Directed Evolution of 4-Hydroxyphenylpyruvate Biosensors Based on a Dual Selection System
Abstract
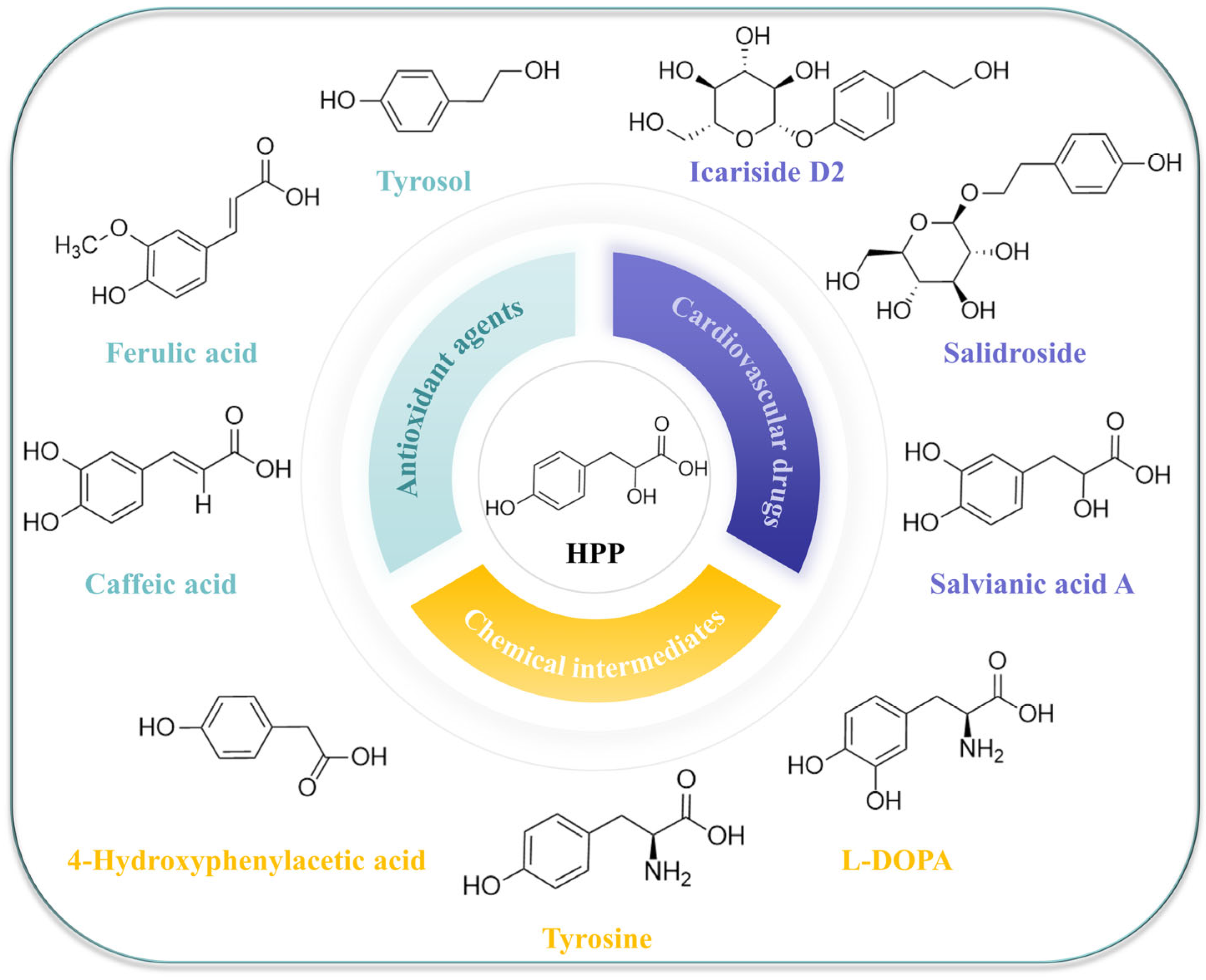
:1. Introduction
2. Results
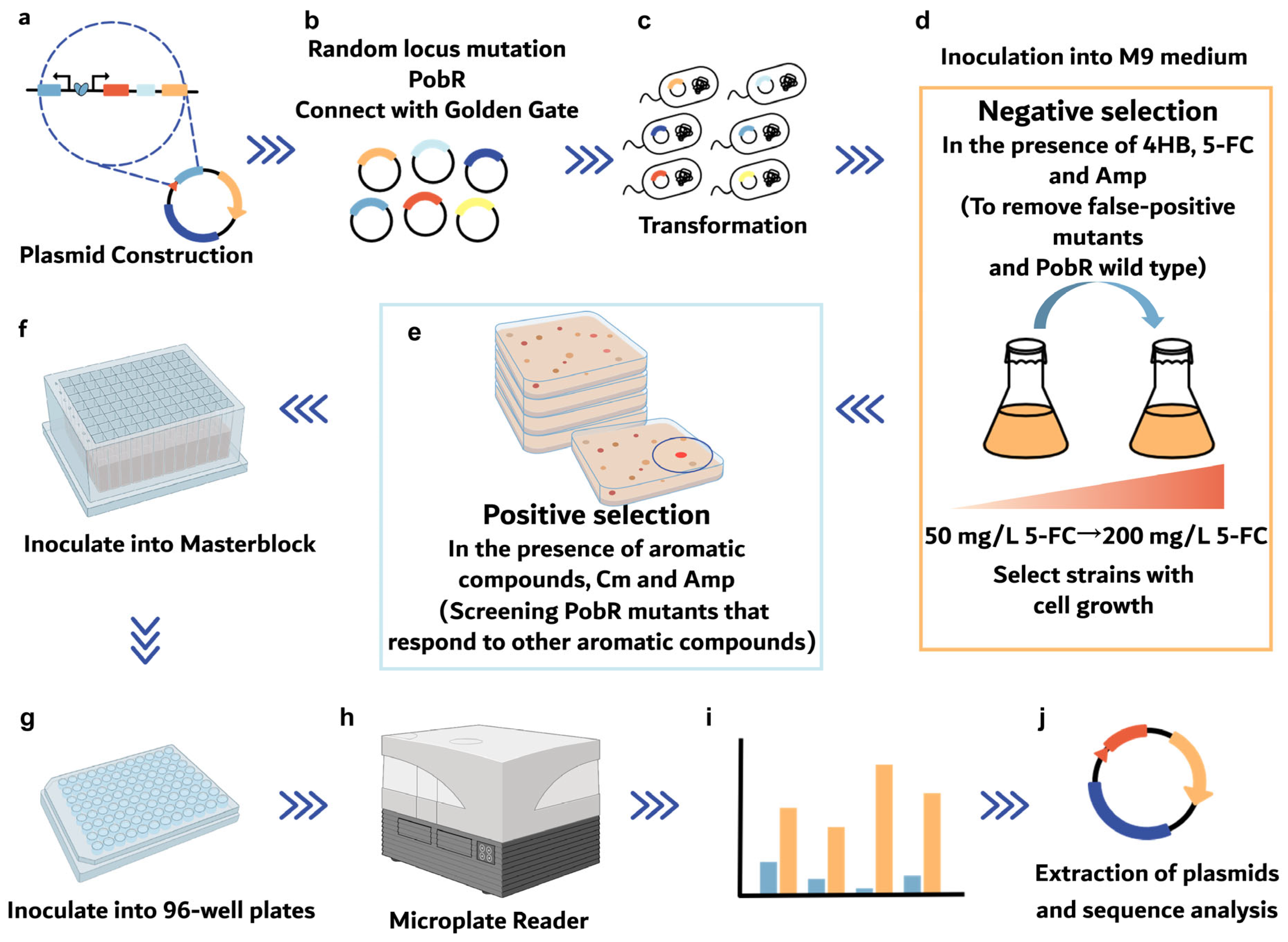
2.1. Design of a Dual Selection System
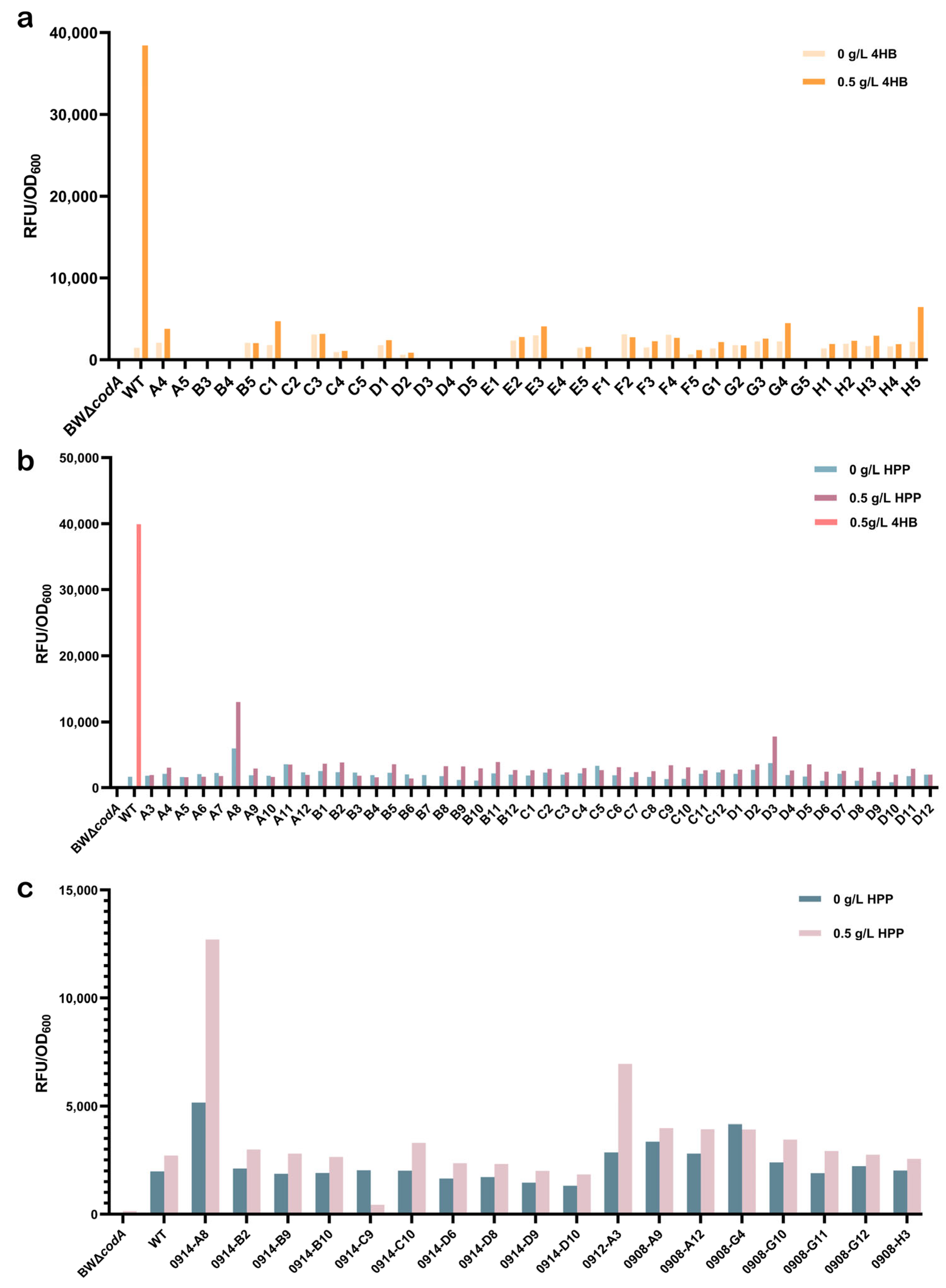
2.2. Directed Evolution of PobR
2.3. Screening for HPP-Responsive PobR Mutants
2.4. The Specificity of PobR177 and Screening of PobR Mutants Responsive to Other Ligands
2.5. Kinetic Simulation and Ligand Docking
3. Discussion
4. Methods and Materials
4.1. Bacterial Strains, Media, Chemicals and Other Materials
4.2. Construction of the gYB2a-pobR-mCherry-codA-cmr Plasmid
4.3. Design and Construction of the PobR Mutant Library
4.4. Counter-Selection Using CDase
4.5. Positive Screening Using Cm
4.6. Modeling Docking and Kinetic Simulation
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Effendi, S.S.W.; Ng, I.S. Challenges and opportunities for engineered Escherichia coli as a pivotal chassis toward versatile tyrosine-derived chemicals production. Biotechnol. Adv. 2023, 69, 108270. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Chen, J.; Hu, M.; Fang, F.; Zhou, J. Promoting FADH(2) Regeneration of Hydroxylation for High-Level Production of Hydroxytyrosol from Glycerol in Escherichia coli. J. Agric. Food Chem. 2023, 71, 16681–16690. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.Q.; Ju, X.L. Advances in Research on Anticancer Properties of Salidroside. Chin. J. Integr. Med. 2021, 27, 153–160. [Google Scholar] [CrossRef]
