SkQ3 Exhibits the Most Pronounced Antioxidant Effect on Isolated Rat Liver Mitochondria and Yeast Cells
Abstract
:1. Introduction
2. Results
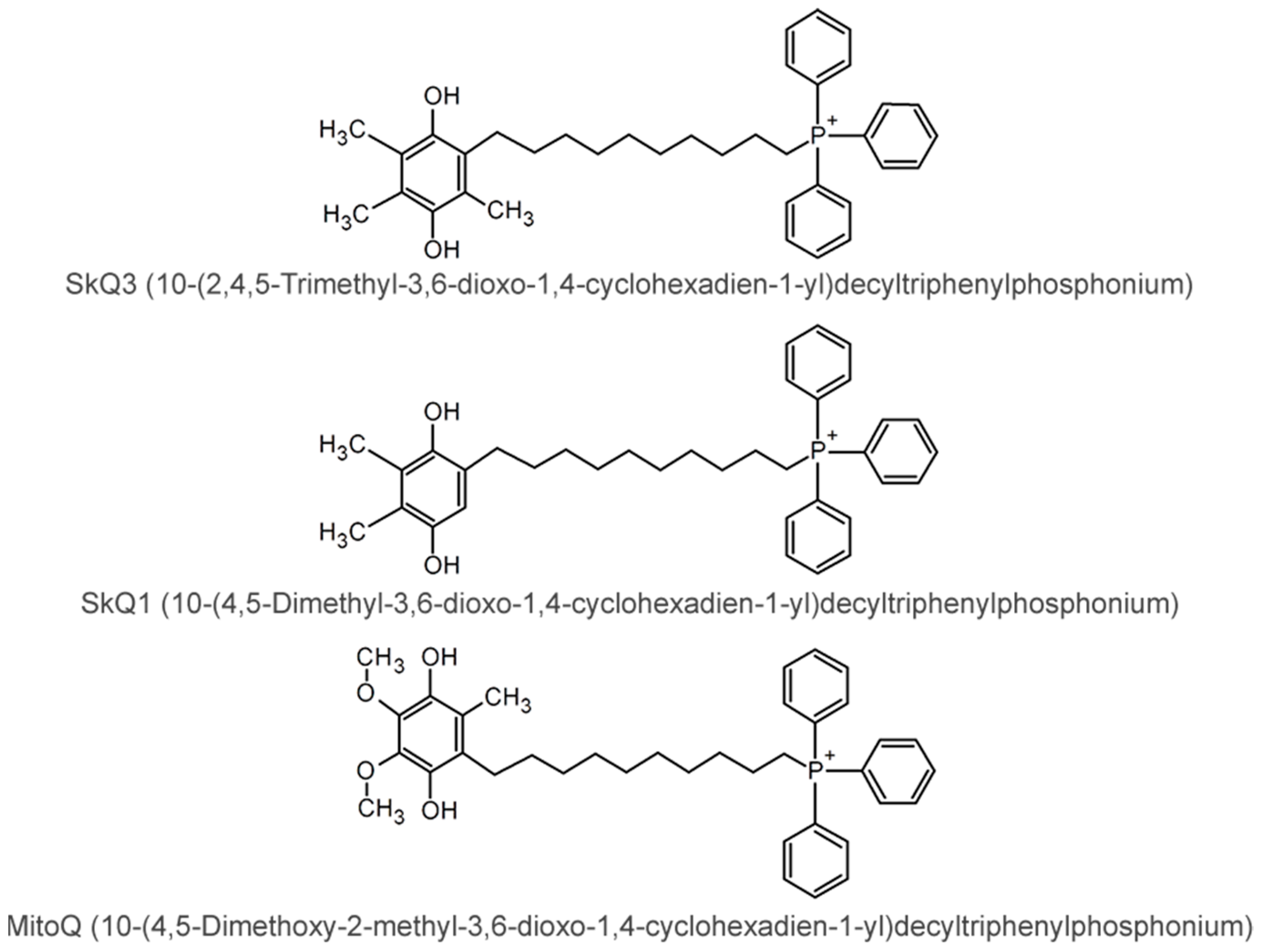
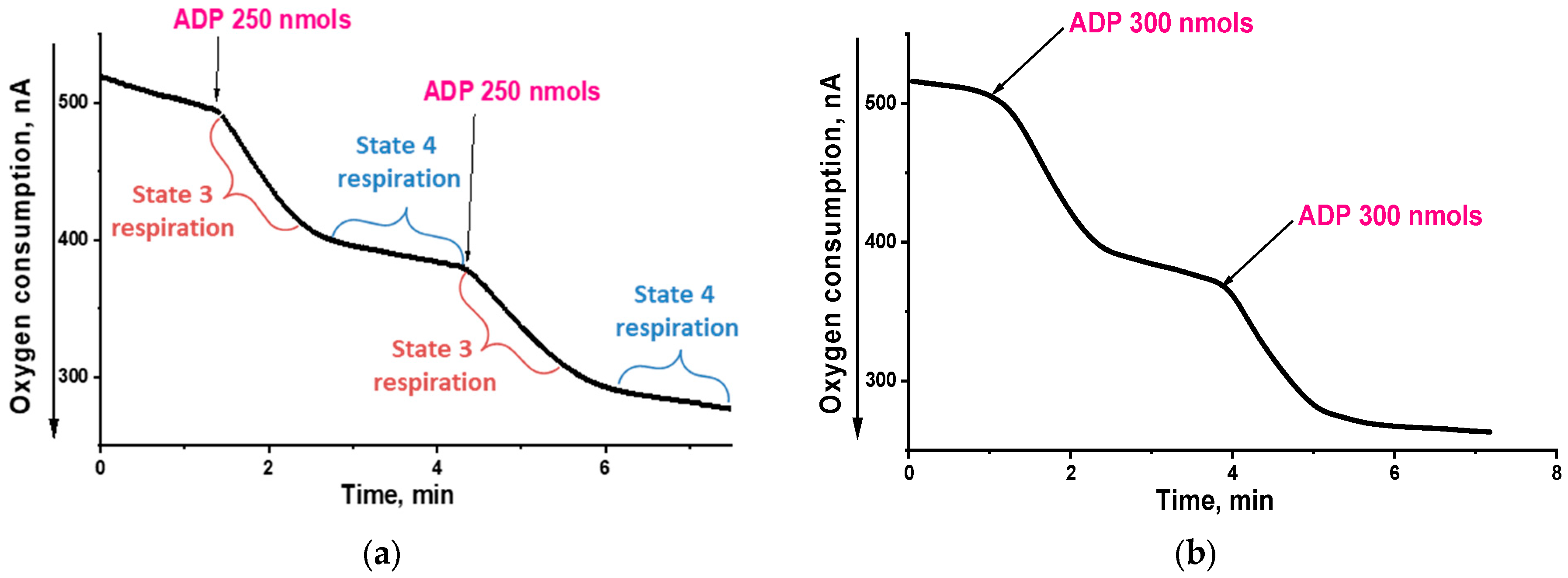
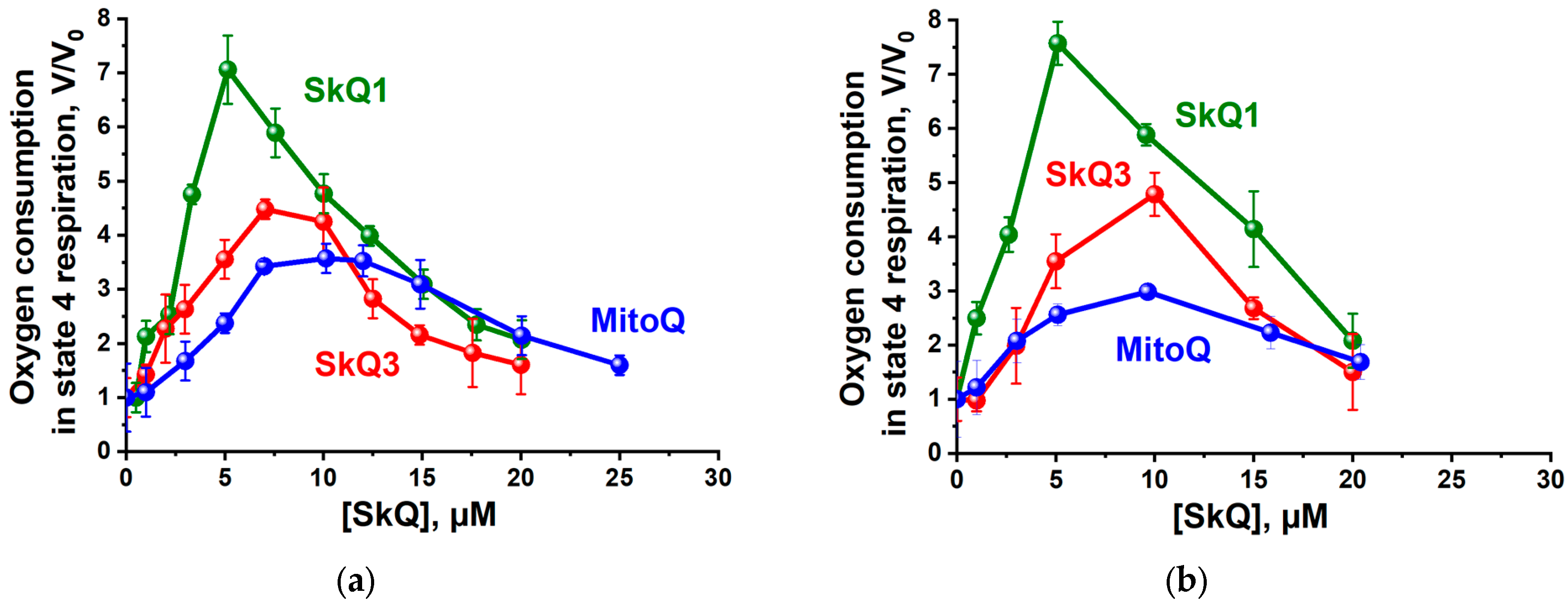
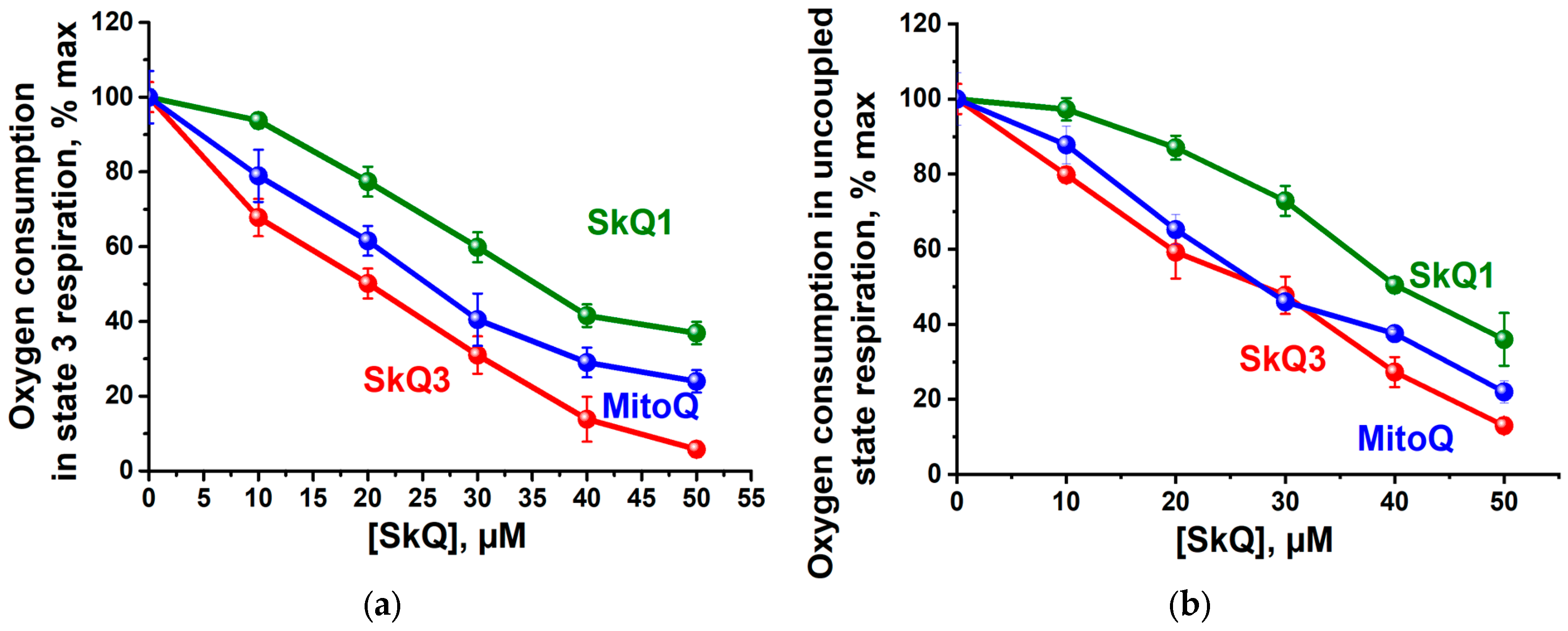
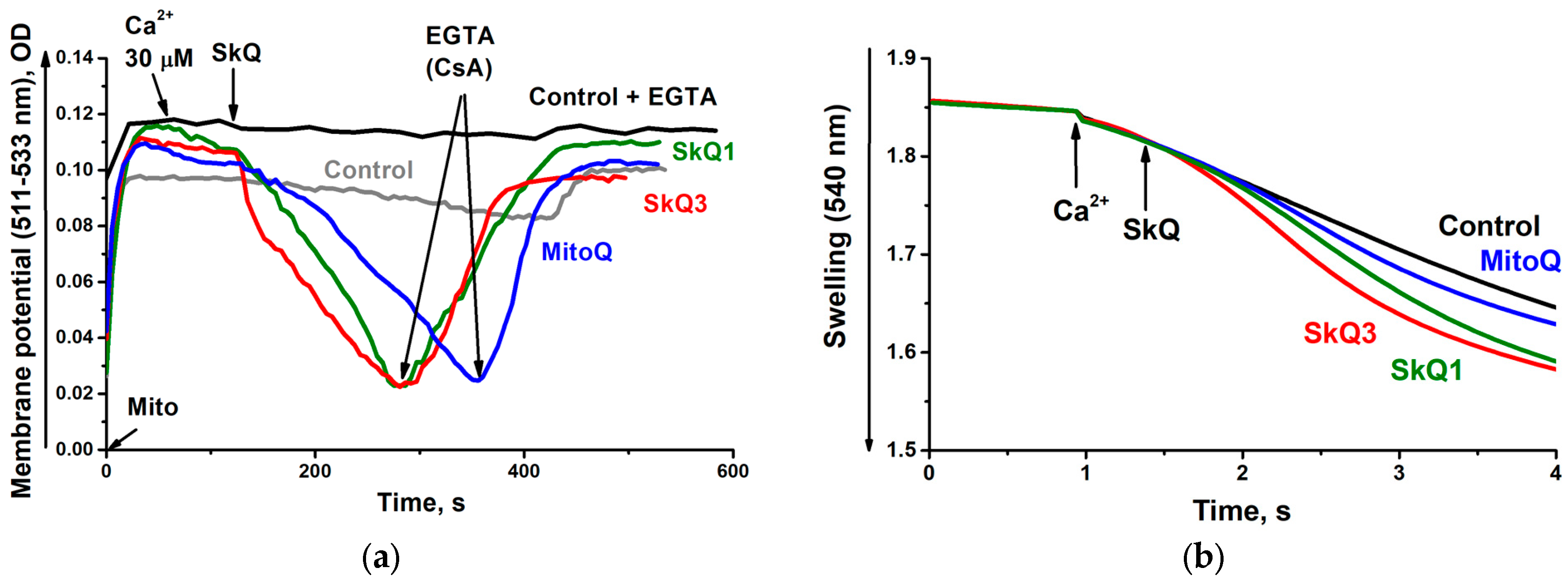
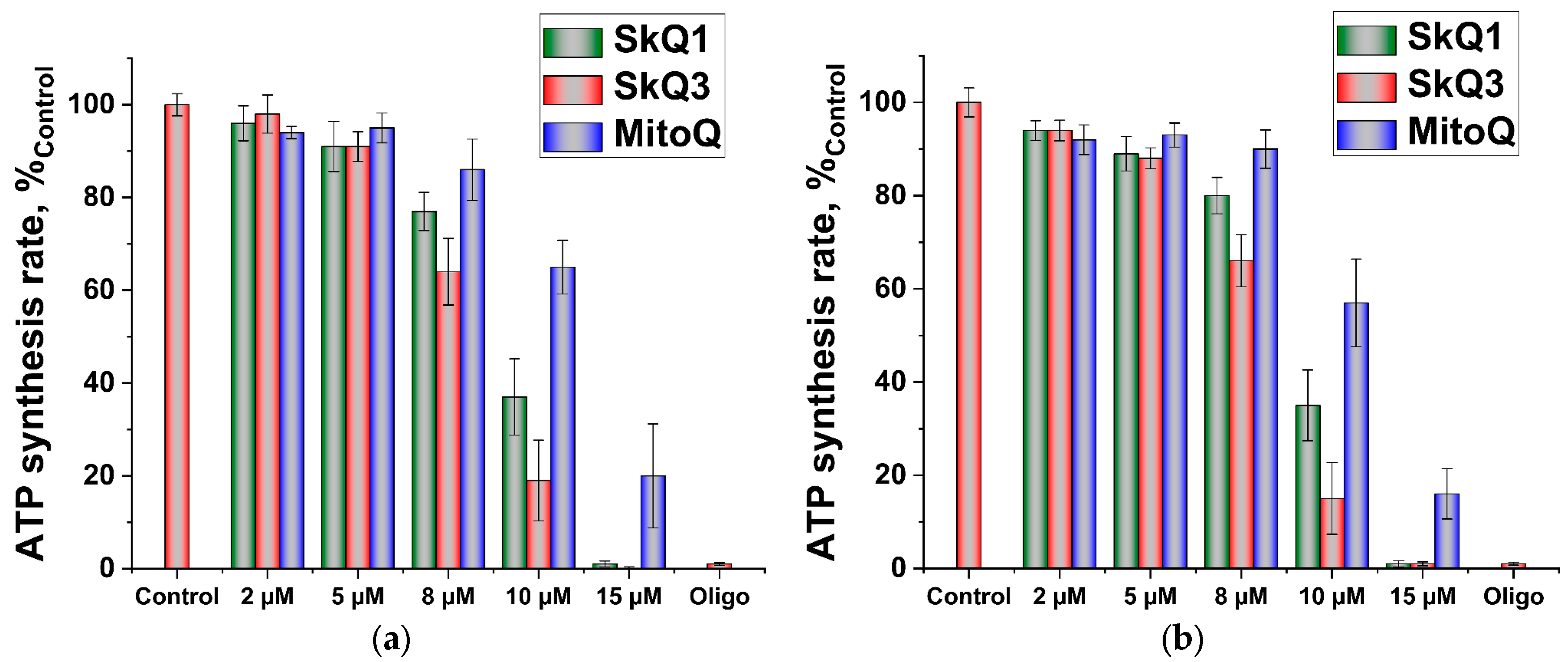
2.1. Effects of SkQ3, SkQ1 and MitoQ Supplementation on Isolated Rat Liver Mitochondria
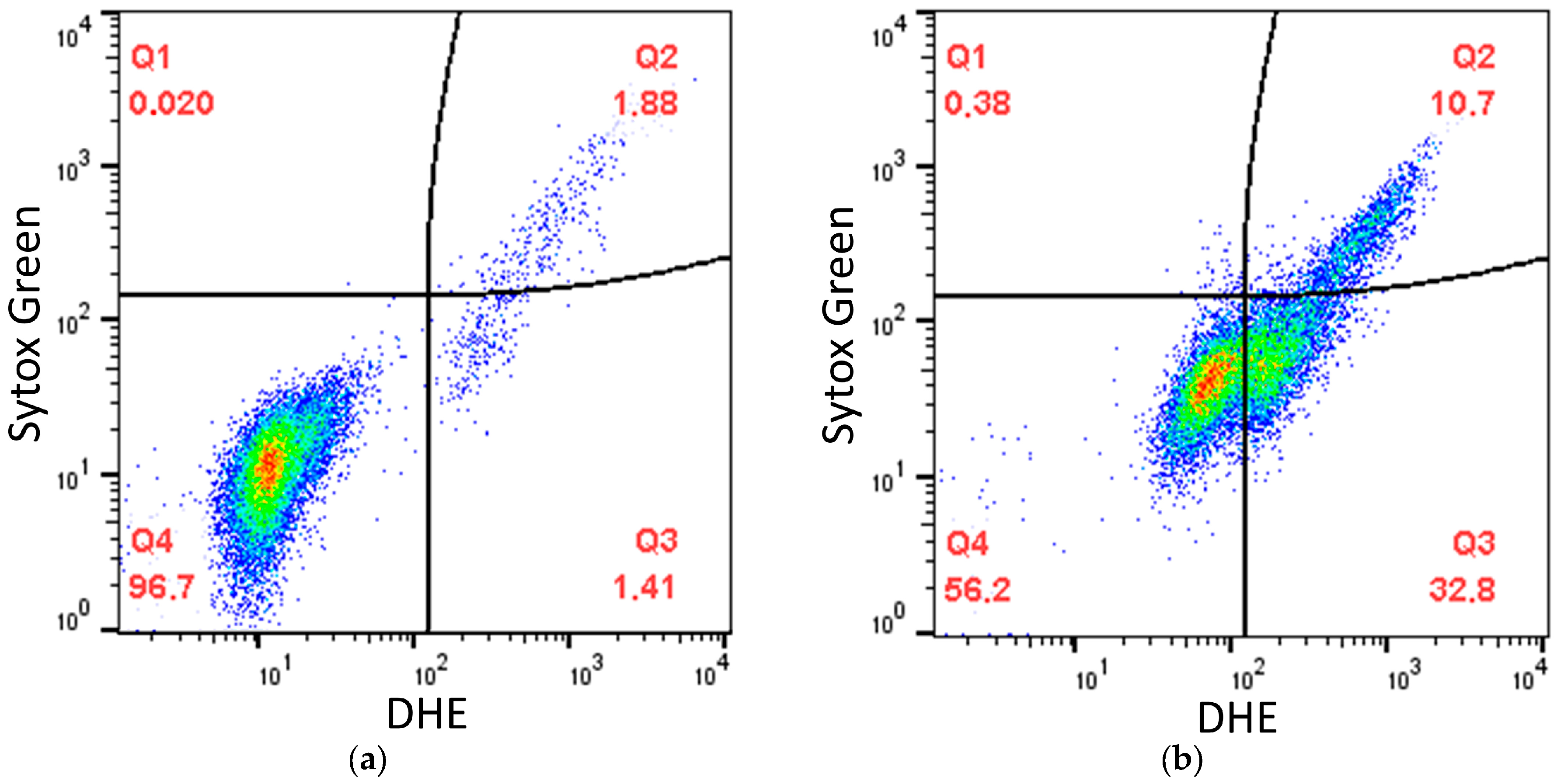
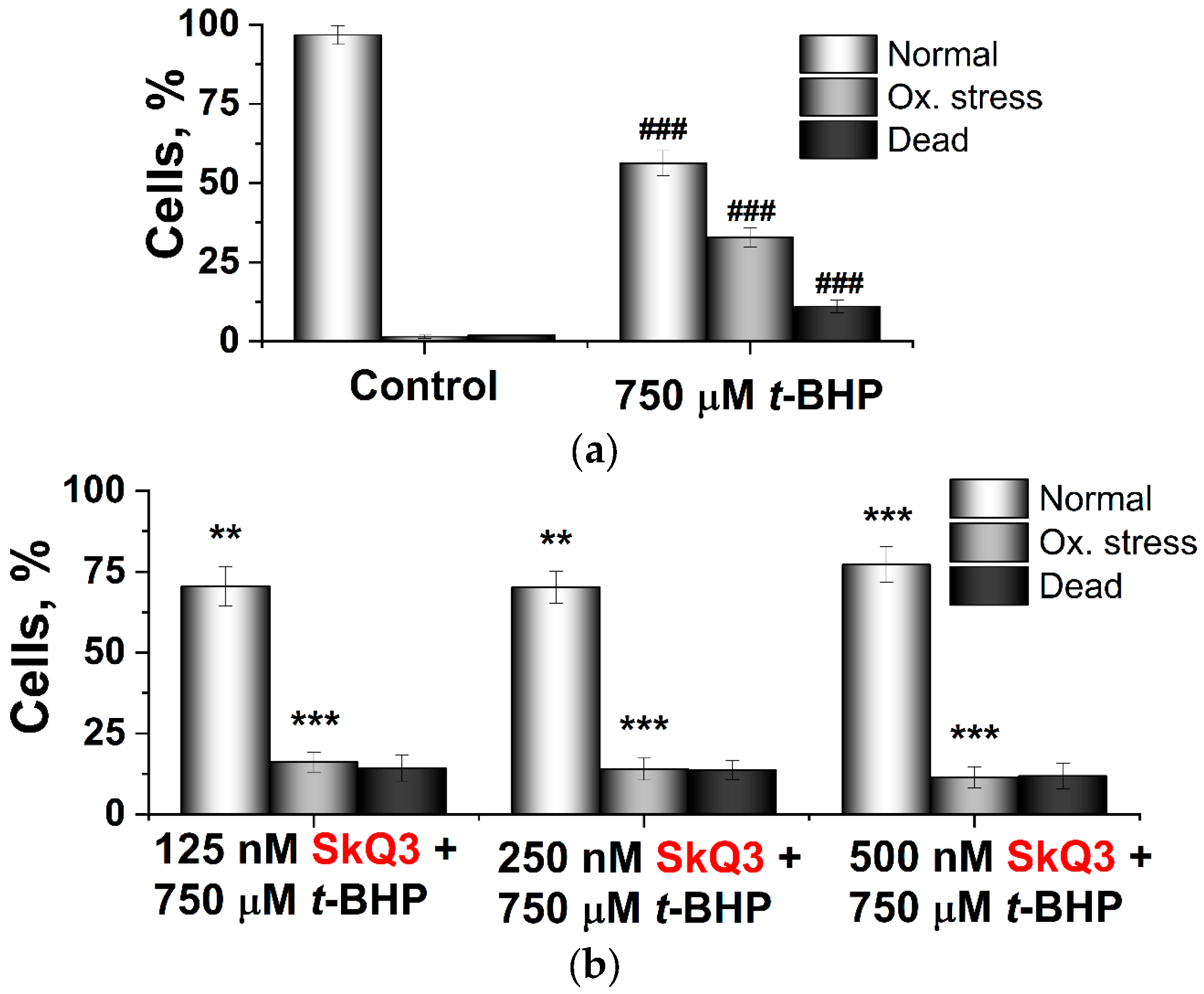
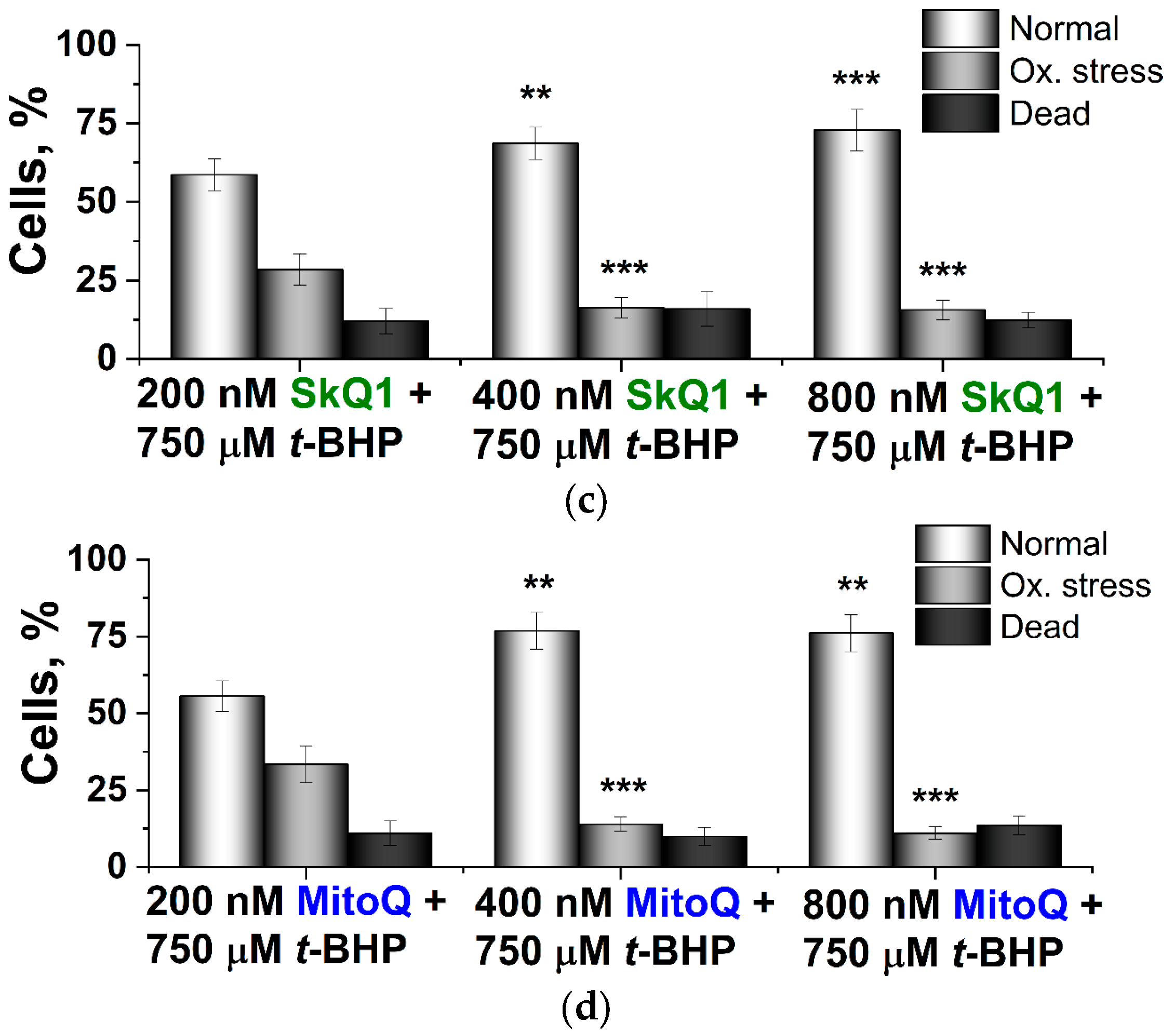
2.2. Effects of SkQ3, SkQ1 and MitoQ Suplementation on D. magnusii Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Isolation of RLM
4.3. Mitochondrial Protein Assay
4.4. Oxygen Consumption Assay
4.5. Transmembrane Potential Measurement
4.6. Swelling of RLM
4.7. Opening of the Nonspecific Ca2+/Pi-Dependent Pore
4.8. ATP Synthesis by RLM
4.9. Hydrogen Peroxide Production by RLM
4.10. Yeast Cell Culture
4.11. Visualization of Mitochondria in Cells
4.12. ROS Generation and Determination
4.13. Cell Viability Assay
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, O.M.; Gorman, G.S.; Lightowlers, R.N.; Turnbull, D.M. Mitochondrial Diseases: Hope for the Future. Cell 2020, 181, 168–188. [Google Scholar] [CrossRef]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886. [Google Scholar] [CrossRef] [PubMed]
- Lott, M.T.; Leipzig, J.N.; Derbeneva, O.; Xie, H.M.; Chalkia, D.; Sarmady, M.; Procaccio, V.; Wallace, D.C. mtDNA Variation and Analysis Using Mitomap and Mitomaster. Curr. Protoc. Bioinform. 2013, 44, 1.23.1–1.23.26. [Google Scholar] [CrossRef] [PubMed]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Craven, L.; Russell, O.M.; Turnbull, D.M.; Vincent, A.E. Diagnosis and Treatment of Mitochondrial Myopathies. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2018, 15, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Gattolliat, C.H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef] [PubMed]
