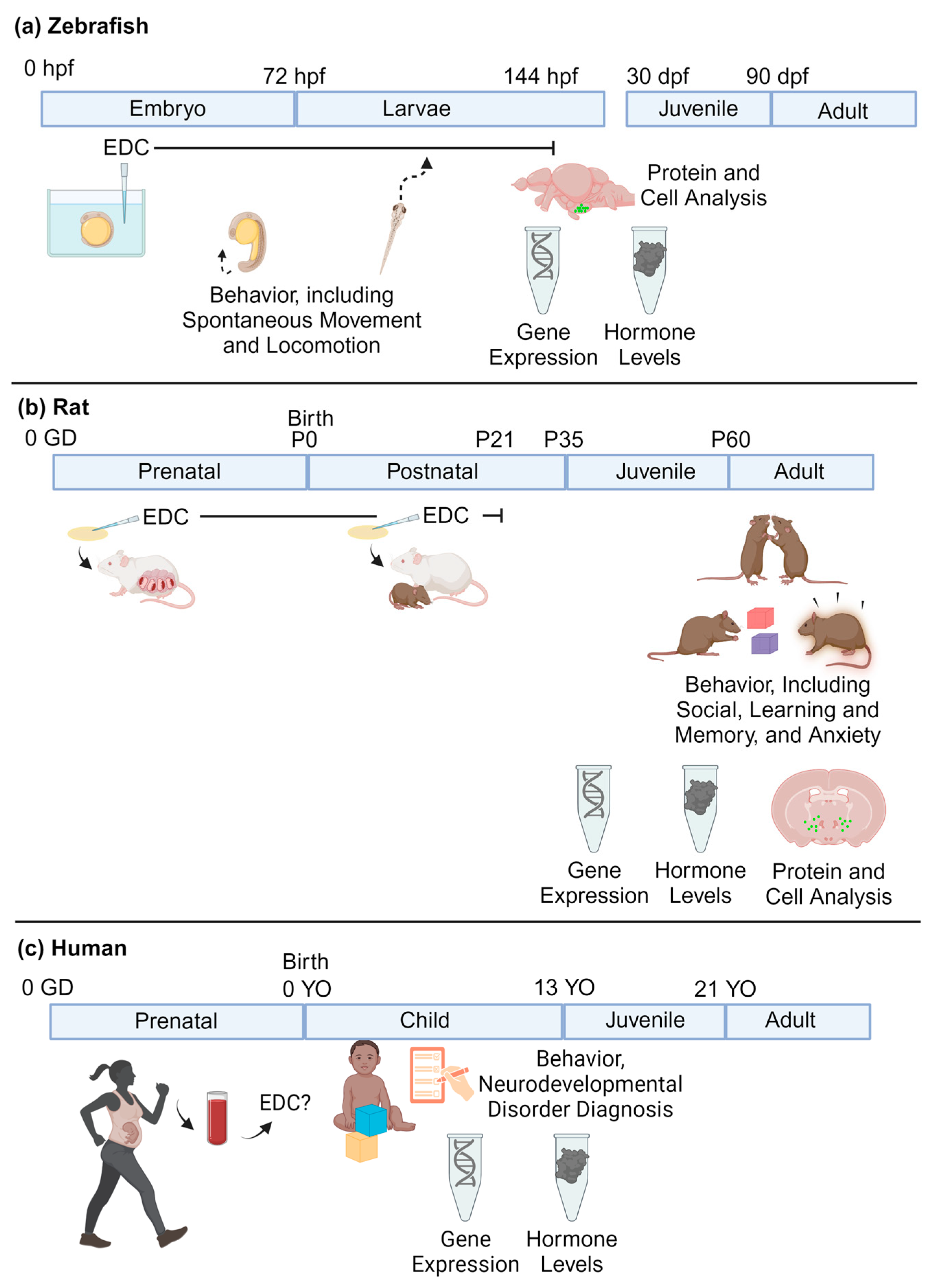
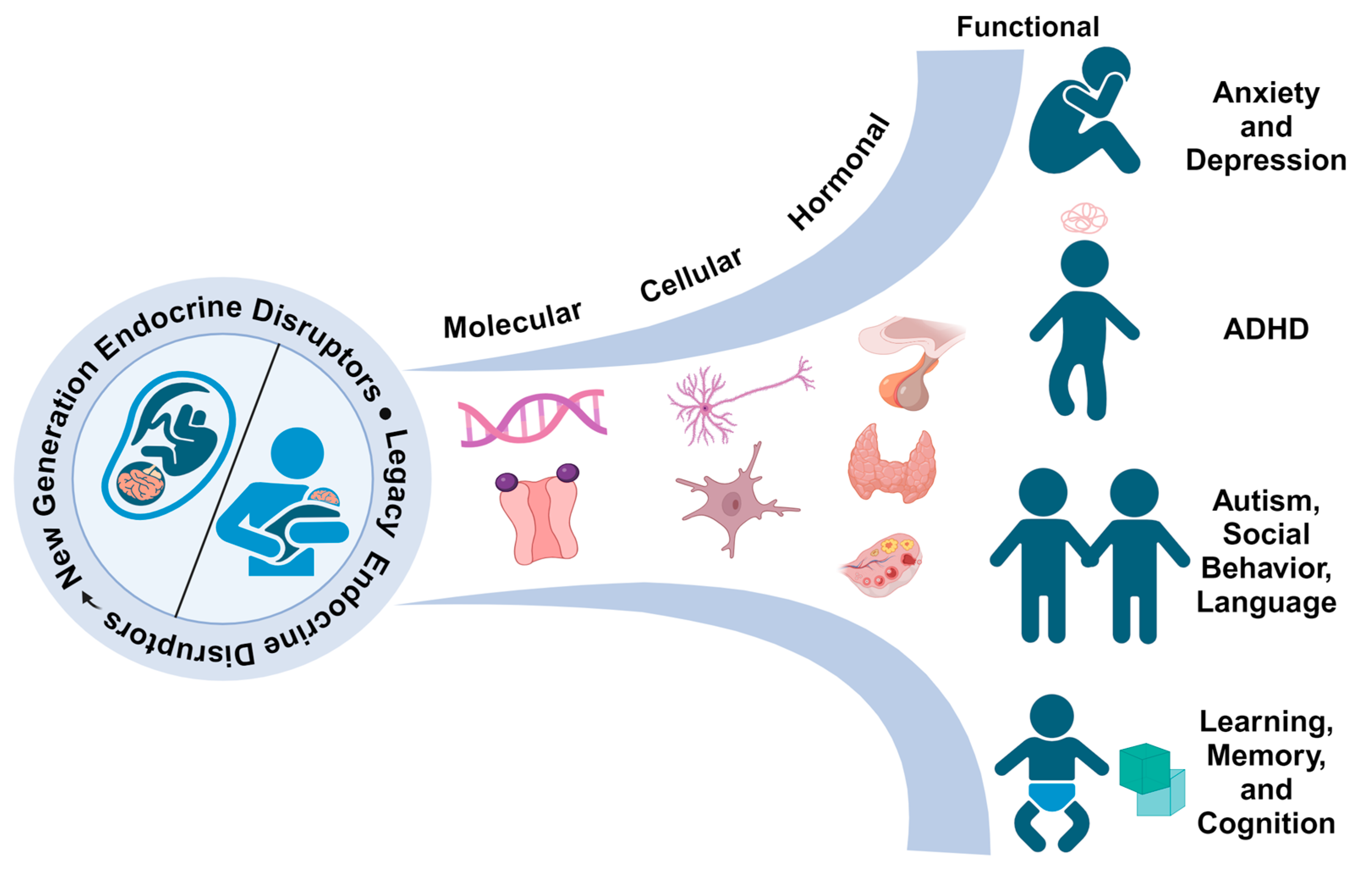
| Bisphenols | ||
|---|---|---|
| Abbreviation | Common Name | CASRN |
| BPAF | Bisphenol AF | 1478-61-1 |
| BPAP | Bisphenol AP | 1571-75-1 |
| BPB | Bisphenol B | 77-40-7 |
| BPF | Bisphenol F | 620-92-8 |
| BPP | Bisphenol P | 2167-51-3 |
| BPS | Bisphenol S | 80-09-1 |
| BPS-MAE | - | 97042-18-7 |
| BPZ | Bisphenol Z | 843-55-0 |
| TCBPF | Tetrachlorobisphenol A | 79-95-8 |
| TMBPF | Tetramethylbisphenol F | 5384-21-4 |
| Per- and polyfluoroalkyl substances (PFAS) | ||
| 6:2 Cl-PFESA/F-53B | 6:2 chlorinated polyfluorooctane ether sulfonic acid | 73606-19-6 |
| 8:2 Cl-PFESA/F-53B | 8:2 chlorinated polyfluorooctane ether sulfonic acid | 83329-89-9 |
| ADONA | 4,8-dioxa-3H-perfluorononanoic | 919005-14-4 |
| EtFOSAA/NEtFOSAA | 2-(N-ethyl-perfluorooctane sulfonamido) acetic acid | 2991-50-6 |
| FOSA | Perfluorooctanesulfonamide | 754-91-6 |
| GenX/HFPO-DA | Perfluoro-2-methyl-3-oxahexanoic acid | 13252-13-6 |
| MeFOSAA/NMeFOSA/ Me-PFOSA-AcOH | 2-(N-methyl-perfluorooctane sulfonamido) acetic acid | 2355-31-9 |
| PFBA | Perfluorobutanoic acid | 375-22-4 |
| PFBS | Perfluorobutanesulfonic acid | 375-73-5 |
| PFDA/PFDeA | Perfluorodecanoic acid | 335-76-2 |
| PFDoA/PFDoDA | Perfluorododecanoic acid | 307-55-1 |
| PFHpA | Perfluoroheptanoic acid | 375-85-9 |
| PFHpS | Perfluoroheptanesulfonic acid | 375-92-8 |
| PFNA | Perfluorononanoic acid | 375-95-1 |
| PFTrDA | Perfluorotridecanoic acid | 72629-94-8 |
| PFUA/PFUdA/PFUnA, PFUnDA | Perfluoroundecanoic acid | 2058-94-8 |
| Phthalate esters | ||
| DINCH | 1,2-cyclohexane dicarboxylic acid diisononyl ester | 166412-78-8 |
| ATBC | Acetyl tributyl citrate | 77-90-7 |
| ATEC | Triethyl 2-acetylcitrate | 77-89-4 |
| DGD | Dipropylene glycol dibenzoate | 27138-31-4 |
| GTA | Glyceryl triacetate | 102-76-1 |
| DEHA/DOA | Di-(2-ethylhexy) adipate | 103-23-1 |
| DEHT/DOTP/DEHTP | Di-(2-ethylhexyl) terephthalate | 6422-86-2 |
| EDC | Time of Exposure Measured | Conc. Measured | Findings | Reference |
|---|---|---|---|---|
| BPAF | 12 months old | <0.01–1.456 ng/mL | ↓ Association with social developmental quotient in girls No association with other developmental quotient domains (gross and fine motor, adaptive, and language domains) | Xia et al., 2023 [119] |
| BPF | 6 and 8 years old | Urinary Mean (ug/g creatinine): 6 years: 0.2 8 years: 0.4 | ↑ Association with ADHD rating scores, higher in girls than boys | Kim et al., 2022 [128] |
