Association of Plasma Claudin-5 with Age and Alzheimer Disease
Abstract
:1. Introduction
2. Results
2.1. Establishment of an Immunoassay System Using Simoa
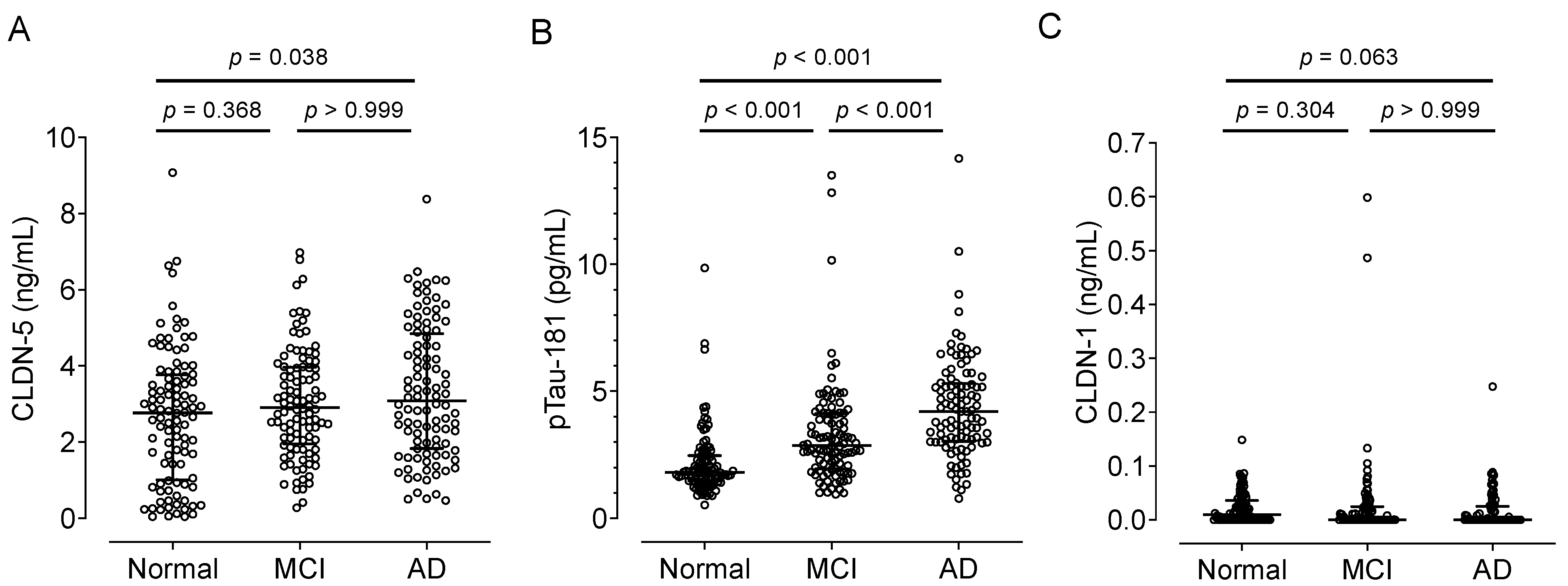
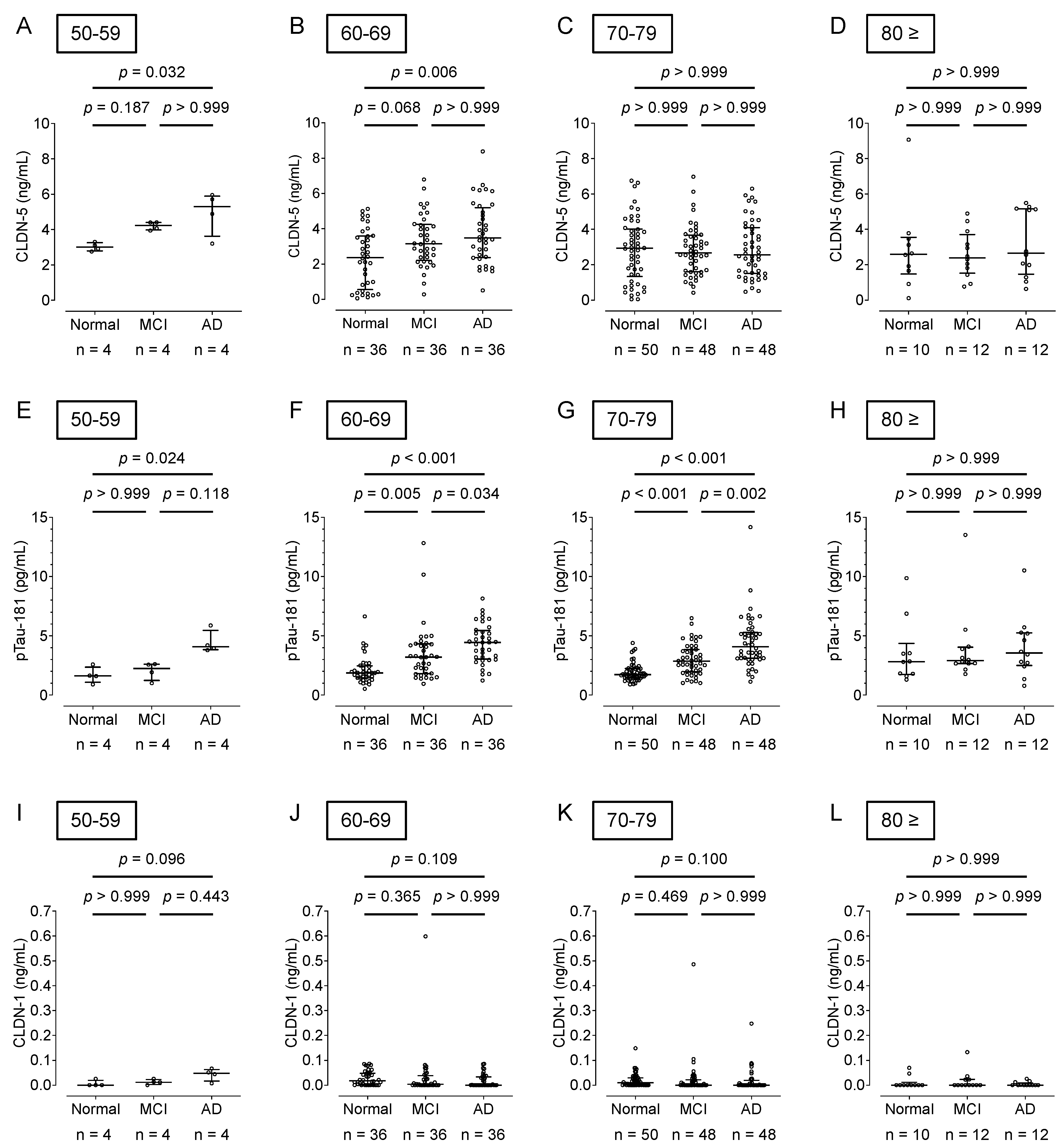
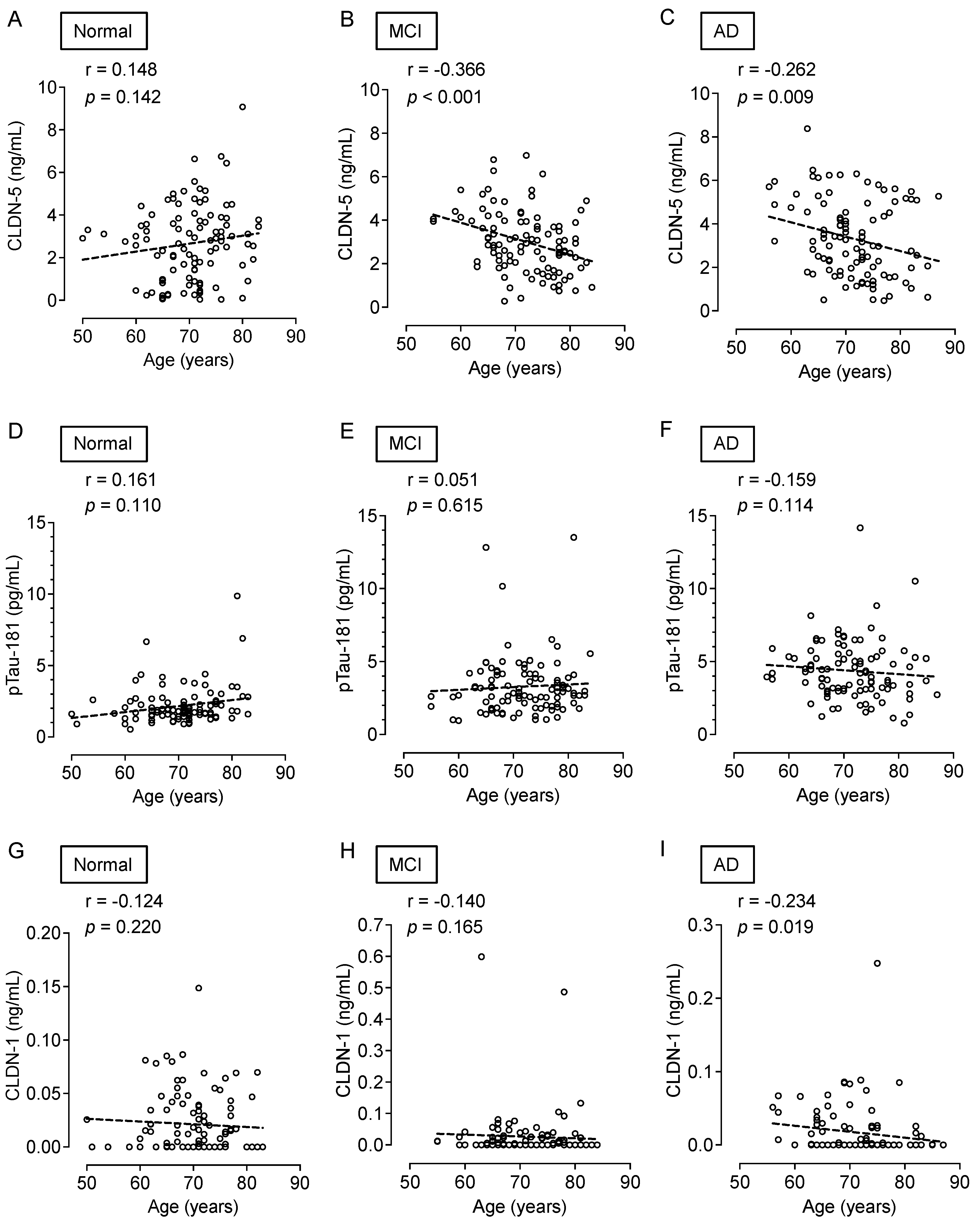
2.2. Association of Plasma CLDN-5 Levels with the Stages of Cognitive Decline
3. Discussion
4. Materials and Methods
4.1. Clinical Samples
4.2. Purification of Anti-CLDNs mAbs
4.3. Preparation of CLDNs
4.4. Simoa Assay for CLDNs
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauthier, S.; Webster, C.; Servaes, S.; Morais, J.; Rosa-Neto, P. World-Alzheimer-Report 2022: Life after Diagnosis: Navigating Treatment, Care and Support; Alzheimer’s Disease International: London, UK, 2022. [Google Scholar]
- Hameed, S.; Fuh, J.L.; Senanarong, V.; Ebenezer, E.G.M.; Looi, I.; Dominguez, J.C.; Park, K.W.; Karanam, A.K.; Simon, O. Role of fluid biomarkers and PET imaging in early diagnosis and its clinical implication in the management of Alzheimer’s disease. J. Alzheimer’s Dis. Rep. 2020, 4, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.E.Z.N.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2022. Alzheimer’s Dement. 2022, 8, e12295. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, J.; Xu, H.; Luo, T.; Li, Y.; Jiang, K.; Shentu, Y.; Tong, Z. New insights into Alzheimer’s disease: Novel pathogenesis, drug target and delivery. Pharmaceutics 2023, 15, 1133. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Rabinovici, G.D.; Gatsonis, C.; Apgar, C.; Chaudhary, K.; Gareen, I.; Hanna, L.; Hendrix, J.; Hillner, B.E.; Olson, C.; Lesman-Segev, O.H.; et al. Association of Amyloid Positron Emission Tomography with Subsequent Change in Clinical Management Among Medicare