- Boyman, L.; Karbowski, M.; Lederer, W.J. Regulation of Mitochondrial ATP Production: Ca(2+) Signaling and Quality Control. Trends Mol. Med. 2020, 26, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Nikkanen, J.; Yatsuga, S.; Jackson, C.; Wang, L.; Pradhan, S.; Kivelä, R.; Pessia, A.; Velagapudi, V.; Suomalainen, A. mTORC1 Regulates Mitochondrial Integrated Stress Response and Mitochondrial Myopathy Progression. Cell Metab. 2017, 26, 419–428.e5. [Google Scholar] [CrossRef]
- Ma, X.; McKeen, T.; Zhang, J.; Ding, W.X. Role and Mechanisms of Mitophagy in Liver Diseases. Cells 2020, 9, 837. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, M.; Jiang, J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion 2019, 49, 35–45. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H. Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review. Nutrients 2018, 10, 1137. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.; Smith, N.; Saunders, D.; Ranjit, R.; Kneis, P.; Towner, R.A.; Van Remmen, H. Using MRI to measure in vivo free radical production and perfusion dynamics in a mouse model of elevated oxidative stress and neurogenic atrophy. Redox Biol. 2019, 26, 101308. [Google Scholar] [CrossRef]
- Fetisova, E.; Chernyak, B.; Korshunova, G.; Muntyan, M.; Skulachev, V. Mitochondria-targeted Antioxidants as a Prospective Therapeutic Strategy for Multiple Sclerosis. Curr. Med. Chem. 2017, 24, 2086–2114. [Google Scholar] [CrossRef] [PubMed]
- Demyanenko, I.A.; Zakharova, V.V.; Ilyinskaya, O.P.; Vasilieva, T.V.; Fedorov, A.V.; Manskikh, V.N.; Zinovkin, R.A.; Pletjushkina, O.Y.; Chernyak, B.V.; Skulachev, V.P.; et al. Mitochondria-Targeted Antioxidant SkQ1 Improves Dermal Wound Healing in Genetically Diabetic Mice. Oxidative Med. Cell. Longev. 2017, 2017, 6408278. [Google Scholar]
