Targeting Human Glucocorticoid Receptors in Fear Learning: A Multiscale Integrated Approach to Study Functional Connectivity
Abstract
1. Introduction
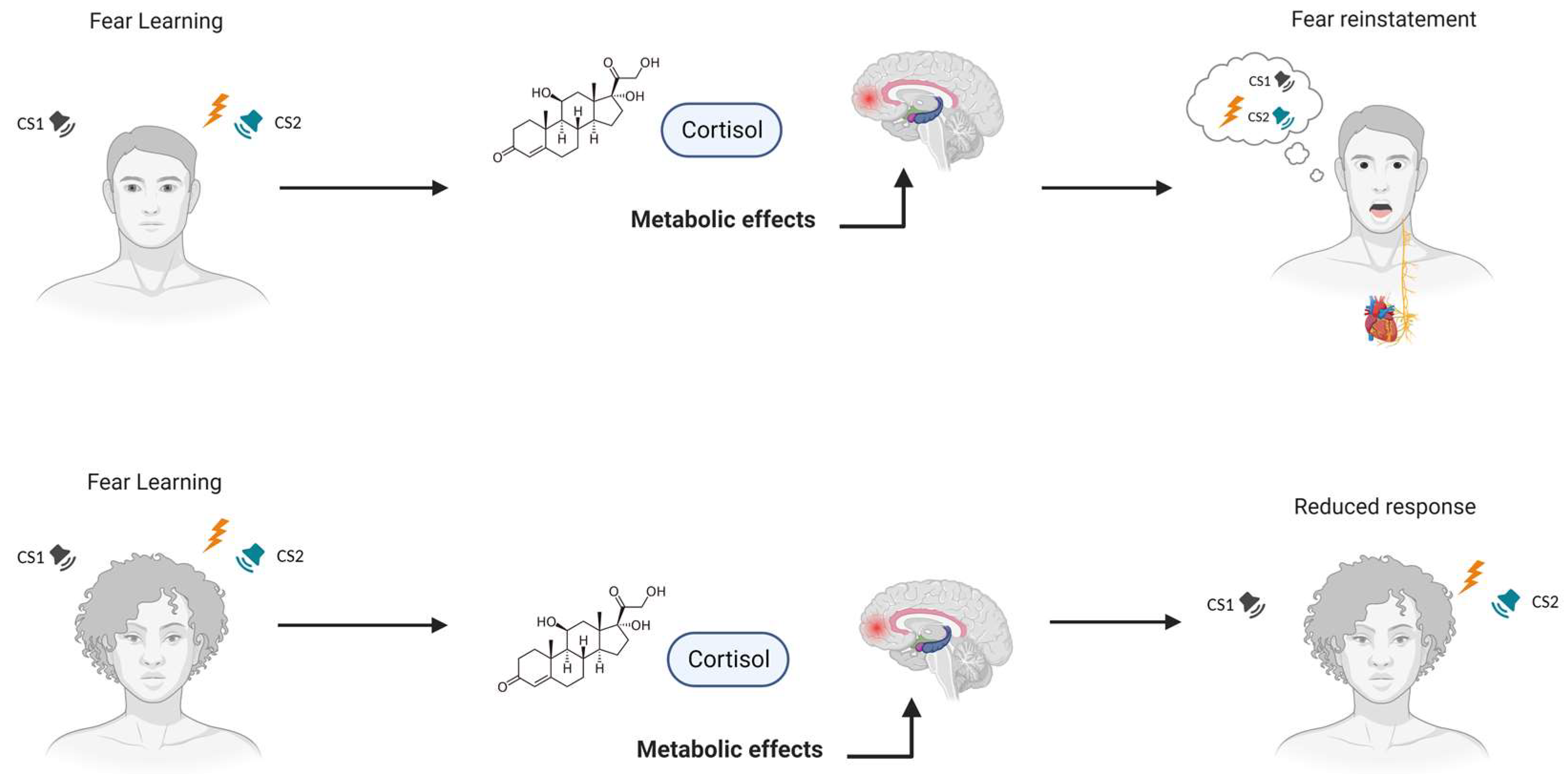
2. Understanding the Effects of Glucocorticoids on Fear Learning
3. Glucocorticoids and Gender Influence on Fear Extinction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NS | neutral stimulus |
| US | unconditioned stimulus |
| CS+ | conditioned stimulus |
| CS− | conditioned stimulus never paired with the US |
| ROF | return of fear |
| SCR | skin conductance response |
| EMG | electromyography |
| fMRI | functional magnetic resonance imaging |
| ROIs | regions of interest |
| BOLD | blood-oxygen-level-dependent |
| PTSD | post-traumatic stress disorder |
| HPA | hypothalamic–pituitary–adrenal axis |
| GRs | glucocorticoid receptors |
| DEX | dexamethasone |
| HC | hydrocortisone |
| FPS | fear-potentiated startle |
| BMI | body mass index |
| vmPFC | ventromedial prefrontal cortex |
| dACC | dorsal anterior cingulate cortex |
| RE + CORT | reactivation + cortisol |
| RE | reactivation |
| CORT | cortisol |
| SPM | statistical parametric mapping |
References
- Pessoa, L. Neural Dynamics of Emotion and Cognition: From Trajectories to Underlying Neural Geometry. Neural Netw. Off. J. Int. Neural Netw. Soc. 2019, 120, 158–166. [Google Scholar] [CrossRef]
- LeDoux, J.E. Evolution of Human Emotion: A View through Fear. Prog. Brain Res. 2012, 195, 431–442. [Google Scholar] [CrossRef]
- Adolphs, R. The Biology of Fear. Curr. Biol. 2013, 23, R79–R93. [Google Scholar] [CrossRef]
- Milad, M.R.; Quirk, G.J. Fear Extinction as a Model for Translational Neuroscience: Ten Years of Progress. Annu. Rev. Psychol. 2012, 63, 129–151. [Google Scholar] [CrossRef]
- Bressler, S.L.; Menon, V. Large-Scale Brain Networks in Cognition: Emerging Methods and Principles. Trends Cogn. Sci. 2010, 14, 277–290. [Google Scholar] [CrossRef]
- LeDoux, J.E. Emotion Circuits in the Brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef]
- Quirk, G.J.; Mueller, D. Neural Mechanisms of Extinction Learning and Retrieval. Neuropsychopharmacology 2008, 33, 56–72. [Google Scholar] [CrossRef]
- Phelps, E.A.; Delgado, M.R.; Nearing, K.I.; Ledoux, J.E. Extinction Learning in Humans: Role of the Amygdala and VmPFC. Neuron 2004, 43, 897–905. [Google Scholar] [CrossRef]
- Quirk, G.J.; Beer, J.S. Prefrontal Involvement in the Regulation of Emotion: Convergence of Rat and Human Studies. Curr. Opin. Neurobiol. 2006, 16, 723–727. [Google Scholar] [CrossRef]
- Milad, M.R.; Wright, C.I.; Orr, S.P.; Pitman, R.K.; Quirk, G.J.; Rauch, S.L. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biol. Psychiatry 2007, 62, 446–454. [Google Scholar] [CrossRef]
- Quirk, G.J.; Gehlert, D.R. Inhibition of the Amygdala: Key to Pathological States? Ann. N. Y. Acad. Sci. 2003, 985, 263–272. [Google Scholar] [CrossRef]
- Kim, M.J.; Gee, D.G.; Loucks, R.A.; Davis, F.C.; Whalen, P.J. Anxiety Dissociates Dorsal and Ventral Medial Prefrontal Cortex Functional Connectivity with the Amygdala at Rest. Cereb. Cortex 2011, 21, 1667–1673. [Google Scholar] [CrossRef]
- Bouton, M.E. Context and Behavioral Processes in Extinction. Learn. Mem. 2004, 11, 485–494. [Google Scholar] [CrossRef]
- Bechara, A.; Damasio, H.; Damasio, A.R.; Lee, G.P. Different Contributions of the Human Amygdala and Ventromedial Prefrontal Cortex to Decision-Making. J. Neurosci. 1999, 19, 5473–5481. [Google Scholar] [CrossRef]
- Lonsdorf, T.B.; Menz, M.M.; Andreatta, M.; Fullana, M.A.; Golkar, A.; Haaker, J.; Heitland, I.; Hermann, A.; Kuhn, M.; Kruse, O.; et al. Don’t Fear ‘Fear Conditioning’: Methodological Considerations for the Design and Analysis of Studies on Human Fear Acquisition, Extinction, and Return of Fear. Neurosci. Biobehav. Rev. 2017, 77, 247–285. [Google Scholar] [CrossRef]
- Maren, S.; Quirk, G.J. Neuronal Signalling of Fear Memory. Nat. Rev. Neurosci. 2004, 5, 844–852. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories Are Not Written in Stone: Re-Writing Fear Memories by Means of Non-Invasive Brain Stimulation and Optogenetic Manipulations. Neurosci. Biobehav. Rev. 2021, 127, 334–352. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Fear-Induced Bradycardia in Mental Disorders: Foundations, Current Advances, Future Perspectives. Neurosci. Biobehav. Rev. 2023, 149, 105163. [Google Scholar] [CrossRef]
- Battaglia, S.; Thayer, J.F. Functional Interplay between Central and Autonomic Nervous Systems in Human Fear Conditioning. Trends Neurosci. 2022, 45, 4. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Heart’s Tale of Trauma: Fear-Conditioned Heart Rate Changes in Post-Traumatic Stress Disorder. Acta Psychiatr. Scand. 2023, 148, 463–466. [Google Scholar] [CrossRef]
- Phelps, E.A.; Ling, S.; Carrasco, M. Emotion Facilitates Perception and Potentiates the Perceptual Benefits of Attention. Psychol. Sci. 2006, 17, 292–299. [Google Scholar] [CrossRef]
- Myers, K.M.; Davis, M. Behavioral and Neural Analysis of Extinction. Neuron 2002, 36, 567–584. [Google Scholar] [CrossRef]
- Maren, S. Neurobiology of Pavlovian Fear Conditioning. Annu. Rev. Neurosci. 2001, 24, 897–931. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Garofalo, S.; Tortora, F.; Avenanti, A.; di Pellegrino, G. State-Dependent TMS over Prefrontal Cortex Disrupts Fear-Memory Reconsolidation and Prevents the Return of Fear. Curr. Biol. 2020, 30, 3672–3679.e4. [Google Scholar] [CrossRef]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the Human Ventromedial Prefrontal Cortex Support Fear Learning, Fear Extinction or Both? A Commentary on Subregional Contributions. Mol. Psychiatry 2021, 27, 784–786. [Google Scholar] [CrossRef]
- Fullana, M.A.; Harrison, B.J.; Soriano-Mas, C.; Vervliet, B.; Cardoner, N.; Àvila-Parcet, A.; Radua, J. Neural Signatures of Human Fear Conditioning: An Updated and Extended Meta-Analysis of FMRI Studies. Mol. Psychiatry 2016, 21, 500–508. [Google Scholar] [CrossRef]
- Haaker, J.; Lonsdorf, T.B.; Schümann, D.; Menz, M.; Brassen, S.; Bunzeck, N.; Gamer, M.; Kalisch, R. Deficient Inhibitory Processing in Trait Anxiety: Evidence from Context-Dependent Fear Learning, Extinction Recall and Renewal. Biol. Psychol. 2015, 111, 65–72. [Google Scholar] [CrossRef]
- Battaglia, S.; Orsolini, S.; Borgomaneri, S.; Barbieri, R.; Diciotti, S.; di Pellegrino, G. Characterizing Cardiac Autonomic Dynamics of Fear Learning in Humans. Psychophysiology 2022, 59, e14122. [Google Scholar] [CrossRef]