Beneficiaries with Mild Cognitive Impairment or Dementia. JAMA 2019, 321, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, E.H.; La Joie, R.; Wolf, A.; Strom, A.; Wang, P.; Iaccarino, L.; Bourakova, V.; Cobigo, Y.; Heuer, H.; Spina, A.; et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 2020, 26, 387–397. [Google Scholar] [CrossRef]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Fowler, C.; Li, Q.X.; Martins, R.; Rowe, C.; et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and dysfunction of the blood-brain barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef]
- van de Haar, H.J.; Burgmans, S.; Jansen, J.F.; van Osch, M.J.; van Buchem, M.A.; Muller, M.; Hofman, P.A.M.; Verhey, F.R.J.; Backes, W.H. Blood-brain barrier leakage in patients with early Alzheimer disease. Radiology 2016, 281, 527–535. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Tsukita, S.; Tanaka, H.; Tamura, A. The claudins: From tight junctions to biological systems. Trends Biochem. Sci. 2019, 44, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Mandel, I.; Paperna, T.; Glass-Marmor, L.; Volkowich, A.; Badarny, S.; Schwartz, I.; Vardi, P.; Koren, I.; Miller, A. Tight junction proteins expression and modulation in immune cells and multiple sclerosis. J. Cell. Mol. Med. 2012, 16, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA 2009, 106, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Shirakura, K.; Okada, Y.; Takeda, H.; Endo, K.; Tamura, M.; Watari, A.; Sadamura, Y.; Sawasaki, T.; Doi, T.; et al. Claudin-5-binders enhance permeation of solutes across the blood-brain barrier in a mammalian model. J. Pharmacol. Exp. Ther. 2017, 363, 275–283. [Google Scholar] [CrossRef]