- Milke, L.; Aschenbrenner, J.; Marienhagen, J.; Kallscheuer, N. Production of plant-derived polyphenols in microorganisms: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Park, S.Y.; Park, Y.S.; Eun, H.; Lee, S.Y. Metabolic Engineering of Escherichia coli for Natural Product Biosynthesis. Trends Biotechnol. 2020, 38, 745–765. [Google Scholar] [CrossRef]
- Liu, Y.; Nielsen, J. Recent trends in metabolic engineering of microbial chemical factories. Curr. Opin. Biotechnol. 2019, 60, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.G.; De Mey, M.; Lim, C.G.; Ajikumar, P.K.; Stephanopoulos, G. The future of metabolic engineering and synthetic biology: Towards a systematic practice. Metab. Eng. 2012, 14, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Chen, J.; Qin, Z.; Yu, S.; Zhou, J. Coordinating caffeic acid and salvianic acid A pathways for efficient production of rosmarinic acid in Escherichia coli. Metab Eng. 2023, 76, 29–38. [Google Scholar] [CrossRef]
- Lai, W.; Shi, F.; Tan, S.; Liu, H.; Li, Y.; Xiang, Y. Dynamic control of 4-hydroxyisoleucine biosynthesis by multi-biosensor in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2022, 106, 5105–5121. [Google Scholar] [CrossRef]
- Mitchler, M.M.; Garcia, J.M.; Montero, N.E.; Williams, G.J. Transcription factor-based biosensors: A molecular-guided approach for natural product engineering. Curr. Opin. Biotechnol. 2021, 69, 172–181. [Google Scholar] [CrossRef]
- Ding, N.; Zhou, S.; Deng, Y. Transcription-Factor-based Biosensor Engineering for Applications in Synthetic Biology. ACS Synth. Biol. 2021, 10, 911–922. [Google Scholar] [CrossRef]
- Wan, X.; Volpetti, F.; Petrova, E.; French, C.; Maerkl, S.J.; Wang, B. Cascaded amplifying circuits enable ultrasensitive cellular sensors for toxic metals. Nat. Chem. Biol. 2019, 15, 540–548. [Google Scholar] [CrossRef]