- Battaglia, S.; Di Fazio, C.; Vicario, C.M.; Avenanti, A. Neuropharmacological Modulation of N-Methyl-D-Aspartate, Noradrenaline and Endocannabinoid Receptors in Fear Extinction Learning: Synaptic Transmission and Plasticity. Int. J. Mol. Sci. 2023, 24, 5926. [Google Scholar] [CrossRef]
- Di Gregorio, F.; Battaglia, S. Advances in EEG-Based Functional Connectivity Approaches to the Study of the Central Nervous System in Health and Disease. Adv. Clin. Exp. Med. 2023, 32, 607–612. [Google Scholar] [CrossRef]
- Battaglia, S.; Serio, G.; Scarpazza, C.; D’Ausilio, A.; Borgomaneri, S. Frozen in (e)motion: How Reactive Motor Inhibition is Influenced by the Emotional Content of Stimuli in Healthy and Psychiatric Populations. Behav. Res. Ther. 2021, 146, 103963. [Google Scholar] [CrossRef]
- Battaglia, S.; Fabius, J.H.; Moravkova, K.; Fracasso, A.; Borgomaneri, S. The Neurobiological Correlates of Gaze Perception in Healthy Individuals and Neurologic Patients. Biomedicines 2022, 10, 627. [Google Scholar] [CrossRef]
- Nader, K.; Hardt, O. A Single Standard for Memory: The Case for Reconsolidation. Nat. Rev. Neurosci. 2009, 10, 224–234. [Google Scholar] [CrossRef]
- Bouton, M.E.; King, D.A. Contextual Control of the Extinction of Conditioned Fear: Tests for the Associative Value of the Context. J. Exp. Psychol. Anim. Behav. Process. 1983, 9, 248–265. [Google Scholar] [CrossRef]
- Battaglia, S. Neurobiological Advances of Learned Fear in Humans. Adv. Clin. Exp. Med. 2022, 31, 217–221. [Google Scholar] [CrossRef]
- Kalisch, R.; Korenfeld, E.; Stephan, K.E.; Weiskopf, N.; Seymour, B.; Dolan, R.J. Context-Dependent Human Extinction Memory is Mediated by a Ventromedial Prefrontal and Hippocampal Network. J. Neurosci. 2006, 26, 9503–9511. [Google Scholar] [CrossRef]
- Taschereau-Dumouchel, V.; Kawato, M.; Lau, H. Multivoxel Pattern Analysis Reveals Dissociations between Subjective Fear and its Physiological Correlates. Mol. Psychiatry 2020, 25, 2342–2354. [Google Scholar] [CrossRef]
- Battaglia, S.; Garofalo, S.; di Pellegrino, G.; Starita, F. Revaluing the Role of VmPFC in the Acquisition of Pavlovian Threat Conditioning in Humans. J. Neurosci. 2020, 40, 8491–8500. [Google Scholar] [CrossRef]
- Rescorla, R.A. Spontaneous Recovery. Learn. Mem. 2004, 11, 501–509. [Google Scholar] [CrossRef]
- Vervliet, B.; Indekeu, E. Low-Cost Avoidance Behaviors are Resistant to Fear Extinction in Humans. Front. Behav. Neurosci. 2015, 9, 351. [Google Scholar] [CrossRef]
- Bouton, M.E. Context, Ambiguity, and Unlearning: Sources of Relapse after Behavioral Extinction. Biol. Psychiatry 2002, 52, 976–986. [Google Scholar] [CrossRef]
- Myers, K.M.; Davis, M. Mechanisms of Fear Extinction. Mol. Psychiatry 2007, 12, 120–150. [Google Scholar] [CrossRef]
- Maren, S.; Phan, K.L.; Liberzon, I. The Contextual Brain: Implications for Fear Conditioning, Extinction and Psychopathology. Nat. Rev. Neurosci. 2013, 14, 417–428. [Google Scholar] [CrossRef]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. The Influence of Vicarious Fear-Learning in ‘Infecting’ Reactive Action Inhibition. Front. Behav. Neurosci. 2022, in press. [Google Scholar]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. Stopping in (e)Motion: Reactive Action Inhibition When Facing Valence-Independent Emotional Stimuli. Front. Behav. Neurosci. 2022, 16, 998714. [Google Scholar] [CrossRef]
- Raij, T.; Nummenmaa, A.; Marin, M.F.; Porter, D.; Furtak, S.; Setsompop, K.; Milad, M.R. Prefrontal Cortex Stimulation Enhances Fear Extinction Memory in Humans. Biol. Psychiatry 2018, 84, 129–137. [Google Scholar] [CrossRef]
- Maren, S. Seeking a Spotless Mind: Extinction, Deconsolidation, and Erasure of Fear Memory. Neuron 2011, 70, 830–845. [Google Scholar] [CrossRef]
- Roozendaal, B.; McEwen, B.S.; Chattarji, S. Stress, Memory and the Amygdala. Nat. Rev. Neurosci. 2009, 10, 423–433. [Google Scholar] [CrossRef]
- Tanaka, M.; Schally, A.V.; Telegdy, G. Neurotransmission of the Antidepressant-like Effects of the Growth Hormone-Releasing Hormone Antagonist MZ-4-71. Behav. Brain Res. 2012, 228, 388–391. [Google Scholar] [CrossRef]
- Palotai, M.; Telegdy, G.; Tanaka, M.; Bagosi, Z.; Jászberényi, M. Neuropeptide AF Induces Anxiety-like and Antidepressant-like Behavior in Mice. Behav. Brain Res. 2014, 274, 264–269. [Google Scholar] [CrossRef]
- Telegdy, G.; Adamik, A.; Tanaka, M.; Schally, A.V. Effects of the LHRH Antagonist Cetrorelix on Affective and Cognitive Functions in Rats. Regul. Pept. 2010, 159, 142–147. [Google Scholar] [CrossRef]
- Tanaka, M.; Telegdy, G. Neurotransmissions of Antidepressant-like Effects of Neuromedin U-23 in Mice. Behav. Brain Res. 2014, 259, 196–199. [Google Scholar] [CrossRef]
- Soravia, L.M.; Heinrichs, M.; Aerni, A.; Maroni, C.; Schelling, G.; Ehlert, U.; Roozendaal, B.; de Quervain, D.J.-F. Glucocorticoids Reduce Phobic Fear in Humans. Proc. Natl. Acad. Sci. USA 2006, 103, 5585–5590. [Google Scholar] [CrossRef]
- de Souza-Talarico, J.N.; Marin, M.-F.; Sindi, S.; Lupien, S.J. Effects of Stress Hormones on the Brain and Cognition: Evidence from Normal to Pathological Aging. Dement. Neuropsychol. 2011, 5, 8–16. [Google Scholar] [CrossRef]
- Lupien, S.J.; Juster, R.-P.; Raymond, C.; Marin, M.-F. The Effects of Chronic Stress on the Human Brain: From Neurotoxicity, to Vulnerability, to Opportunity. Front. Neuroendocrinol. 2018, 49, 91–105. [Google Scholar] [CrossRef]
- Schwabe, L.; Joëls, M.; Roozendaal, B.; Wolf, O.T.; Oitzl, M.S. Stress Effects on Memory: An Update and Integration. Neurosci. Biobehav. Rev. 2012, 36, 1740–1749. [Google Scholar] [CrossRef]
- Liberzon, I.; Sripada, C.S. The Functional Neuroanatomy of PTSD: A Critical Review. Prog. Brain Res. 2008, 167, 151–169. [Google Scholar] [CrossRef]
- Maren, S.; Holmes, A. Stress and Fear Extinction. Neuropsychopharmacology 2016, 41, 58–79. [Google Scholar] [CrossRef]
- Joëls, M.; Fernandez, G.; Roozendaal, B. Stress and Emotional Memory: A Matter of Timing. Trends Cogn. Sci. 2011, 15, 280–288. [Google Scholar] [CrossRef]
- Kim, J.J.; Song, E.Y.; Kosten, T.A. Stress Effects in the Hippocampus: Synaptic Plasticity and Memory. Stress 2006, 9, 1–11. [Google Scholar] [CrossRef]