- Berndt, P.; Winkler, L.; Cording, J.; Breitkreuz-Korff, O.; Rex, A.; Dithmer, S.; Rausch, V.; Blasig, R.; Richter, M.; Sporbert, A.; et al. Tight junction proteins at the blood-brain barrier: Far more than claudin-5. Cell. Mol. Life Sci. 2019, 76, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Feng, S.; Cen, J.; Huang, Y.; Shen, H.; Yao, L.; Wang, Y.; Chen, Z. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS ONE 2011, 6, e20599. [Google Scholar] [CrossRef]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef]
- Stewart, P.A.; Hayakawa, K.; Akers, M.A.; Vinters, H.V. A morphometric study of the blood-brain barrier in Alzheimer’s disease. Lab. Investig. 1992, 67, 734–742. [Google Scholar]
- Kazmierski, R.; Michalak, S.; Wencel-Warot, A.; Nowinski, W.L. Serum tight-junction proteins predict hemorrhagic transformation in ischemic stroke patients. Neurology 2012, 79, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, F.; Işık, Ü.; Demirdaş, A.; Doğuç, D.K.; Bozkurt, M. Serum zonulin and claudin-5 levels in patients with bipolar disorder. J. Affect. Disord. 2020, 266, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Işık, Ü.; Aydoğan Avşar, P.; Aktepe, E.; Doğuç, D.K.; Kılıç, F.; Büyükbayram, H. Serum zonulin and claudin-5 levels in children with obsessive-compulsive disorder. Nord. J. Psychiatry 2020, 74, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.A.; Magliocco, M.; Hayakawa, K.; Farrell, C.L.; Del Maestro, R.F.; Girvin, J.; Kaufmann, J.C.; Vinters, H.V.; Gilbert, J. A quantitative analysis of blood-brain barrier ultrastructure in the aging human. Microvasc. Res. 1987, 33, 270–282. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Shinohara, M.; Shinohara, M.; Yamazaki, A.; Murray, M.E.; Liesinger, A.M.; Heckman, M.G.; Lesser, E.R.; Parisi, J.E.; Petersen, R.C.; et al. Selective loss of cortical endothelial tight junction proteins during Alzheimer’s disease progression. Brain 2019, 142, 1077–1092. [Google Scholar] [CrossRef]