- Bowman, E.K.; Alper, H.S. Microdroplet-Assisted Screening of Biomolecule Production for Metabolic Engineering Applications. Trends Biotechnol. 2020, 38, 701–714. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, S. Transcription Factor-Based Biosensor for Dynamic Control in Yeast for Natural Product Synthesis. Front. Bioeng. Biotechnol. 2021, 9, 635265. [Google Scholar] [CrossRef]
- Yu, X.; Shi, F.; Liu, H.; Tan, S.; Li, Y. Programming adaptive laboratory evolution of 4-hydroxyisoleucine production driven by a lysine biosensor in Corynebacterium glutamicum. AMB Express 2021, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Ma, Y.; Bu, R.; Zhao, T.; Wu, K. Directed evolution of a transcription factor PbrR to improve lead selectivity and reduce zinc interference through dual selection. AMB Express 2020, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.K.; Narayanan, N.; Pandey, N.; Bingen, J.M.; Kern, T.L.; Johnson, C.W.; Strauss, C.E.M.; Beckham, G.T.; Hennelly, S.P.; Dale, T. Sensor-Enabled Alleviation of Product Inhibition in Chorismate Pyruvate-Lyase. ACS Synth. Biol. 2019, 8, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Mannan, A.A.; Liu, D.; Zhang, F.; Oyarzún, D.A. Fundamental Design Principles for Transcription-Factor-Based Metabolite Biosensors. ACS Synth. Biol. 2017, 6, 1851–1859. [Google Scholar] [CrossRef]
- Chen, X.F.; Xia, X.X.; Lee, S.Y.; Qian, Z.G. Engineering tunable biosensors for monitoring putrescine in Escherichia coli. Biotechnol. Bioeng. 2018, 115, 1014–1027. [Google Scholar] [CrossRef]