- Lefranc, C.; Friederich-Persson, M.; Palacios-Ramirez, R.; Nguyen Dinh Cat, A. Mitochondrial oxidative stress in obesity: Role of the mineralocorticoid receptor. J. Endocrinol. 2018, 238, R143–R159. [Google Scholar] [CrossRef]
- Shabalina, I.G.; Vyssokikh, M.Y.; Gibanova, N.; Csikasz, R.I.; Edgar, D.; Hallden-Waldemarson, A.; Rozhdestvenskaya, Z.; Bakeeva, L.E.; Vays, V.B.; Pustovidko, A.V.; et al. Improved health-span and lifespan in mtDNA mutator mice treated with the mitochondrially targeted antioxidant SkQ1. Aging 2017, 9, 315–339. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar]
- Ekoue, D.N.; He, C.; Diamond, A.M.; Bonini, M.G. Manganese superoxide dismutase and glutathione peroxidase-1 contribute to the rise and fall of mitochondrial reactive oxygen species which drive oncogenesis. Biochim. Biophys. Acta. Bioenerg. 2017, 1858, 628–632. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxidative Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Huynh, K.; Heshu, S.R.; Yeap, S.K.; Hazilawati, H.; Roselina, K. Water extract of brewers’ rice induces apoptosis in human colorectal cancer cells via activation of caspase-3 and caspase-8 and downregulates the Wnt/β-catenin downstream signaling pathway in brewers’ rice-treated rats with azoxymethane-induced colon carcinogenesis. BMC Complement. Altern. Med. 2015, 15, 205. [Google Scholar]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimer’s Dis. JAD 2014, 42 (Suppl. 3), S125–S152. [Google Scholar] [CrossRef] [PubMed]
- Flemming, N.B.; Gallo, L.A.; Forbes, J.M. Mitochondrial Dysfunction and Signaling in Diabetic Kidney Disease: Oxidative Stress and Beyond. Semin. Nephrol. 2018, 38, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, B.V.; Antonenko, Y.N.; Galimov, E.R.; Domnina, L.V.; Dugina, V.B.; Zvyagilskaya, R.A.; Ivanova, O.Y.; Izyumov, D.S.; Lyamzaev, K.G.; Pustovidko, A.V.; et al. Novel mitochondria-targeted compounds composed of natural constituents: Conjugates of plant alkaloids berberine and palmatine with plastoquinone. Biochem. Biokhimiia 2012, 77, 983–995. [Google Scholar] [CrossRef]