- Raio, C.M.; Brignoni-Perez, E.; Goldman, R.; Phelps, E.A. Acute Stress Impairs the Retrieval of Extinction Memory in Humans. Neurobiol. Learn. Mem. 2014, 112, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Hartley, C.A.; Gorun, A.; Reddan, M.C.; Ramirez, F.; Phelps, E.A. Stressor Controllability Modulates Fear Extinction in Humans. Neurobiol. Learn. Mem. 2014, 113, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Merz, C.J.; Hamacher-Dang, T.C.; Wolf, O.T. Exposure to Stress Attenuates Fear Retrieval in Healthy Men. Psychoneuroendocrinology 2014, 41, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Merz, C.J.; Hamacher-Dang, T.C.; Stark, R.; Wolf, O.T.; Hermann, A. Neural Underpinnings of Cortisol Effects on Fear Extinction. Neuropsychopharmacology 2018, 43, 384–392. [Google Scholar] [CrossRef] [PubMed]
- van Ast, V.A.; Cornelisse, S.; Marin, M.-F.; Ackermann, S.; Garfinkel, S.N.; Abercrombie, H.C. Modulatory Mechanisms of Cortisol Effects on Emotional Learning and Memory: Novel Perspectives. Psychoneuroendocrinology 2013, 38, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.; Wolf, O.T.; Tabbert, K.; Kagerer, S.; Zimmermann, M.; Kirsch, P.; Schienle, A.; Vaitl, D. Influence of the Stress Hormone Cortisol on Fear Conditioning in Humans: Evidence for Sex Differences in the Response of the Prefrontal Cortex. Neuroimage 2006, 32, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the Brain: From Adaptation to Disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Mercado, D.; Padilla-Coreano, N.; Quirk, G.J. Dissociable Roles of Prelimbic and Infralimbic Cortices, Ventral Hippocampus, and Basolateral Amygdala in the Expression and Extinction of Conditioned Fear. Neuropsychopharmacology 2011, 36, 529–538. [Google Scholar] [CrossRef]
- Vafaei, A.A.; Rashidy-Pour, A.; Trahomi, P.; Omoumi, S.; Dadkhah, M. Role of Amygdala-Infralimbic Cortex Circuitry in Glucocorticoid-Induced Facilitation of Auditory Fear Memory Extinction. Basic Clin. Neurosci. 2022, 13, 193–205. [Google Scholar] [CrossRef]
- Aubry, A.V.; Serrano, P.A.; Burghardt, N.S. Molecular Mechanisms of Stress-Induced Increases in Fear Memory Consolidation within the Amygdala. Front. Behav. Neurosci. 2016, 10, 191. [Google Scholar] [CrossRef]
- Yehuda, R. Status of Glucocorticoid Alterations in Post-Traumatic Stress Disorder. Ann. N. Y. Acad. Sci. 2009, 1179, 56–69. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Monitoring the Kynurenine System: Concentrations, Ratios or What Else? Adv. Clin. Exp. Med. 2021, 30, 775–778. [Google Scholar] [CrossRef]
- Balogh, L.; Tanaka, M.; Török, N.; Taguchi, S. Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders. Biomedicines 2021, 9, 340. [Google Scholar] [CrossRef]
- Scherholz, M.L.; Schlesinger, N.; Androulakis, I.P. Chronopharmacology of Glucocorticoids. Adv. Drug Deliv. Rev. 2019, 151, 245–261. [Google Scholar] [CrossRef]
- Elston, M.S.; Conaglen, H.M.; Hughes, C.; Tamatea, J.A.U.; Meyer-Rochow, G.Y.; Conaglen, J.V. Duration of Cortisol Suppression Following a Single Dose of Dexamethasone in Healthy Volunteers: A Randomised Double-Blind Placebo-Controlled Trial. Anaesth. Intensive Care 2013, 41, 596–601. [Google Scholar] [CrossRef]
- Williams, D.M. Clinical Pharmacology of Corticosteroids. Respir. Care 2018, 63, 655–670. [Google Scholar] [CrossRef]
- McEwen, B.S.; Morrison, J.H. The Brain on Stress: Vulnerability and Plasticity of the Prefrontal Cortex over the Life Course. Neuron 2013, 79, 16–29. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Preclinical Modeling in Depression and Anxiety: Current Challenges and Future Research Directions. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2023, 32, 505–509. [Google Scholar] [CrossRef]
- Polyák, H.; Galla, Z.; Nánási, N.; Cseh, E.K.; Rajda, C.; Veres, G.; Spekker, E.; Szabó, Á.; Klivényi, P.; Tanaka, M.; et al. The Tryptophan-Kynurenine Metabolic System Is Suppressed in Cuprizone-Induced Model of Demyelination Simulating Progressive Multiple Sclerosis. Biomedicines 2023, 11, 945. [Google Scholar] [CrossRef]
- Tajti, J.; Szok, D.; Csáti, A.; Szabó, Á.; Tanaka, M.; Vécsei, L. Exploring Novel Therapeutic Targets in the Common Pathogenic Factors in Migraine and Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 4114. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Integrating Armchair, Bench, and Bedside Research for Behavioral Neurology and Neuropsychiatry: Editorial. Biomedicines 2022, 10, 2999. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial Impairment: A Common Motif in Neuropsychiatric Presentation? The Link to the Tryptophan-Kynurenine Metabolic System. Cells 2022, 11, 2607. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue “Dissecting Neurological and Neuropsychiatric Diseases: Neurodegeneration and Neuroprotection”. Int. J. Mol. Sci. 2022, 23, 6991. [Google Scholar] [CrossRef]
- Ressler, K.J.; Mayberg, H.S. Targeting Abnormal Neural Circuits in Mood and Anxiety Disorders: From the Laboratory to the Clinic. Nat. Neurosci. 2007, 10, 1116–1124. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Körtési, T.; Szok, D.; Tajti, J.; Vécsei, L. From CGRP to PACAP, VIP, and Beyond: Unraveling the Next Chapters in Migraine Treatment. Cells 2023, 12, 2649. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, T.; Norrholm, S.D.; Fennell, J.E.; Keyes, M.; Fiallos, A.M.; Myers, K.M.; Davis, M.; Duncan, E.J. Posttraumatic Stress Disorder May Be Associated with Impaired Fear Inhibition: Relation to Symptom Severity. Psychiatry Res. 2009, 167, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L.; Giménez-Llort, L. Emerging Translational Research in Neurological and Psychiatric Diseases: From In Vitro to In Vivo Models. Int. J. Mol. Sci. 2023, 24, 15739. [Google Scholar] [CrossRef]
- Pace, T.W.W.; Heim, C.M. A Short Review on the Psychoneuroimmunology of Posttraumatic Stress Disorder: From Risk Factors to Medical Comorbidities. Brain. Behav. Immun. 2011, 25, 6–13. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E.; Zhou, E.S. If It Goes up, Must It Come down? Chronic Stress and the Hypothalamic-Pituitary-Adrenocortical Axis in Humans. Psychol. Bull. 2007, 133, 25–45. [Google Scholar] [CrossRef]