| BPF | Gestation (~10 weeks) | Urinary Mean (ng/mL): 0.16 ng/mL | ↓ Association with cognitive function, including verbal comprehension, perceptual reasoning, working memory, and processing speed in boys | Bornehag et al., 2021 [127] |
| BPF | Gestation (<12 weeks) | Urinary Mean (ng/mL): 0.16 | ↓ Association with IQ in boys | Tanner et al., 2020 [121] |
| BPF | Gestation (~13 weeks) | Urinary Mean (ug/L): 0.47 | ↓ Association with scores of IQ, perceptual reasoning index, and verbal comprehension index among boys | Chen et al., 2023 [120] |
| BPS | 6 and 8 years old | Urinary Mean (ug/g creatinine): 6 years: 0.1 8 years: 0.16 | ↑ Association with ADHD rating scores, higher in girls than boys | Kim et al., 2022 [128] |
| BPS | 16–19 weeks of pregnancy | Urinary Range (ng/mL): 0.1–3.5 | ↑ Association with emotionally reactive behaviors in girls | Geiger et al., 2023 [125] |
| BPS | Gestation (<16 weeks) | Urinary Range (ug/L): ~0.18–0.89 | ↓ Association with psychomotor development index in boys No association with mental development | Jiang et al., 2020 [123] |
| TCBPA | Gestation (~13 weeks) | Urinary Range (ug/L): ~0.01–0.08 | ↑ Association with IQ and perceptual reasoning index scores No association with verbal comprehension | Chen et al., 2023 [120] |
| BPF | Gestation (<16 weeks) | Urinary Range (ug/L): ~0.38–1.44 | No association with infant neurodevelopment (mental development, psychomotor development) | Jiang et al., 2020 [123] |
| BPF, BPS | Gestation (16–34 weeks) | Urinary Range (ng/mL): 0.1–82.7 (BPF), 0–46.7 (BPS) | No association with nonverbal IQ | van den Dries et al., 2020 [124] |
| BPS | Gestation (~17 weeks) | Urinary Mean (ug/g creatinine): 0.23 | No association with neurodevelopment test assessing cognitive, language, motor, social, emotional, and adaptive skills | Liu et al., 2021 [126] |
| BPS | Gestation (≤18 weeks) | Urinary Median (ng/mL): 0.34 | No association with cord blood or childhood TSH and FT4. | Derakhshan et al., 2021 [118] |
| BPS | Gestation (~10 weeks) | Urinary Mean (ng/mL): 0.07 | No association with cognitive function | Bornehag et al., 2021 [127] |
| BPS | Gestation (<12 weeks) | Urinary Mean (ng/mL): 0.07 | No association with IQ | Tanner et al., 2020 [121] |
| BPS | Gestation (~13 weeks) | Urinary Range (ug/L creatinine): ~0.03–0.13 (BPAF), ~0–0.002 (BPS) | No association with scores of IQ, perceptual reasoning index, and verbal comprehension index | Chen et al., 2023 [120] |
| BPS, BPB, BPAP, BPP, BPZ | 12 months old | Urinary Range (ng/mL): <0.01–54.605 (BPS), <0.01–0.686 (BPB), <0.01–16.195 (BPAP), <0.01–0.511 (BPP), <0.01–0.512 (BPZ) | No association with developmental quotients (gross and fine motor, adaptive, language, and social domains) | Xia et al., 2023 [119] |