- Andjelkovic, A.V.; Situ, M.; Citalan-Madrid, A.F.; Stamatovic, S.M.; Xiang, J.; Keep, R.F. Blood-brain barrier dysfunction in normal aging and neurodegeneration: Mechanisms, impact, and treatments. Stroke 2023, 54, 661–672. [Google Scholar] [CrossRef]
- Morita, K.; Furuse, M.; Fujimoto, K.; Tsukita, S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. USA 1999, 96, 96511–96516. [Google Scholar] [CrossRef]
- Scalise, A.A.; Kakogiannos, N.; Zanardi, F.; Iannelli, F.; Giannotta, M. The blood-brain and gut-vascular barriers: From the perspective of claudins. Tissue Barriers 2021, 9, 1926190. [Google Scholar] [CrossRef]
- Greene, C.; Hanley, N.; Reschke, C.R.; Reddy, A.; Mäe, M.A.; Connolly, R.; Behan, C.; O’Keeffe, E.; Bolger, I.; Hudson, N.; et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat. Commun. 2022, 13, 2003. [Google Scholar] [CrossRef]
- Greene, C.; Kealy, J.; Humphries, M.M.; Gong, Y.; Hou, J.; Hudson, N.; Cassidy, L.M.; Martiniano, R.; Shashi, V.; Hooper, S.R.; et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol. Psychiatry. 2018, 23, 2156–2166. [Google Scholar] [CrossRef] [PubMed]
- Dion-Albert, L.; Cadoret, A.; Doney, E.; Kaufmann, F.N.; Dudek, K.A.; Daigle, B.; Parise, L.F.; Cathomas, F.; Samba, N.; Hudson, N.; et al. Vascular and blood-brain barrier-related changes underlie stress responses and resilience in female mice and depression in human tissue. Nat. Commun. 2022, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, D.; Wagner, C.A.; Martino, G.; Nedergaard, M.; Zoccali, C.; Unwin, R.; Capasso, G. Mechanisms of cognitive dysfunction in CKD. Nat. Rev. Nephrol. 