- Liberzon, I.; Abelson, J.L. Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron 2016, 92, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, G.; Bertaccini, R.; Tarasi, L.; Di Gregorio, F.; Trajkovic, J.; Battaglia, S.; Romei, V. The Role of Alpha Oscillations among the Main Neuropsychiatric Disorders in the Adult and Developing Human Brain: Evidence from the Last 10 Years of Research. Biomedicines 2022, 10, 3189. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, F.; La Porta, F.; Petrone, V.; Battaglia, S.; Orlandi, S.; Ippolito, G.; Romei, V.; Piperno, R.; Lullini, G. Accuracy of EEG Biomarkers in the Detection of Clinical Outcome in Disorders of Consciousness after Severe Acquired Brain Injury: Preliminary Results of a Pilot Study Using a Machine Learning Approach. Biomedicines 2022, 10, 1897. [Google Scholar] [CrossRef] [PubMed]
- Tortora, F.; Hadipour, A.L.; Battaglia, S.; Falzone, A.; Avenanti, A.; Vicario, C.M. The Role of Serotonin in Fear Learning and Memory: A Systematic Review of Human Studies. Brain Sci. 2023, 13, 1197. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.R.; Di Fazio, C.; Battaglia, S. Activated Tryptophan-Kynurenine Metabolic System in the Human Brain Is Associated with Learned Fear. Front. Mol. Neurosci. 2023, 16, 1217090. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Török, N.; Fanni, T.; Szab, Á. Co-Players in Chronic Pain: Neuroinflammation and the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines 2022, 10, 76. [Google Scholar] [CrossRef]
- Roozendaal, B.; Okuda, S.; Van der Zee, E.A.; McGaugh, J.L. Glucocorticoid Enhancement of Memory Requires Arousal-Induced Noradrenergic Activation in the Basolateral Amygdala. Proc. Natl. Acad. Sci. USA 2006, 103, 6741–6746. [Google Scholar] [CrossRef]
- Herman, J.P.; Ostrander, M.M.; Mueller, N.K.; Figueiredo, H. Limbic System Mechanisms of Stress Regulation: Hypothalamo-Pituitary-Adrenocortical Axis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 1201–1213. [Google Scholar] [CrossRef]
- McEwen, B.S.; Weiss, J.M.; Schwartz, L.S. Selective Retention of Corticosterone by Limbic Structures in Rat Brain. Nature 1968, 220, 911–912. [Google Scholar] [CrossRef]
- Makino, S.; Gold, P.W.; Schulkin, J. Effects of Corticosterone on CRH mRNA and Content in the Bed Nucleus of the Stria Terminalis; Comparison with the Effects in the Central Nucleus of the Amygdala and the Paraventricular Nucleus of the Hypothalamus. Brain Res. 1994, 657, 141–149. [Google Scholar] [CrossRef]
- Roozendaal, B.; Castello, N.A.; Vedana, G.; Barsegyan, A.; McGaugh, J.L. Noradrenergic Activation of the Basolateral Amygdala Modulates Consolidation of Object Recognition Memory. Neurobiol. Learn. Mem. 2008, 90, 576–579. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. The Neurobiology of Stress: From Serendipity to Clinical Relevance. Brain Res. 2000, 886, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, J.J.; Mailliet, F.; Almeida, O.F.X.; Jay, T.M.; Sousa, N. The Prefrontal Cortex as a Key Target of the Maladaptive Response to Stress. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Madalena, K.M.; Lerch, J.K. The Effect of Glucocorticoid and Glucocorticoid Receptor Interactions on Brain, Spinal Cord, and Glial Cell Plasticity. Neural Plast. 2017, 2017, 8640970. [Google Scholar] [CrossRef]
- Myers, B.; McKlveen, J.M.; Herman, J.P. Glucocorticoid Actions on Synapses, Circuits, and Behavior: Implications for the Energetics of Stress. Front. Neuroendocrinol. 2014, 35, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Cruceanu, C.; Dony, L.; Krontira, A.C.; Fischer, D.S.; Roeh, S.; Di Giaimo, R.; Kyrousi, C.; Kaspar, L.; Arloth, J.; Czamara, D.; et al. Cell-Type-Specific Impact of Glucocorticoid Receptor Activation on the Developing Brain: A Cerebral Organoid Study. Am. J. Psychiatry 2022, 179, 375–387. [Google Scholar] [CrossRef]
- Hill, A.R.; Spencer-Segal, J.L. Glucocorticoids and the Brain after Critical Illness. Endocrinology 2021, 162, bqaa242. [Google Scholar] [CrossRef]
- Conrad, C.D. Chronic Stress-Induced Hippocampal Vulnerability: The Glucocorticoid Vulnerability Hypothesis. Rev. Neurosci. 2008, 19, 395–411. [Google Scholar] [CrossRef]
- Cornelisse, S.; van Ast, V.A.; Joëls, M.; Kindt, M. Delayed Effects of Cortisol Enhance Fear Memory of Trace Conditioning. Psychoneuroendocrinology 2014, 40, 257–268. [Google Scholar] [CrossRef]
- Merz, C.J.; Hermann, A.; Stark, R.; Wolf, O.T. Cortisol Modifies Extinction Learning of Recently Acquired Fear in Men. Soc. Cogn. Affect. Neurosci. 2014, 9, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Kinner, V.L.; Wolf, O.T.; Merz, C.J. Cortisol Increases the Return of Fear by Strengthening Amygdala Signaling in Men. Psychoneuroendocrinology 2018, 91, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Jung, M.W. Neural Circuits and Mechanisms Involved in Pavlovian Fear Conditioning: A Critical Review. Neurosci. Biobehav. Rev. 2006, 30, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Lebron-Milad, K.; Milad, M.R. Sex Differences, Gonadal Hormones and the Fear Extinction Network: Implications for Anxiety Disorders. Biol. Mood Anxiety Disord. 2012, 2, 3. [Google Scholar] [CrossRef]
- Kogler, L.; Müller, V.I.; Seidel, E.-M.; Boubela, R.; Kalcher, K.; Moser, E.; Habel, U.; Gur, R.C.; Eickhoff, S.B.; Derntl, B. Sex Differences in the Functional Connectivity of the Amygdalae in Association with Cortisol. Neuroimage 2016, 134, 410–423. [Google Scholar] [CrossRef]
- Tabbert, K.; Merz, C.J.; Klucken, T.; Schweckendiek, J.; Vaitl, D.; Wolf, O.T.; Stark, R. Cortisol Enhances Neural Differentiation during Fear Acquisition and Extinction in Contingency Aware Young Women. Neurobiol. Learn. Mem. 2010, 94, 392–401. [Google Scholar] [CrossRef]
- Hagedorn, B.; Wolf, O.T.; Merz, C.J. Stimulus-Based Extinction Generalization: Neural Correlates and Modulation by Cortisol. Int. J. Neuropsychopharmacol. 2021, 24, 354–365. [Google Scholar] [CrossRef]
- Brueckner, A.H.; Lass-Hennemann, J.; Wilhelm, F.H.; Ferreira de Sá, D.S.; Michael, T. Cortisol Administration after Extinction in a Fear-Conditioning Paradigm with Traumatic Film Clips Prevents Return of Fear. Transl. Psychiatry 2019, 9, 128. [Google Scholar] [CrossRef]
- Hagedorn, B.; Wolf, O.T.; Merz, C.J. Cortisol before Extinction Generalization Alters Its Neural Correlates during Retrieval. Psychoneuroendocrinology 2022, 136, 105607. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural Regulation of Endocrine and Autonomic Stress Responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Kalsbeek, A.; van der Spek, R.; Lei, J.; Endert, E.; Buijs, R.M.; Fliers, E. Circadian Rhythms in the Hypothalamo-Pituitary-Adrenal (HPA) Axis. Mol. Cell. Endocrinol. 2012, 349, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, B. Stress and Memory: Opposing Effects of Glucocorticoids on Memory Consolidation and Memory Retrieval. Neurobiol. Learn. Mem. 2002, 78, 578–595. [Google Scholar] [CrossRef]