- Zhang, F.; Carothers, J.M.; Keasling, J.D. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat. Biotechnol. 2012, 30, 354–359. [Google Scholar] [CrossRef]
- Skjoedt, M.L.; Snoek, T.; Kildegaard, K.R.; Arsovska, D.; Eichenberger, M.; Goedecke, T.J.; Rajkumar, A.S.; Zhang, J.; Kristensen, M.; Lehka, B.J.; et al. Engineering prokaryotic transcriptional activators as metabolite biosensors in yeast. Nat. Chem. Biol. 2016, 12, 951–958. [Google Scholar] [CrossRef]
- Bayer, T.; Hänel, L.; Husarcikova, J.; Kunzendorf, A.; Bornscheuer, U.T. In Vivo Detection of Low Molecular Weight Platform Chemicals and Environmental Contaminants by Genetically Encoded Biosensors. ACS Omega 2023, 8, 23227–23239. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.; Rogers, J.K.; Taylor, N.D.; Church, G.M. Evolution-guided optimization of biosynthetic pathways. Proc. Natl. Acad. Sci. USA 2014, 111, 17803–17808. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Song, J.; Zhang, H.; Lin, Y.; Han, S.; Huang, Y.; Zheng, S. Development of a Transcription Factor-Based Diamine Biosensor in Corynebacterium glutamicum. ACS Synth. Biol. 2021, 10, 3074–3083. [Google Scholar] [CrossRef] [PubMed]
- Bahls, M.O.; Platz, L.; Morgado, G.; Schmidt, G.W.; Panke, S.J.M.E. Directed evolution of biofuel-responsive biosensors for automated optimization of branched-chain alcohol biosynthesis. Metab. Eng. 2022, 69, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.A.; Shis, D.L.; Alikhani, A.; Keasling, J.D. Transcription factor-based screens and synthetic selections for microbial small-molecule biosynthesis. ACS Synth. Biol. 2013, 2, 47–58. [Google Scholar] [CrossRef]