- Goleva, T.N.; Rogov, A.G.; Korshunova, G.A.; Trendeleva, T.A.; Mamaev, D.V.; Aliverdieva, D.A.; Zvyagilskaya, R.A. SkQThy, a novel and promising mitochondria-targeted antioxidant. Mitochondrion 2019, 49, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Rogov, A.G.; Goleva, T.N.; Trendeleva, T.A.; Ovchenkova, A.P.; Aliverdieva, D.A.; Zvyagilskaya, R.A. New Data on Effects of SkQ1 and SkQT1 on Rat Liver Mitochondria and Yeast Cells. Biochem. Biokhimiia 2018, 83, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Severina, I.I.; Severin, F.F.; Korshunova, G.A.; Sumbatyan, N.V.; Ilyasova, T.M.; Simonyan, R.A.; Rogov, A.G.; Trendeleva, T.A.; Zvyagilskaya, R.A.; Dugina, V.B.; et al. In search of novel highly active mitochondria-targeted antioxidants: Thymoquinone and its cationic derivatives. FEBS Lett. 2013, 587, 2018–2024. [Google Scholar] [CrossRef]
- Skulachev, V.P. Cationic antioxidants as a powerful tool against mitochondrial oxidative stress. Biochem. Biophys. Res. Commun. 2013, 441, 275–279. [Google Scholar] [CrossRef]
- Skulachev, V.P.; Anisimov, V.N.; Antonenko, Y.N.; Bakeeva, L.E.; Chernyak, B.V.; Erichev, V.P.; Filenko, O.F.; Kalinina, N.I.; Kapelko, V.I.; Kolosova, N.G.; et al. An attempt to prevent senescence: A mitochondrial approach. Biochim. Biophys. Acta 2009, 1787, 437–461. [Google Scholar] [CrossRef]
- Kelso, G.F.; Porteous, C.M.; Coulter, C.V.; Hughes, G.; Porteous, W.K.; Ledgerwood, E.C.; Smith, R.A.; Murphy, M.P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J. Biol. Chem. 2001, 276, 4588–4596. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P. A biochemical approach to the problem of aging: “megaproject” on membrane-penetrating ions. The first results and prospects. Biochem. Biokhimiia 2007, 72, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Green, D.E. The electromechanochemical model for energy coupling in mitochondria. Biochim. Biophys. Acta 1974, 346, 27–78. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, Y.N.; Avetisyan, A.V.; Bakeeva, L.E.; Chernyak, B.V.; Chertkov, V.A.; Domnina, L.V.; Ivanova, O.Y.; Izyumov, D.S.; Khailova, L.S.; Klishin, S.S.; et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: Synthesis and in vitro studies. Biochem. Biokhimiia 2008, 73, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V.N.; Bakeeva, L.E.; Egormin, P.A.; Filenko, O.F.; Isakova, E.F.; Manskikh, V.N.; Mikhelson, V.M.; Panteleeva, A.A.; Pasyukova, E.G.; Pilipenko, D.I.; et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 5. SkQ1 prolongs lifespan and prevents development of traits of senescence. Biochem. Biokhimiia 2008, 73, 1329–1342. [Google Scholar] [CrossRef]
- Bakeeva, L.E.; Barskov, I.V.; Egorov, M.V.; Isaev, N.K.; Kapelko, V.I.; Kazachenko, A.V.; Kirpatovsky, V.I.; Kozlovsky, S.V.; Lakomkin, V.L.; Levina, S.B.; et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 2. Treatment of some ROS- and age-related diseases (heart arrhythmia, heart infarctions, kidney ischemia, and stroke). Biochem. Biokhimiia 2008, 73, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Agapova, L.S.; Chernyak, B.V.; Domnina, L.V.; Dugina, V.B.; Efimenko, A.Y.; Fetisova, E.K.; Ivanova, O.Y.; Kalinina, N.I.; Khromova, N.V.; Kopnin, B.P.; et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 3. Inhibitory effect of SkQ1 on tumor development from p53-deficient cells. Biochem. Biokhimiia 2008, 73, 1300–1316. [Google Scholar] [CrossRef]
- Neroev, V.V.; Archipova, M.M.; Bakeeva, L.E.; Fursova, A.; Grigorian, E.N.; Grishanova, A.Y.; Iomdina, E.N.; Ivashchenko, Z.N.; Katargina, L.A.; Khoroshilova-Maslova, I.P.; et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 4. Age-related eye disease. SkQ1 returns vision to blind animals. Biochem. Biokhimiia 2008, 73, 1317–1328. [Google Scholar] [CrossRef]