- de Quervain, D.J.-F.; Aerni, A.; Schelling, G.; Roozendaal, B. Glucocorticoids and the Regulation of Memory in Health and Disease. Front. Neuroendocrinol. 2009, 30, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Lebron-Milad, K.; Abbs, B.; Milad, M.R.; Linnman, C.; Rougemount-Bücking, A.; Zeidan, M.A.; Holt, D.J.; Goldstein, J.M. Sex Differences in the Neurobiology of Fear Conditioning and Extinction: A Preliminary fMRI Study of Shared Sex Differences with Stress-Arousal Circuitry. Biol. Mood Anxiety Disord. 2012, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Milad, M.R.; Zeidan, M.A.; Contero, A.; Pitman, R.K.; Klibanski, A.; Rauch, S.L.; Goldstein, J.M. The Influence of Gonadal Hormones on Conditioned Fear Extinction in Healthy Humans. Neuroscience 2010, 168, 652–658. [Google Scholar] [CrossRef]
- Bangasser, D.A.; Valentino, R.J. Sex Differences in Stress-Related Psychiatric Disorders: Neurobiological Perspectives. Front. Neuroendocrinol. 2014, 35, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A.; Felmingham, K.L.; Silove, D.; Creamer, M.; O’Donnell, M.; McFarlane, A.C. The Association between Menstrual Cycle and Traumatic Memories. J. Affect. Disord. 2011, 131, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.M.; Milad, M.R. Blockade of Estrogen by Hormonal Contraceptives Impairs Fear Extinction in Female Rats and Women. Biol. Psychiatry 2013, 73, 371–378. [Google Scholar] [CrossRef]
- Shansky, R.M. Are Hormones a “Female Problem” for Animal Research? Science 2019, 364, 825–826. [Google Scholar] [CrossRef]
- Beery, A.K.; Zucker, I. Sex Bias in Neuroscience and Biomedical Research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef]
- Abercrombie, H.C.; Kalin, N.H.; Thurow, M.E.; Rosenkranz, M.A.; Davidson, R.J. Cortisol Variation in Humans Affects Memory for Emotionally Laden and Neutral Information. Behav. Neurosci. 2003, 117, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Maren, S. Synaptic Mechanisms of Associative Memory in the Amygdala. Neuron 2005, 47, 783–786. [Google Scholar] [CrossRef]
- Goosens, K.A.; Maren, S. NMDA Receptors Are Essential for the Acquisition, but Not Expression, of Conditional Fear and Associative Spike Firing in the Lateral Amygdala. Eur. J. Neurosci. 2004, 20, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Merz, C.J.; Tabbert, K.; Schweckendiek, J.; Klucken, T.; Vaitl, D.; Stark, R.; Wolf, O.T. Investigating the Impact of Sex and Cortisol on Implicit Fear Conditioning with fMRI. Psychoneuroendocrinology 2010, 35, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Merz, C.J.; Stark, R.; Vaitl, D.; Tabbert, K.; Wolf, O.T. Stress Hormones Are Associated with the Neuronal Correlates of Instructed Fear Conditioning. Biol. Psychol. 2013, 92, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Meir Drexler, S.; Merz, C.J.; Hamacher-Dang, T.C.; Tegenthoff, M.; Wolf, O.T. Effects of Cortisol on Reconsolidation of Reactivated Fear Memories. Neuropsychopharmacology 2015, 40, 3036–3043. [Google Scholar] [CrossRef] [PubMed]
- Meir Drexler, S.; Merz, C.J.; Hamacher-Dang, T.C.; Wolf, O.T. Cortisol Effects on Fear Memory Reconsolidation in Women. Psychopharmacology 2016, 233, 2687–2697. [Google Scholar] [CrossRef] [PubMed]
- Meir Drexler, S.; Merz, C.J.; Lissek, S.; Tegenthoff, M.; Wolf, O.T. Reactivation of the Unconditioned Stimulus Inhibits the Return of Fear Independent of Cortisol. Front. Behav. Neurosci. 2019, 13, 254. [Google Scholar] [CrossRef]
- Kindt, M.; Soeter, M.; Vervliet, B. Beyond Extinction: Erasing Human Fear Responses and Preventing the Return of Fear. Nat. Neurosci. 2009, 12, 256–258. [Google Scholar] [CrossRef]
- Etkin, A.; Egner, T.; Kalisch, R. Emotional Processing in Anterior Cingulate and Medial Prefrontal Cortex. Trends Cogn. Sci. 2011, 15, 85–93. [Google Scholar] [CrossRef]
- Lesuis, S.L.; Brosens, N.; Immerzeel, N.; van der Loo, R.J.; Mitrić, M.; Bielefeld, P.; Fitzsimons, C.P.; Lucassen, P.J.; Kushner, S.A.; van den Oever, M.C.; et al. Glucocorticoids Promote Fear Generalization by Increasing the Size of a Dentate Gyrus Engram Cell Population. Biol. Psychiatry 2021, 90, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Purves, K.L.; Krebs, G.; McGregor, T.; Constantinou, E.; Lester, K.J.; Barry, T.J.; Craske, M.G.; Young, K.S.; Breen, G.; Eley, T.C. Evidence for Distinct Genetic and Environmental Influences on Fear Acquisition and Extinction. Psychol. Med. 2023, 53, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Fredrikson, M.; Annas, P.; Hettema, J.M. Different Genetic Factors Underlie Fear Conditioning and Episodic Memory. Psychiatr. Genet. 2015, 25, 155–162. [Google Scholar] [CrossRef]
- Lucifora, C.; Grasso, G.M.; Nitsche, M.A.; D’Italia, G.; Sortino, M.; Salehinejad, M.A.; Falzone, A.; Avenanti, A.; Vicario, C.M. Enhanced Fear Acquisition in Individuals with Evening Chronotype. A Virtual Reality Fear Conditioning/Extinction Study. J. Affect. Disord. 2022, 311, 344–352. [Google Scholar] [CrossRef]
- Vicario, C.M.; Makris, S.; Culicetto, L.; Lucifora, C.; Falzone, A.; Martino, G.; Ferraioli, F.; Nitsche, M.A.; Avenanti, A.; Craparo, G. Evidence of Altered Fear Extinction Learning in Individuals with High Vaccine Hesitancy During COVID-19 Pandemic. Clin. Neuropsychiatry 2023, 20, 364–369. [Google Scholar] [CrossRef]
- Stockhorst, U.; Antov, M.I. Modulation of Fear Extinction by Stress, Stress Hormones and Estradiol: A Review. Front. Behav. Neurosci. 2015, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Shechner, T.; Hong, M.; Britton, J.C.; Pine, D.S.; Fox, N.A. Fear Conditioning and Extinction Across Development: Evidence from Human Studies and Animal models. Biol. Psychol. 2014, 100, 1–12. [Google Scholar] [CrossRef]
- Suzuki, A.; Josselyn, S.A.; Frankland, P.W.; Masushige, S.; Silva, A.J.; Kida, S. Memory Reconsolidation and Extinction Have Distinct Temporal and Biochemical Signatures. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 4787–4795. [Google Scholar] [CrossRef]
- Quirk, G.J.; Paré, D.; Richardson, R.; Herry, C.; Monfils, M.H.; Schiller, D.; Vicentic, A. Erasing Fear Memories with Extinction Training. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 14993–14997. [Google Scholar] [CrossRef]