2020, 16, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Winblad, B.; Marengoni, A.; Klarin, I.; Fastbom, J.; Fratiglioni, L. Heart failure and risk of dementia and Alzheimer disease: A population-based cohort study. Arch. Intern. Med. 2006, 166, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Manemann, S.M.; Knopman, D.S.; St Sauver, J.; Bielinski, S.J.; Chamberlain, A.M.; Weston, S.A.; Jiang, R.; Roger, V.L. Alzheimer’s disease and related dementias and heart failure: A community study. J. Am. Geriatr. Soc. 2022, 70, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, M.A.; Dean, D.C.; Bendlin, B.B.; Jarjour, N.N.; Esnault, S.; Zetterberg, H.; Heslegrave, A.; Evans, M.D.; Davidson, R.J.; Busse, W.W. Neuroimaging and biomarker evidence of neurodegeneration in asthma. J. Allergy Clin. Immunol. 2022, 149, 589–598.e6. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.L.; Tybjærg-Hansen, A.; Nordestgaard, B.G.; Frikke-schmidt, R. Plasma levels of apolipoprotein E, APOE genotype, and all-cause and cause-specific mortality in 105,949 individuals from a white general population cohort. Eur. Heart J. 2019, 40, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Thorin, E. Hypertension and Alzheimer disease: Another brick in the wall of awareness. Hypertension 2015, 65, 36–38. [Google Scholar] [CrossRef]
- Choi, D.; Choi, S.; Park, S.M. Effect of smoking cessation on the risk of dementia: A longitudinal study. Ann. Clin. Transl. Neurol. 2018, 5, 1192–1199. [Google Scholar] [CrossRef]
- Cirovic, A.; Cirovic, A.; Orisakwe, O.E.; Lima, R.R. Local and systemic hypoxia as inductors of increased aluminum and iron brain accumulation promoting the onset of Alzheimer’s disease. Biol. Trace Elem. Res. 2023, 201, 5134–5142. [Google Scholar] [CrossRef]