- Obukhova, L.A.; Skulachev, V.P.; Kolosova, N.G. Mitochondria-targeted antioxidant SkQ1 inhibits age-dependent involution of the thymus in normal and senescence-prone rats. Aging 2009, 1, 389–401. [Google Scholar] [CrossRef]
- Petrosillo, G.; Moro, N.; Ruggiero, F.M.; Paradies, G. Melatonin inhibits cardiolipin peroxidation in mitochondria and prevents the mitochondrial permeability transition and cytochrome c release. Free Radic. Biol. Med. 2009, 47, 969–974. [Google Scholar] [CrossRef]
- Kiselevsky, D.B.; Frolova, O.Y.; Solovyev, A.G.; Dorokhov, Y.L.; Morozov, S.Y.; Samuilov, V.D. Plant cell death caused by fungal, bacterial, and viral elicitors: Protective effect of mitochondria-targeted quinones. Biochem. Biokhimiia 2014, 79, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, E.I.; Trendeleva, T.A.; Zvyagilskaya, R.A. Interaction of yeast mitochondria with fatty acids and mitochondria-targeted lipophilic cations. Biochem. Biokhimiia 2010, 75, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Bazhenova, E.N.; Deryabina, Y.I.; Eriksson, O.; Zvyagilskaya, R.A.; Saris, N.E. Characterization of a high capacity calcium transport system in mitochondria of the yeast Endomyces magnusii. J. Biol. Chem. 1998, 273, 4372–4377. [Google Scholar] [CrossRef] [PubMed]
- Deryabina, Y.I.; Bazhenova, E.N.; Saris, N.E.; Zvyagilskaya, R.A. Ca(2+) efflux in mitochondria from the yeast Endomyces magnusii. J. Biol. Chem. 2001, 276, 47801–47806. [Google Scholar] [CrossRef] [PubMed]
- Rogov, A.G.; Ovchenkova, A.P.; Goleva, T.N.; Kireev, I.I.; Zvyagilskaya, R.A. New yeast models for studying mitochondrial morphology as affected by oxidative stress and other factors. Anal. Biochem. 2018, 552, 24–29. [Google Scholar] [CrossRef]