- Jones, C.E.; Monfils, M.-H. Chapter Eight—Using Reconsolidation and Extinction to Weaken Fear Memories in Animal Models. In Memory Reconsolidation; Alberini, C.M., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 165–184. [Google Scholar] [CrossRef]
- Soeter, M.; Kindt, M. Disrupting Reconsolidation: Pharmacological and Behavioral Manipulations. Learn. Mem. 2011, 18, 357–366. [Google Scholar] [CrossRef]
- Quinones, M.M.; Gallegos, A.M.; Lin, F.V.; Heffner, K. Dysregulation of Inflammation, Neurobiology, and Cognitive Function in PTSD: An Integrative Review. Cogn. Affect. Behav. Neurosci. 2020, 20, 455–480. [Google Scholar] [CrossRef]
- Lee, H.; Kaang, B.-K. How Engram Mediates Learning, Extinction, and Relapse. Curr. Opin. Neurobiol. 2023, 81, 102723. [Google Scholar] [CrossRef]
- Raber, J.; Arzy, S.; Bertolus, J.B.; Depue, B.; Haas, H.E.; Hofmann, S.G.; Kangas, M.; Kensinger, E.; Lowry, C.A.; Marusak, H.A.; et al. Current Understanding of Fear Learning and Memory in Humans and Animal Models and the Value of a Linguistic Approach for Analyzing Fear Learning and Memory in Humans. Neurosci. Biobehav. Rev. 2019, 105, 136–177. [Google Scholar] [CrossRef]
- Finsterwald, C.; Alberini, C.M. Stress and Glucocorticoid Receptor-Dependent Mechanisms in Long-Term Memory: From Adaptive Responses to Psychopathologies. Neurobiol. Learn. Mem. 2014, 112, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Monari, S.; Guillot de Suduiraut, I.; Grosse, J.; Zanoletti, O.; Walker, S.E.; Mesquita, M.; Wood, T.C.; Cash, D.; Astori, S.; Sandi, C. Blunted Glucocorticoid Responsiveness to Stress Causes Behavioral and Biological Alterations That Lead to Posttraumatic Stress Disorder Vulnerability. Biol. Psychiatry 2023, in press. [CrossRef] [PubMed]
- Adzic, M.; Glavonic, E.; Nesic, M.J.; Milosavljevic, M.; Mihaljevic, M.; Petrovic, Z.; Pavlovic, Z.; Brkic, Z.; Francija, E.; Soldatovic, I.; et al. Glucocorticoid Receptor Alpha Translational Isoforms as Mediators of Early Adversities and Negative Emotional States. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 90, 288–299. [Google Scholar] [CrossRef]
- Krugers, H.J.; Zhou, M.; Joëls, M.; Kindt, M. Regulation of Excitatory Synapses and Fearful Memories by Stress Hormones. Front. Behav. Neurosci. 2011, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Likhtik, E.; Popa, D.; Apergis-Schoute, J.; Fidacaro, G.A.; Paré, D. Amygdala Intercalated Neurons Are Required for Expression of Fear Extinction. Nature 2008, 454, 642–645. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex Brain Networks: Graph Theoretical Analysis of Structural and Functional Systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Buckner, R.L.; Sepulcre, J.; Talukdar, T.; Krienen, F.M.; Liu, H.; Hedden, T.; Andrews-Hanna, J.R.; Sperling, R.A.; Johnson, K.A. Cortical Hubs Revealed by Intrinsic Functional Connectivity: Mapping, Assessment of Stability, and Relation to Alzheimer’s Disease. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 1860–1873. [Google Scholar] [CrossRef]
- Chiappini, E.; Silvanto, J.; Hibbard, P.B.; Avenanti, A.; Romei, V. Strengthening Functionally Specific Neural Pathways with Transcranial Brain Stimulation. Curr. Biol. 2018, 28, R735–R736. [Google Scholar] [CrossRef]
- Romei, V.; Chiappini, E.; Hibbard, P.B.; Avenanti, A. Empowering Reentrant Projections from V5 to V1 Boosts Sensitivity to Motion. Curr. Biol. 2016, 26, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Vicario, C.M.; Nitsche, M.A.; Hoysted, I.; Yavari, F.; Avenanti, A.; Salehinejad, M.A.; Felmingham, K.L. Anodal Transcranial Direct Current Stimulation over the Ventromedial Prefrontal Cortex Enhances Fear Extinction in Healthy Humans: A Single Blind Sham-Controlled Study. Brain Stimul. 2020, 13, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, P.; Yang, K.; Zhou, Q.; Zhan, S.; Lin, H.; Li, L.; Wang, L.; Wang, Y. Efficacy of Repetitive Dual-Site Paired Associative Transcranial Magnetic Stimulation in the Treatment of Generalized Anxiety Disorder. Brain Stimul. 2020, 13, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Zanon, M.; Di Luzio, P.; Romei, V.; Tamietto, M.; Avenanti, A. Driving Associative Plasticity in Temporo-occipital Back-Projections Improves Visual Recognition of Emotional Expressions. Nat. Commun. 2022. under review. [Google Scholar]
- Turrini, S.; Bevacqua, N.; Cataneo, A.; Chiappini, E.; Fiori, F.; Battaglia, S.; Romei, V.; Avenanti, A. Neurophysiological Markers of Premotor-Motor Network Plasticity Predict Motor Performance in Young and Older Adults. Biomedicines 2023, 11, 1464. [Google Scholar] [CrossRef]
- Turrini, S.; Fiori, F.; Chiappini, E.; Lucero, B.; Santarnecchi, E.; Avenanti, A. Cortico-Cortical Paired Associative Stimulation (CcPAS) over Premotor-Motor Areas Affects Local Circuitries in the Human Motor Cortex Via Hebbian Plasticity. Neuroimage 2023, 271, 120027. [Google Scholar] [CrossRef]
- Shin, L.M.; Liberzon, I. The Neurocircuitry of Fear, Stress, and Anxiety Disorders. Neuropsychopharmacology 2010, 35, 169–191. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. Stress Signalling Pathways that Impair Prefrontal Cortex Structure and Function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- LaBar, K.S.; Cabeza, R. Cognitive Neuroscience of Emotional Memory. Nat. Rev. Neurosci. 2006, 7, 54–64. [Google Scholar] [CrossRef]
- Morey, R.A.; Dunsmoor, J.E.; Haswell, C.C.; Brown, V.M.; Vora, A.; Weiner, J.; Stjepanovic, D.; Wagner, H.R., 3rd; LaBar, K.S. Fear Learning Circuitry Is Biased toward Generalization of Fear Associations in Posttraumatic Stress Disorder. Transl. Psychiatry 2015, 5, e700. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Battaglia, S.; Avenanti, A.; di Pellegrino, G. Don’t Hurt Me No More: State-Dependent Transcranial Magnetic Stimulation for the Treatment of Specific Phobia. J. Affect. Disord. 2021, 286, 78–79. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Central Effects of Stress Hormones in Health and Disease: Understanding the Protective and Damaging Effects of Stress and Stress Mediators. Eur. J. Pharmacol. 2008, 583, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Pitman, R.K.; Rasmusson, A.M.; Koenen, K.C.; Shin, L.M.; Orr, S.P.; Gilbertson, M.W.; Milad, M.R.; Liberzon, I. Biological Studies of Post-Traumatic Stress Disorder. Nat. Rev. Neurosci. 2012, 13, 769–787. [Google Scholar] [CrossRef] [PubMed]