- Shen, W.; Li, S.; Chung, S.H.; Zhu, L.; Stayt, J.; Su, T.; Couraud, P.; Romero, I.A.; Weksler, B.; Gillies, M.C. Tyrosine phosphorylation of VE-cadherin and claudin-5 is associated with TGF-β1-induced permeability of centrally derived vascular endothelium. Eur. J. Cell Biol. 2011, 90, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Awan, F.M.; Anjum, S.; Obaid, A.; Ali, A.; Paracha, R.Z.; Janjua, H.A. In-silico analysis of claudin-5 reveals novel putative sites for post-translational modifications: Insights into potential molecular determinants of blood-brain barrier breach during HIV-1 infiltration. Infect. Genet. Evol. 2014, 27, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, P.; Cioni, C.; Masi, G.; Tassi, M.; Marotta, G.; Severi, S. Fingolimod reduces circulating tight-junction protein levels and in vitro peripheral blood mononuclear cells migration in multiple sclerosis patients. Sci. Rep. 2018, 8, 15371. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, J.; Teng, T.; Yin, B.; He, Y.; Jiang, Y.; Liu, X.; Yu, Y.; Li, X.; Zhou, X. Biomarkers of intestinal permeability and blood-brain barrier permeability in adolescents with major depressive disorder. J. Affect. Disord. 2023, 323, 659–666. [Google Scholar] [CrossRef]
- Usta, A.; Kılıç, F.; Demirdaş, A.; Işık, Ü.; Doğuç, D.K.; Bozkurt, M. Serum zonulin and claudin-5 levels in patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 767–773. [Google Scholar] [CrossRef]
- Jongen, P.J.; Ter Horst, A.T.; Brands, A.M. Cognitive impairment in multiple sclerosis. Minerva. Med. 2012, 103, 73–96. [Google Scholar]
- Yamamoto, K.S.; Utshigisawa, T.; Ogura, H.; Aoki, T.; Kawakami, T.; Ohga, S.; Ohara, A.; Ito, E.; Yamamoto, T.; Kanno, H. Clinical and genetic diagnosis of thirteen Japanese patients with hereditary spherocytosis. Hum. Genome Var. 2022, 9, 1. [Google Scholar] [CrossRef]