- Snow, B.J.; Rolfe, F.L.; Lockhart, M.M.; Frampton, C.M.; O’Sullivan, J.D.; Fung, V.; Smith, R.A.; Murphy, M.P.; Taylor, K.M. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov. Disord. 2010, 25, 1670–1674. [Google Scholar] [CrossRef]
- Brzheskiy, V.V.; Efimova, E.L.; Vorontsova, T.N.; Alekseev, V.N.; Gusarevich, O.G.; Shaidurova, K.N.; Ryabtseva, A.A.; Andryukhina, O.M.; Kamenskikh, T.G.; Sumarokova, E.S.; et al. Results of a Multicenter, Randomized, Double-Masked, Placebo-Controlled Clinical Study of the Efficacy and Safety of Visomitin Eye Drops in Patients with Dry Eye Syndrome. Adv. Ther. 2015, 32, 1263–1279. [Google Scholar] [CrossRef]
- Baiula, M.; Spampinato, S. Experimental Pharmacotherapy for Dry Eye Disease: A Review. J. Exp. Pharmacol. 2021, 13, 345–358. [Google Scholar] [CrossRef]
- Chance, B.; Williams, G.R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J. Biol. Chem. 1955, 217, 409–427. [Google Scholar] [CrossRef]
- Chance, B.; Williams, G.R.; Holmes, W.F.; Higgins, J. Respiratory Enzymes in Oxidative Phosphorylation, V. a Mechanism for Oxidative Phosphorylation. J. Biol. Chem. 1955, 217, 439–452. [Google Scholar] [CrossRef]
- Bernardi, P.; Forte, M. The mitochondrial permeability transition pore. Novartis Found. Symp. 2007, 287, 157–164, discussion 164–169. [Google Scholar] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals; Institute for Laboratory Animal Research. The National Academies Collection: Reports funded by National Institutes of Health. In Guide for the Care and Use of Laboratory Animals; National Academies Press, National Academy of Sciences: Washington, DC, USA, 2011. [Google Scholar]
- Chance, B.; Williams, G.R. A simple and rapid assay of oxidative phosphorylation. Nature 1955, 175, 1120–1121. [Google Scholar] [CrossRef]