| EDC | Time of Exposure (hpf) | Dose | Findings | Reference |
|---|---|---|---|---|
| 1H,1H,2H,2H-Perfluorohexyl iodide | 6–120 | 0.015–100 μM | ↓ Larvae photomotor behavior in the light period No effect on embryonic photomotor behavior | Truong et al., 2022 [135] |
| 1H,1H,8H,8H-Perfluorooctane-1,8-diol | 6–120 | 0.015–100 μM | ↓ Embryonic photomotor behavior ↓ Larvae photomotor behavior in the light and dark periods | Truong et al., 2022 [135] |
| 6:2 FTOH | 3–120 | 0.2, 2, 20 μM | ↑ Larvae locomotion at 2 μM and 20 μM ↑ Expression of bdnf and ap1s1 at 20 μM (neurodevelopment-related genes) | Annnunziato et al., 2019 [136] |
| 6:2 FTSA | ~4–144 | 0.006–180 μM | ↑ Larvae locomotion in dark period at 180 μM No effect on burst activity (movements > 6 mm) or “startle response” (peak distance − mean distance) | Menger et al., 2020 [134] |
| Six PFASs, including GenX, PFHpS (see paper for details) | 8–120 | 0.35–0.92 μM | ↓ Larvae photomotor behavior in light period No effect on embryonic photomotor behavior and larvae startle response | Rericha et al., 2021 [131] |
| 8:8 PFPiA | 4–144 | 0.0116–5.79 μM | ↓ Larvae locomotion at 0.343–5.79 μM in the dark periods ↑ Expression of chrb, dio3a, and tshr (HPT axis) at 5.79 μM; No effect on expression of dio3b, thraa, and thrb (axis) ↓ Expression of elavl3 at 1.35–5.79 μM (neurodevelopment) No effect on expression of mbp, syn2a, shha, and tuba1 (neurodevelopment) | Kim et al., 2020 [137] |
| DiSAMPAP | 8–120 | 0.20 μM | ↑ Larvae photomotor behavior in dark period No effect on embryonic photomotor behavior and larvae startle response | Rericha et al., 2021 [131] |
| Five PFASs (see paper for details) | 6–120 | 0.015–100 μM | ↑ Embryonic photomotor behavior No effect on larvae photomotor behavior | Truong et al., 2022 [135] |
| Five PFASs (see paper for details) | 6–120 | 0.015–100 μM | ↓ Larvae photomotor behavior in the dark period No effects on embryonic photomotor behavior | Truong et al., 2022 [135] |
| FOSA | 6–120 | 0.015–100 μM | ↑ Embryonic photomotor behavior ↓ Larvae photomotor behavior in the dark period | Truong et al., 2022 [135] |
| Four PFASs (see paper for details) | 6–120 | 0.015–100 μM | ↓ Embryonic photomotor behavior No effect on larvae photomotor response | Truong et al., 2022 [135] |
| Four PFASs, including PFUA (see paper for details) | 6–120 | 0.015–100 μM | ↑ Larvae photomotor behavior in the light and dark periods No effect on embryonic photomotor behavior | Truong et al., 2022 [135] |