- Tachibana, K.; Hashimoto, Y.; Shirakura, K.; Okada, Y.; Hirayama, R.; Iwashita, Y.; Nishino, I.; Ago, Y.; Takeda, H.; Kuniyasu, H.; et al. Safety and efficacy of an anti-claudin-5 monoclonal antibody to increase blood-brain barrier permeability for drug delivery to the brain in a non-human primate. J. Control. Release 2021, 336, 105–111. [Google Scholar] [CrossRef]
- Fukasawa, M.; Nagase, S.; Shirasago, Y.; Iida, M.; Yamashita, M.; Endo, K.; Yagi, K.; Suzuki, T.; Wakita, T.; Hanada, K.; et al. Monoclonal antibodies against extracellular domains of claudin-1 block hepatitis C virus infection in a mouse model. J. Virol. 2015, 89, 4866–4879. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Zhou, W.; Hamauchi, K.; Shirakura, K.; Doi, T.; Yagi, K.; Sawasaki, T.; Okada, Y.; Kondoh, M.; Takeda, H. Engineered membrane protein antigens successfully induce antibodies against extracellular regions of claudin-5. Sci. Rep. 2018, 8, 8383. [Google Scholar] [CrossRef]
- Zhou, W.; Takeda, H. Cell-free production of proteoliposomes for functional analysis and antibody development targeting membrane proteins. J. Vis. Exp. 2020, 163, e61871. [Google Scholar]
- Wilson, D.H.; Rissin, D.M.; Kan, C.W.; Fournier, D.R.; Piech, T.; Campbell, T.G.; Meyer, R.E.; Fishburn, M.W.; Cabrera, C.; Patel, P.P.; et al. The Simoa HD-1 Analyzer: A novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J. Lab. Autom. 2016, 21, 533–547. [Google Scholar] [CrossRef] [PubMed]
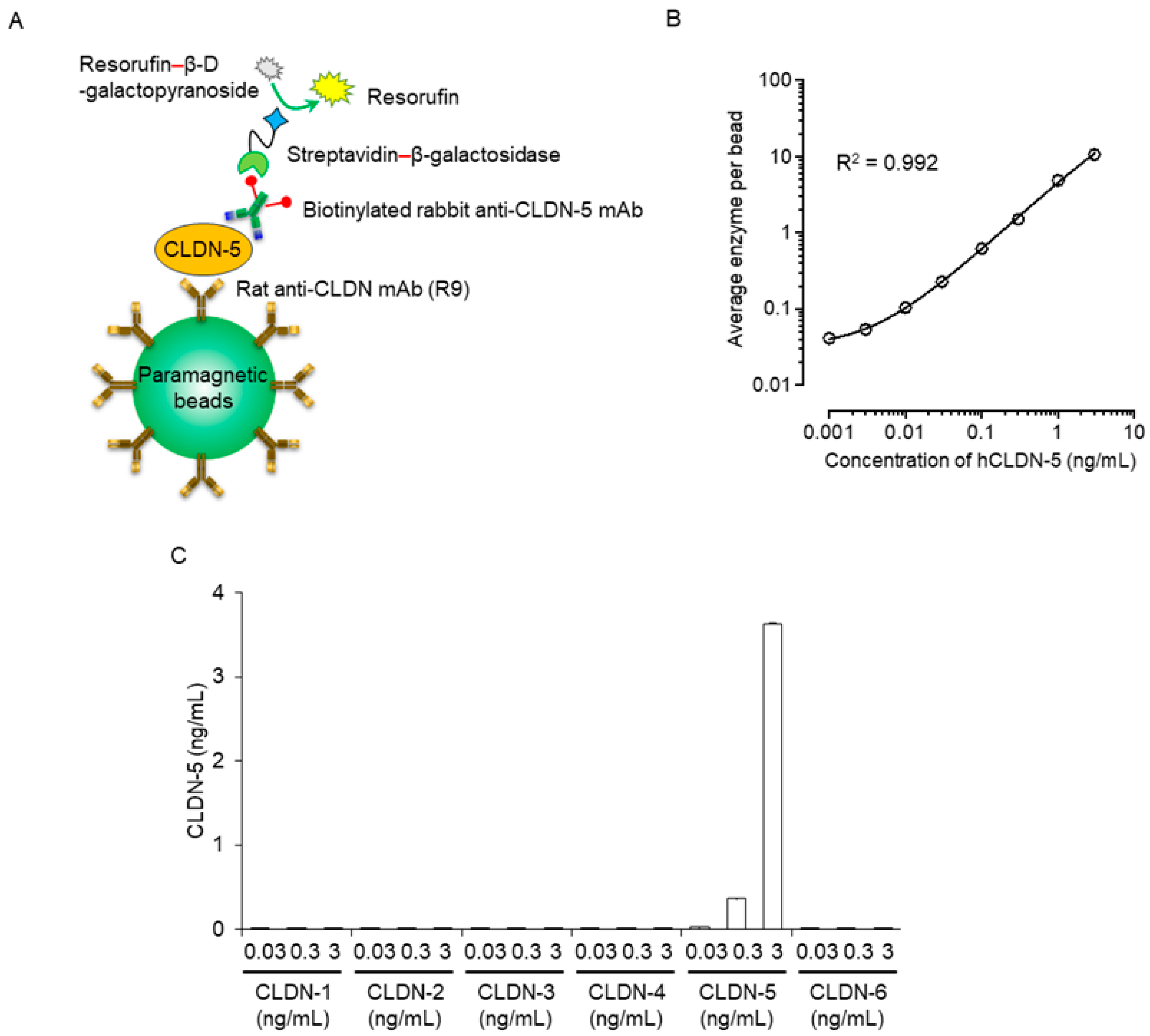

| Theoretical Concentration (ng/mL) | Actual Concentration (ng/mL) | Mean (ng/mL) | SD | CV (%) | Accuracy | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||