- Goleva, T.N.; Lyamzaev, K.G.; Rogov, A.G.; Khailova, L.S.; Epremyan, K.K.; Shumakovich, G.P.; Domnina, L.V.; Ivanova, O.Y.; Marmiy, N.V.; Zinevich, T.V.; et al. Mitochondria-targeted 1,4-naphthoquinone (SkQN) is a powerful prooxidant and cytotoxic agent. Biochim. Biophys. Acta. Bioenerg. 2020, 1861, 148210. [Google Scholar] [CrossRef] [PubMed]
- Zviagil’skaia, R.A.; Zelenshchikova, V.A.; Ural’skaia, L.A.; Kotel’nikova, A.V. Respiratory system of Endomyces magnusii. Properties of mitochondria from cells grown on glycerol. Biokhimiia 1981, 46, 3–10. [Google Scholar]
- Agnello, M.; Morici, G.; Rinaldi, A.M. A method for measuring mitochondrial mass and activity. Cytotechnology 2008, 56, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Epremyan, K.K.; Rogov, A.G.; Goleva, T.N.; Lavrushkina, S.V.; Zinovkin, R.A.; Zvyagilskaya, R.A. Altered Mitochondrial Morphology and Bioenergetics in a New Yeast Model Expressing Aβ42. Int. J. Mol. Sci. 2023, 24, 900. [Google Scholar] [CrossRef]
- Thakur, S.; Cattoni, D.I.; Nollmann, M. The fluorescence properties and binding mechanism of SYTOX green, a bright, low photo-damage DNA intercalating agent. Eur. Biophys. J. 2015, 44, 337–348. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogov, A.G.; Goleva, T.N.; Aliverdieva, D.A.; Zvyagilskaya, R.A. SkQ3 Exhibits the Most Pronounced Antioxidant Effect on Isolated Rat Liver Mitochondria and Yeast Cells. Int. J. Mol. Sci. 2024, 25, 1107. https://doi.org/10.3390/ijms25021107
Rogov AG, Goleva TN, Aliverdieva DA, Zvyagilskaya RA. SkQ3 Exhibits the Most Pronounced Antioxidant Effect on Isolated Rat Liver Mitochondria and Yeast Cells. International Journal of Molecular Sciences. 2024; 25(2):1107. https://doi.org/10.3390/ijms25021107
Chicago/Turabian StyleRogov, Anton G., Tatyana N. Goleva, Dinara A. Aliverdieva, and Renata A. Zvyagilskaya. 2024. "SkQ3 Exhibits the Most Pronounced Antioxidant Effect on Isolated Rat Liver Mitochondria and Yeast Cells" International Journal of Molecular Sciences 25, no. 2: 1107. https://doi.org/10.3390/ijms25021107