- Ogawa, Y.; Katsuyama, Y.; Ueno, K.; Ohnishi, Y. Switching the Ligand Specificity of the Biosensor XylS from meta to para-Toluic Acid through Directed Evolution Exploiting a Dual Selection System. ACS Synth. Biol. 2019, 8, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Qian, S.; Akinterinwa, O.; Frei, C.S.; Gredell, J.A.; Cirino, P.C. Screening for enhanced triacetic acid lactone production by recombinant Escherichia coli expressing a designed triacetic acid lactone reporter. J. Am. Chem. Soc. 2013, 135, 10099–10103. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Fazelinia, H.; Cirino, P.C. AraC regulatory protein mutants with altered effector specificity. J. Am. Chem. Soc. 2008, 130, 5267–5271. [Google Scholar] [CrossRef]
- Tang, S.Y.; Cirino, P.C. Design and application of a mevalonate-responsive regulatory protein. Angew. Chem. (Int. Ed. Engl.) 2011, 50, 1084–1086. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, K.; Shen, L.; Ma, J.; Bao, L.; Chen, D.; Wei, L.; Wei, N.; Liu, B.; Wu, Y.; et al. Evolved Biosensor with High Sensitivity and Specificity for Measuring Cadmium in Actual Environmental Samples. Environ. Sci. Technol. 2022, 56, 10062–10071. [Google Scholar] [CrossRef]
- Xiong, D.; Lu, S.; Wu, J.; Liang, C.; Wang, W.; Wang, W.; Jin, J.M.; Tang, S.Y. Improving key enzyme activity in phenylpropanoid pathway with a designed biosensor. Metab. Eng. 2017, 40, 115–123. [Google Scholar] [CrossRef]
- Zhou, S.; Yuan, S.F.; Nair, P.H.; Alper, H.S.; Deng, Y.; Zhou, J. Development of a growth coupled and multi-layered dynamic regulation network balancing malonyl-CoA node to enhance (2S)-naringenin biosynthesis in Escherichia coli. Metab. Eng. 2021, 67, 41–52. [Google Scholar] [CrossRef]