| 0 | ND | ND | ND | 0.000 | ND | 0.000 | NA | NA | NA |
| 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 13.80 | 117.18 |
| 0.003 | 0.003 | 0.004 | 0.003 | 0.003 | 0.003 | 0.003 | 0.000 | 15.51 | 95.37 |
| 0.01 | 0.010 | 0.011 | 0.011 | 0.010 | 0.009 | 0.010 | 0.001 | 7.61 | 102.21 |
| 0.03 | 0.029 | 0.033 | 0.030 | 0.029 | 0.029 | 0.030 | 0.002 | 5.74 | 100.19 |
| 0.1 | 0.103 | 0.108 | 0.096 | 0.105 | 0.090 | 0.100 | 0.007 | 7.47 | 100.27 |
| 0.3 | 0.290 | 0.315 | 0.293 | 0.280 | 0.208 | 0.277 | 0.041 | 14.73 | 92.46 |
| 1 | 1.094 | 1.152 | 1.082 | 1.083 | 1.048 | 1.092 | 0.038 | 3.47 | 109.19 |
| 3 | 3.008 | 3.180 | 2.888 | 2.517 | 2.981 | 2.915 | 0.246 | 8.44 | 97.16 |
| Characteristic | Cognitively Normal (n = 100) | MCI (n = 100) | AD (n = 100) |
|---|---|---|---|
| Age, y | 70.3 ± 6.7 | 71.7 ± 6.6 | 71.5 ± 6.7 |
| Female, n (%) | 50 (50.0) | 50 (50.0) | 50 (50.0) |
| MMSE score | 29.5 ± 0.7 | 22.2 ± 1.9 | 16.0 ± 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tachibana, K.; Hirayama, R.; Sato, N.; Hattori, K.; Kato, T.; Takeda, H.; Kondoh, M. Association of Plasma Claudin-5 with Age and Alzheimer Disease. Int. J. Mol. Sci. 2024, 25, 1419. https://doi.org/10.3390/ijms25031419
Tachibana K, Hirayama R, Sato N, Hattori K, Kato T, Takeda H, Kondoh M. Association of Plasma Claudin-5 with Age and Alzheimer Disease. International Journal of Molecular Sciences. 2024; 25(3):1419. https://doi.org/10.3390/ijms25031419
Chicago/Turabian StyleTachibana, Keisuke, Ryuichi Hirayama, Naoyuki Sato, Kotaro Hattori, Takashi Kato, Hiroyuki Takeda, and Masuo Kondoh. 2024. "Association of Plasma Claudin-5 with Age and Alzheimer Disease" International Journal of Molecular Sciences 25, no. 3: 1419. https://doi.org/10.3390/ijms25031419