| GenX | 6–168 | 0.1–10,000 μg/L | ↑ Expression of elavl3, gap43, tubb (neuron differentiation and growth), gfap (astrocytes); ↓ expression of manf (neurotrophic signals) at 0.1 and 1 μg/L (other doses not measured) No effect on expression of mbp (axon function), nestin (neuron structure) at 0.1 and 1 μg/L (other doses not measured) ↓ Larvae locomotion in dark period at 1000 μg/L; in light periods at 100 μg/L No effect in light-dark preference test (anxiety) | Ivantsova et al., 2023 [129] |
| GenX | 1–120 | 4–4000 ppb | ↑ Larvae locomotion at 40–4000 ppb in the dark periods; ↓ at 4000 ppb in the light period ↑ Dopamine at 40 ppb | Wasel et al., 2023 [130] |
| Methyl 3H-perfluoroisopropyl ether | 6–120 | 0.015–100 μM | ↓ Larvae photomotor behavior in the light and dark period | Truong et al., 2022 [135] |
| PFBA | 8–120 | 0.98–98 μM | ↑ Larvae photomotor behavior in the light period ↑ Larvae photomotor behavior at all doses in the dark period and at 2.49, 6.32, and 34.3–98 μM in the light period No effect on embryonic photomotor behavior and larvae startle response | Rericha et al., 2021 [131] |
| PFBA | 1–120 | 23,360–46,720 μM | ↓ Larvae locomotion at 0.4 ppb in the light; at 400, 4000 ppb in the dark; ↑ at 4 in the dark. ↑ Head width at 400 ppb ↑ Enrichment of pathways associated with neurological disorders, behavior, and nervous system development and function | Wasel et al., 2022 [132] |
| PFBS | 1–120 | 4–4000 ppb | ↑ Larvae locomotion at 4–4000 ppb in the dark period; at 40–4000 ppb in the light period ↓ Dopamine at 400 ppb | Wasel et al., 2023 [130] |
| PFBS | ~4–144 | 10–3000 mg/L | ↓ Larvae locomotion at 1000 μg/L (3000 mg/L not measured) | Ulhaq et al., 2013 [133] |
| PFDA | 8–120 | 0–100 μM | No effect on embryonic or larvae photomotor behavior and larvae startle response ↑ Larvae photomotor behavior in the dark period at 2.49 and 34.3 μM, and in the light at 2.5 μM ↓ at 16.07 and 100 μM | Rericha et al., 2021 [131] |
| PFDMMOBA | 4–168 | 100–300 ppm | ↑ Listing (falling to one side) | Gebreab et al., 2020 [138] |
| PFDoA | ~4–120 | 0, 0.24, 1.2, 6 mg/L | ↓ Larvae locomotion at all doses ↓ GFP neurons at 6 mg/L ↓ ACh at 6 mg/L and AChE activity at 1.2 or 6 mg/L ↑ Dopamine at 1.2 and 6 mg/L ↓ Expression α1-tubulin, gap43, gfap, shha, syn2a, ache at 6 mg/L; mbp, elavl3 at 1.2 and 6 mg/L; ↑ manf at 6 mg/L | Guo et al., 2018 [139] |
| PFECHS, PFPrS | 8–120 | 0.35–0.50 μM | ↓ Larvae startle response No effect on embryonic or larvae photomotor behavior | Rericha et al., 2021 [131] |