- Glover, E.M.; Jovanovic, T.; Mercer, K.B.; Kerley, K.; Bradley, B.; Ressler, K.J.; Norrholm, S.D. Estrogen Levels Are Associated with Extinction Deficits in Women with Posttraumatic Stress Disorder. Biol. Psychiatry 2012, 72, 19–24. [Google Scholar] [CrossRef]
- Hofmann, S.G.; Meuret, A.E.; Smits, J.A.J.; Simon, N.M.; Pollack, M.H.; Eisenmenger, K.; Shiekh, M.; Otto, M.W. Augmentation of Exposure Therapy with D-Cycloserine for Social Anxiety Disorder. Arch. Gen. Psychiatry 2006, 63, 298–304. [Google Scholar] [CrossRef]
- Schwabe, L.; Wolf, O.T. Stress and Multiple Memory Systems: From “Thinking” to “Doing”. Trends Cogn. Sci. 2013, 17, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; Sapolsky, R.M. Disruption of Fear Memory Through Dual-Hormone Gene Therapy. Biol. Psychiatry 2009, 65, 441–444. [Google Scholar] [CrossRef][Green Version]
- Schwabe, L.; Oitzl, M.S.; Philippsen, C.; Richter, S.; Bohringer, A.; Wippich, W.; Schachinger, H. Stress Modulates the Use of Spatial Versus Stimulus-Response Learning Strategies in Humans. Learn. Mem. 2007, 14, 109–116. [Google Scholar] [CrossRef]
- Smits, J.A.J.; Rosenfield, D.; Otto, M.W.; Marques, L.; Davis, M.L.; Meuret, A.E.; Simon, N.M.; Pollack, M.H.; Hofmann, S.G. D-Cycloserine Enhancement of Exposure Therapy for Social Anxiety Disorder Depends on the Success of Exposure Sessions. J. Psychiatr. Res. 2013, 47, 1455–1461. [Google Scholar] [CrossRef]
- Garfinkel, S.N.; Abelson, J.L.; King, A.P.; Sripada, R.K.; Wang, X.; Gaines, L.M.; Liberzon, I. Impaired Contextual Modulation of Memories in PTSD: An fMRI and Psychophysiological Study of Extinction Retention and Fear Renewal. J. Neurosci. 2014, 34, 13435–13443. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, B.; McReynolds, J.R.; Van der Zee, E.A.; Lee, S.; McGaugh, J.L.; McIntyre, C.K. Glucocorticoid Effects on Memory Consolidation Depend on Functional Interactions between the Medial Prefrontal Cortex and Basolateral Amygdala. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 14299–14308. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L.; Alkire, M.T. Epinephrine Enhancement of Human Memory Consolidation: Interaction with Arousal at Encoding. Neurobiol. Learn. Mem. 2003, 79, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Admon, R.; Milad, M.R.; Hendler, T. A Causal Model of Post-Traumatic Stress Disorder: Disentangling Predisposed from Acquired Neural Abnormalities. Trends Cogn. Sci. 2013, 17, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, T.; Phifer, J.E.; Sicking, K.; Weiss, T.; Norrholm, S.D.; Bradley, B.; Ressler, K.J. Cortisol Suppression by Dexamethasone Reduces Exaggerated Fear Responses in Posttraumatic Stress Disorder. Psychoneuroendocrinology 2011, 36, 1540–1552. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Diamond, D.M. Current Status on Behavioral and Biological Markers of PTSD: A Search for Clarity in a Conflicting Literature. Neurosci. Biobehav. Rev. 2013, 37, 860–895. [Google Scholar] [CrossRef]
- Rothbaum, B.O.; Davis, M. Applying Learning Principles to the Treatment of Post-Trauma Reactions. Ann. N. Y. Acad. Sci. 2003, 1008, 112–121. [Google Scholar] [CrossRef]
- Shin, L.M.; Rauch, S.L.; Pitman, R.K. Amygdala, Medial Prefrontal Cortex, and Hippocampal Function in PTSD. Ann. N. Y. Acad. Sci. 2006, 1071, 67–79. [Google Scholar] [CrossRef]
- LeDoux, J.E. Coming to Terms with Fear. Proc. Natl. Acad. Sci. USA 2014, 111, 2871–2878. [Google Scholar] [CrossRef]
- Etkin, A.; Prater, K.E.; Schatzberg, A.F.; Menon, V.; Greicius, M.D. Disrupted Amygdalar Subregion Functional Connectivity and Evidence of a Compensatory Network in Generalized Anxiety Disorder. Arch. Gen. Psychiatry 2009, 66, 1361–1372. [Google Scholar] [CrossRef]
- Liston, C.; Chen, A.C.; Zebley, B.D.; Drysdale, A.T.; Gordon, R.; Leuchter, B.; Voss, H.U.; Casey, B.J.; Etkin, A.; Dubin, M.J. Default Mode Network Mechanisms of Transcranial Magnetic Stimulation in Depression. Biol. Psychiatry 2014, 76, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Salomons, T.V.; Dunlop, K.; Kennedy, S.H.; Flint, A.; Geraci, J.; Giacobbe, P.; Downar, J. Resting-State Cortico-Thalamic-Striatal Connectivity Predicts Response to Dorsomedial Prefrontal RTMS in Major Depressive Disorder. Neuropsychopharmacology 2014, 39, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.S.W.; Franke, T.F.; Gan, W.-B. Opposite Effects of Fear Conditioning and Extinction on Dendritic Spine Remodelling. Nature 2012, 483, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Joëls, M.; Baram, T.Z. The Neuro-Symphony of Stress. Nat. Rev. Neurosci. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Liston, C.; Cichon, J.M.; Jeanneteau, F.; Jia, Z.; Chao, M.V.; Gan, W.-B. Circadian Glucocorticoid Oscillations Promote Learning-Dependent Synapse Formation and Maintenance. Nat. Neurosci. 2013, 16, 698–705. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Mood Disorders and Allostatic Load. Biol. Psychiatry 2003, 54, 200–207. [Google Scholar] [CrossRef]
- Pattwell, S.S.; Duhoux, S.; Hartley, C.A.; Johnson, D.C.; Jing, D.; Elliott, M.D.; Ruberry, E.J.; Powers, A.; Mehta, N.; Yang, R.R.; et al. Altered Fear Learning across Development in Both Mouse and Human. Proc. Natl. Acad. Sci. USA 2012, 109, 16318–16323. [Google Scholar] [CrossRef]
- Eiland, L.; Romeo, R.D. Stress and the Developing Adolescent Brain. Neuroscience 2013, 249, 162–171. [Google Scholar] [CrossRef]
- Bandelow, B.; Michaelis, S. Epidemiology of Anxiety Disorders in the 21st Century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar] [CrossRef]
- Yehuda, R.; LeDoux, J. Response Variation Following Trauma: A Translational Neuroscience Approach to Understanding PTSD. Neuron 2007, 56, 19–32. [Google Scholar] [CrossRef]
- Smith, E.; Weinberg, A.; Moran, T.; Hajcak, G. Electrocortical Responses to NIMSTIM Facial Expressions of Emotion. Int. J. Psychophysiol. 2013, 88, 17–25. [Google Scholar] [CrossRef] [PubMed]

| Study | Group (N) | Pharmacological Treatment | Mechanism of Action | Phase of Fear Learning | CSs and US | Psychophysiological Measure | Main Findings |
|---|---|---|---|---|---|---|---|
| Cornelisse et al. [110] | ‘Slow cort’ (21) ‘Rapid cort’ (21) Placebo (21) | 10 mg of cortisol Placebo | Cortisol is an agonist of glucocorticoid receptor and Annexin A1 | Acquisition Extinction Reinstatement | CS: Male neutral faces US: Auditory tone | SCR-EMG | No effect of cortisol on SCRs ‘Slow cort’ group showed more differentiation of CS’s trace, compared to placebo group and ‘Rapid cort’ group |