| PFHpA | ~4–144 | 0.002–89 μM | ↑larvae locomotion in light period at 89 μM No effect on burst activity (movements > 6 mm) or “startle response” (peak distance − mean distance) | Menger et al., 2020 [134] |
| PFHpA | 8–120 | 0.69 μM | No effect on embryonic photomotor behavior and larvae startle response ↑ Larvae photomotor behavior at 2.51 and 6.39 μM ↓ at 100 μM in the dark period | Rericha et al., 2021 [131] |
| PFHpS | 0–120 | 1.7–31.4 μM | ↑ Larvae locomotion at 3.1 and 5.5 μM in the light and dark periods | Gaballah et al., 2020 [140] |
| PFHpS, PFOS-K, PFTrDA | 6–120 | 0.015–100 μM | ↑ Larvae photomotor behavior in the light period; ↓ in the dark No effect on embryonic photomotor behavior | Truong et al., 2022 [135] |
| PFHxA | 8–120 | 0.80 μM | ↑ Area under curve analysis (change in movement from light to dark periods) in larvae photomotor behavior ↓ Larvae photomotor behavior at 2.46, 6.32, 34.3, and 73.3 μM in the dark period and ↑ at 2.46 and 16.07 μM in the light period No effect on embryonic photomotor behavior and larvae startle response | Rericha et al., 2021 [131] |
| PFHxA | 0–120 | 4.4–80.0 μM | ↑ Larvae locomotion at 25.1 μM in the light period and at 14 and 25.1 μM in the dark period | Gaballah et al., 2020 [140] |
| PFHxA | 3–120 | 0.2, 2, 20 μM | No effect on larvae locomotion ↑ Expression of bdnf at 20 μM; ap1s1 at 2 and 20 μM (neurodevelopment-related genes) | Annnunziato et al., 2019 [136] |
| PFHxA | 1–120 | 15,921.03–31,842.06 μM | No effect on larvae locomotion ↓ Head length at 40 and 400 ppb No found enrichment of pathways associated with the nervous system and behaviors | Wasel et al., 2022 [132] |
| PFNA | ~4–144 | 0.03–10 mg/L | ↓ Larvae locomotion at 10 mg/L | Ulhaq et al., 2013 [133] |
| PFNA | 3–120 | 1–2.0 μM | ↓ Larvae locomotion at all doses ↑ Time spent in well center at 0.2 and 2.0 μM | Jantzen et al., 2016 [141] |
| PFNA | ~4–144 | 0.001–200 μM | ↓ Larvae locomotion in light period at 48uM (higher doses not measured) ↑ Burst activity (movements > 6 mm) in the dark period at 15 and 48 μM | Menger et al., 2020 [134] |
| PFNA | 8–120 | 0–100 μM | ↓ Larvae startle response at 0.54 μM ↓ Larvae photomotor behavior at 33.95 μM in the dark period and at 2.46 and 33.95 μM in the light period ↑ in the light period at 0.97 and 72.56 μM No effect on embryonic photomotor behavior | Rericha et al., 2021 [131] |
| PFO2DA | 4–168 | 5–100 ppm | ↑ Listing (falling to one side) | Gebreab et al., 2020 [138] |
| PFO3DA | 4–168 | 25–200 ppm | ↑ Listing (falling to one side) | Gebreab et al., 2020 [138] |
| PFO3TDA | 4–168 | 1–40 ppm | ↑ Listing (falling to one side) | Gebreab et al., 2020 [138] |
| PFPeA | 8–120 | 0.97–97 μM | ↑ Larvae photomotor behavior at 15.91, 33.95, and 97 μM in the dark period and 6.26–33.95 μM in the light period ↓Larvae photomotor behavior in the light period at 0.95 μM No effect on embryonic photomotor behavior and larvae startle response | Rericha et al., 2021 [131] |
| PFPeS | 0–120 | 3.1–56.0 μM | ↑ Larvae locomotion at 3.1 and 5.5 μM in the light periods | Gaballah et al., 2020 [140] |
| PFTrDA | 8–120 | 0–35 μM | ↓ Larvae photomotor behavior at 10 μM in the dark period, 35 μM at light period, and ↑ at 10 μM in the light period. No effect on embryonic photomotor behavior and larvae startle response | Rericha et al., 2021 [131] |
| PFUA | 8–120 | 0–75.0 μM | No effect on embryonic photomotor behavior and larvae startle response ↓ Larvae photomotor behavior in dark period at 52.8 μM and in light period at 0.96 and 2.44 μM | Rericha et al., 2021 [131] |
| Six PFASs, including PFBS and PFDoA (see paper for details) | 8–120 | 0.25–0.75 μM (see paper for details) | ↑ Larvae photomotor behavior in light period No effect on embryonic photomotor behavior and larvae startle response | Rericha et al., 2021 [131] |
| TFAA | ~4–144 | 10–3000 mg/L | ↓ Larvae locomotion at 1000 and 3000 mg/L | Ulhaq et al., 2013 [133] |
| Three PFASs (see paper for details) | 6–120 | 0.015–100 μM | ↑ Larvae photomotor behavior in the light period No effect on embryonic photomotor behavior | Truong et al., 2022 [135] |
| Two PFASs (see paper for details) | 6–120 | 0.015–100 μM | ↑ Larvae photomotor behavior in the dark No effect on embryonic photomotor behavior | Truong et al., 2022 [135] |
| Two PFASs (see paper for details) | 6–120 | 0.015–100 μM | ↓ Larvae photomotor behavior in the dark period; ↑ in the light period No effect on embryonic photomotor behavior | Truong et al., 2022 [135] |
| 107 PFASs, including PFBA, PFBS, PFNA, GenX, PFDA, PFHpA, (see paper for details) | 6–120 | 0.015–100 μM | No effect on embryonic or larvae photomotor behavior | Truong et al., 2022 [135] |
| 33 PFASs, including EtFOSAA, PFNA, (see paper for details) | 8–120 | 0.27–1.03 μM (see paper for details) | No effect on embryonic or larvae photomotor behavior and larvae startle response | Rericha et al., 2021 [131] |
| ADONA, GenX | 0–120 | 4.4–80.0 μM | No effect on larvae locomotion | Gaballah et al., 2020 [140] |
| GenX | 5– 120 | 0.1–150 μM | No effect on startle response to light | Satbhai et al., 2022 [142] |