| Merz et al. [111] | Cortisol (16) Placebo (16) | 30 mg of cortisol Placebo | Cortisol is an agonist of glucocorticoid receptor and Annexin A1 | Acquisition Extinction | CS: Geometric figures US: Electric shock | SCR-fMRI | Placebo group showed an enhanced reduction in SCRs from early to late extinction to the CS+, compared to the cortisol group Cortisol group showed diminished activation of the amygdala, MFC, and NAcc during late extinction |
| Merz et al. [64] | Cortisol group (20) Placebo group (20) | 30 mg of cortisol Placebo | Cortisol is an agonist of glucocorticoid receptor and Annexin A1 | Acquisition Extinction Recall | CS: Three lamps US: Electric shock | SCR-fMRI | Cortisol group showed reduced activations in the bilateral amygdala, right anterior parahippocampal gyrus, and right hippocampus during extinction training |
| Hagedorn et al. [117] | Cortisol group (30) Placebo group (30) | 10 mg of cortisol Placebo | Cortisol is an agonist of glucocorticoid receptor and Annexin A1 | Acquisition Extinction Recall Reinstatement | CS: Geometric figures US: Electric shock | SCR-fMRI | Stimulus-based extinction generalization increased fear-related brain activation and altered functional connectivity during both extinction learning and recall, but these effects were reversed by cortisol administration |
| Hagedorn et al. [119] | Cortisol group (30) Placebo group (30) | 20 mg of cortisol Placebo | Cortisol is an agonist of glucocorticoid receptor and Annexin A1 | Acquisition Extinction Recall Reinstatement | CS: Geometric figures US: Electric shock | SCR-fMRI | Cortisol prior to extinction generalization improved the extinction memory during the reinstatement test |
| Brueckner et al. [118] | Cortisol group (25) Placebo group (25) | 30 mg of cortisol Placebo | Cortisol is an agonist of glucocorticoid receptor and Annexin A1 | Acquisition Extinction Reinstatement | CS: Images of neutral male/female faces US: Traumatic clips | SCR-FPS | Cortisol group showed less reinstatement, lower US expectancy for the CS+, and attenuated FPS for the CS+, as compared with the placebo group |
| Study | Group (N) | Pharmacological Treatment | Mechanism of Action | Phase of Fear Learning | CSs | Psychophysiological Measure | Main Findings |
|---|---|---|---|---|---|---|---|
| Stark et al. [66] | Female cortisol (8) Male cortisol (9) Female placebo (9) Male placebo (8) | 30 mg of cortisol Placebo | Cortisol is an agonist of glucocorticoid receptor and Annexin A1 | Acquisition | CS: Geometric figures US: Electric shock | SCR-fMRI | Cortisol abolished the enhanced first interval responses for CS+ in men but increased it in women Men, but not women, in the placebo group showed stronger response to the CS+ than to the CS− |
| Merz et al. [134] | Female cortisol (10) Male cortisol (10) Female placebo (9) Male placebo (10) | 30 mg of cortisol Placebo | Cortisol is an agonist of glucocorticoid receptor and Annexin A1 | Acquisition Extinction | CS: Geometric figures US: Electric shock | SCR-fMRI | Cortisol reduced SCRs to CS in women and enhanced SCRs to both CS in men, compared to the placebo group Cortisol reduced amygdala reactivity to the CS+ both in men and women, but enhanced activity in the right insula only in women |
| Merz et al. [135] | LU women (15) OC women (15) Men group (20) | 30 mg of cortisol Placebo | Cortisol is an agonist of glucocorticoid receptor and Annexin A1 | Acquisition Extinction | CS: Geometric figures US: Electric shock | SCR-fMRI | Men showed higher SCRs to CS+ compared to OC women, whereas LU women did not significantly differ from men LU women showed higher CS+/CS− differentiation in the right amygdala compared to OC women and men |
| Meir Drexler et al. [136] | Reactivation + cortisol (14) Reactivation + placebo (14) No reactivation + cortisol (14) | 30 mg of cortisol Placebo | Cortisol is an agonist of glucocorticoid receptor and Annexin A1 | Acquisition Memory reactivation Extinction Reinstatement | CS: Geometric figures US: Electric shock | SCR | RE + CORT group only had higher SCRs for the reactivated CS1+ in the reinstatement |
| Meir Drexler et al. [137] | Reactivation + cortisol (24) Reactivation + placebo (24) No reactivation + cortisol (24) | 30 mg of cortisol Placebo | Cortisol is an agonist of glucocorticoid receptor and Annexin A1 | Acquisition Memory reactivation Extinction Reinstatement | CS: Geometric figures US: Electric shock | SCR | No differences in the reinstatement of the three CSs in any of the groups |
| Meir Drexler et al. [138] | Reactivation + cortisol (25) Reactivation + placebo (25) No reactivation + cortisol (25) | 20 mg of cortisol Placebo | Cortisol is an agonist of glucocorticoid receptor and Annexin A1 | Acquisition Memory reactivation Extinction Reinstatement | CS: Geometric figures US: Electric shock | SCR | No reactivation group had higher SCRs for the reactivated CS1+ in the reinstatement No reinstatement effect was found in the two reactivation groups, regardless of the pharmacological treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battaglia, S.; Di Fazio, C.; Mazzà, M.; Tamietto, M.; Avenanti, A. Targeting Human Glucocorticoid Receptors in Fear Learning: A Multiscale Integrated Approach to Study Functional Connectivity. Int. J. Mol. Sci. 2024, 25, 864. https://doi.org/10.3390/ijms25020864
Battaglia S, Di Fazio C, Mazzà M, Tamietto M, Avenanti A. Targeting Human Glucocorticoid Receptors in Fear Learning: A Multiscale Integrated Approach to Study Functional Connectivity. International Journal of Molecular Sciences. 2024; 25(2):864. https://doi.org/10.3390/ijms25020864
Chicago/Turabian StyleBattaglia, Simone, Chiara Di Fazio, Matteo Mazzà, Marco Tamietto, and Alessio Avenanti. 2024. "Targeting Human Glucocorticoid Receptors in Fear Learning: A Multiscale Integrated Approach to Study Functional Connectivity" International Journal of Molecular Sciences 25, no. 2: 864. https://doi.org/10.3390/ijms25020864
APA StyleBattaglia, S., Di Fazio, C., Mazzà, M., Tamietto, M., & Avenanti, A. (2024). Targeting Human Glucocorticoid Receptors in Fear Learning: A Multiscale Integrated Approach to Study Functional Connectivity. International Journal of Molecular Sciences, 25(2), 864. https://doi.org/10.3390/ijms25020864