| PFBA, PFDA | ~4–144 | 10–3000 mg/L | No effect on larvae locomotion | Ulhaq et al., 2013 [133] |
| PFBS | 0–5dpf | 5.5–100.0 μM | No effect on larvae locomotion | Gaballah et al., 2020 [140] |
| PFESA1 | 0–120 | 4.4–80.0 μM | No effect on larvae locomotion | Gaballah et al., 2020 [140] |
| PFHxA, PFPeA, PFBS | ~4–144 | 0.002–84 μM (see paper for details) | No effect on larvae locomotion, burst activity (movements > 6 mm), or “startle response” (peak distance − mean distance) | Menger et al., 2020 [134] |
| PFMOBA PFO2HPA GenX | 4–168 | 25–200 ppm | No effect on listing (falling to one side) | Gebreab et al., 2020 [138] |
| EDC | Animal | Time of Exposure | Dose | Findings | Reference |
|---|---|---|---|---|---|
| GenX | Rats | GD14–18 | 62.5–500 mg/kg or 1–30 mg/kg | ↓ maternal T3 at 30–500 mg/kg; T4 at 125–500 mg/kg | Conley et al., 2019 [143] |
| GenX | Rats | GD1.5–11.5 or GD 1.5–17.5 | 2, 10 mg/kg | ↑ placental T4 at 10 mg/kg | Blake et al., 2020 [144] |
| EDC | Time of Exposure (hpf) | Dose | Findings | Reference |
|---|---|---|---|---|
| ATBC | 0–96 | 0.03–300 μg/L | ↓ Locomotion at 300 μg/L in the light and dark phase ↓ AchE at 300 μg/L No effect on dopamine ↓ Expression of ache, gap43, mbpa, and syn2a at 300 μg/L (related to neurodevelopment) No effect on expression of gfap, shha, tuba1b (genes related to neurodevelopment) | Yun et al., 2024 [186] |
| ATBC | 4–120 | 10–1000 μg/L | ↓ Expression of tshβ at 19 and 194.5 μg/L; trα at 194.5 μg/L; dio1 at 194.5 μg/L; dio2 at 71 and 194.5 μg/L (genes related to neurodevelopment) | Horie et al., 2022 [188] |
| ATEC | 0–96 | 0.03–300 μg/L | No effect on locomotion No effect on AChE or dopamine ↓ Expression of ache and mbpa at 300 μg/L (genes related to neurodevelopment) No effect on expression of gap43, gfap, shha, syn2a, tuba1b (genes related to neurodevelopment) | Yun et al., 2024 [186] |
| DGD | 8–48 | 0.000001–1 μM | No effect on thyroid hormone levels ↑ Association with transcriptomics adverse outcome pathway related to thyroid hormone | Tan et al., 2023 [189] |
| DINCH | 2–144 | 0.01–10 μM | ↑ Locomotion at 0.1–10 μM during the light period ↑ Expression of pcsk9 at all doses, dmrt3a at 0.1–10 μM, hmgcs1 at 10 μM, mbpa at 1, 10 μM; ↓ dhcr7 at all doses (genes related to cholesterol biosynthesis, myelin, neurodevelopment) | Saad et al., 2021 [187] |
| DINCH | 8–48 | 10–1000 μg/L | No effect on thyroid hormone levels ↑ Association with transcriptomics adverse outcome pathway related to thyroid hormone | Tan et al., 2023 [189] |
| GTA | 8–48 | 0.001–1000 μM | No effect on thyroid hormone levels ↑ Association with transcriptomics adverse outcome pathway related to thyroid hormone | Tan et al., 2023 [189] |
| EDC | Time EDC Measured | Concentration Measured | Findings | Reference |
|---|---|---|---|---|
| DEHTP | Gestation (~14 weeks) | Mean Urine (metabolites, ng/mL): MECPTP: 28.76 MEHHP: 5.72 | ↓ association with adaptive and cognitive domain in boys; association with communication scores in girls | Park et al., 2023 [191] |
| DINCH | 7–11 years old | Mean Urine (Biomarkers, μg/L): OH-MINCH: 2.3–3.6 cx-MINCH: 1.1–2.3 | No association with IQ | Rosolen et al., 2022 [192] |
| DINCH | Gestation (>14 weeks)] | Mean Urine (biomarkers, ng/mL): MOiNCH: 0.25 | ↑ association with TT3; ↓ association with TT4/TT3 ratio | Derakhshan et al., 2021 [194] |
| DINCH | Gestation (~14 weeks) | Mean Urine (metabolites, ng/mL): MHinCH: 0.47 MCOCH: 0.47 | No association with thyroid hormones (TSH, T3, T4, T3/T4 ratio) | Cathey et al., 2019 [193] |
| DEHT | Gestation (~14 weeks) | Mean Urine (metabolites, ng/mL): MECPTP: 20.5 MEHHTP: 3.72 | ↑ association with T3, T3/T4 ratio No association with TSH or T4 | Cathey et al., 2019 [193] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Grahl, E.; Hilz, E.N.; Gore, A.C. Regrettable Substitutes and the Brain: What Animal Models and Human Studies Tell Us about the Neurodevelopmental Effects of Bisphenol, Per- and Polyfluoroalkyl Substances, and Phthalate Replacements. Int. J. Mol. Sci. 2024, 25, 6887. https://doi.org/10.3390/ijms25136887
Morales-Grahl E, Hilz EN, Gore AC. Regrettable Substitutes and the Brain: What Animal Models and Human Studies Tell Us about the Neurodevelopmental Effects of Bisphenol, Per- and Polyfluoroalkyl Substances, and Phthalate Replacements. International Journal of Molecular Sciences. 2024; 25(13):6887. https://doi.org/10.3390/ijms25136887
Chicago/Turabian StyleMorales-Grahl, Elena, Emily N. Hilz, and Andrea C. Gore. 2024. "Regrettable Substitutes and the Brain: What Animal Models and Human Studies Tell Us about the Neurodevelopmental Effects of Bisphenol, Per- and Polyfluoroalkyl Substances, and Phthalate Replacements" International Journal of Molecular Sciences 25, no. 13: 6887. https://doi.org/10.3390/ijms25136887
APA StyleMorales-Grahl, E., Hilz, E. N., & Gore, A. C. (2024). Regrettable Substitutes and the Brain: What Animal Models and Human Studies Tell Us about the Neurodevelopmental Effects of Bisphenol, Per- and Polyfluoroalkyl Substances, and Phthalate Replacements. International Journal of Molecular Sciences, 25(13), 6887. https://doi.org/10.3390/ijms25136887