- Liang, Y.; Luo, J.; Yang, C.; Guo, S.; Zhang, B.; Chen, F.; Su, K.; Zhang, Y.; Dong, Y.; Wang, Z.; et al. Directed evolution of the PobR allosteric transcription factor to generate a biosensor for 4-hydroxymandelic acid. World J. Microbiol. Biotechnol. 2022, 38, 104. [Google Scholar] [CrossRef]
- De Paepe, B.; Peters, G.; Coussement, P.; Maertens, J.; De Mey, M. Tailor-made transcriptional biosensors for optimizing microbial cell factories. J. Ind. Microbiol. Biotechnol. 2017, 44, 623–645. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, A.C.; Arnold, F.H.; Stuermer, R.; Hauer, B.; Leadbetter, J.R. Directed evolution of Vibrio fischeri LuxR for improved response to butanoyl-homoserine lactone. Appl. Environ. Microbiol. 2007, 73, 5775–5781. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.H.; Leadbetter, J.R.; Arnold, F.H. Dual selection enhances the signaling specificity of a variant of the quorum-sensing transcriptional activator LuxR. Nat. Biotechnol. 2006, 24, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Voziyanov, Y.; Stewart, A.F.; Jayaram, M. A dual reporter screening system identifies the amino acid at position 82 in Flp site-specific recombinase as a determinant for target specificity. Nucleic Acids Res. 2002, 30, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Nearmnala, P.; Thanaburakorn, M.; Panbangred, W.; Chaiyen, P.; Hongdilokkul, N. An in vivo selection system with tightly regulated gene expression enables directed evolution of highly efficient enzymes. Sci. Rep. 2021, 11, 11669. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xiang, C.; Zhou, Y.; Khan, M.S.H.; Liu, W.; Feiler, C.G.; Wei, R.; Weber, G.; Höhne, M.; Bornscheuer, U.T. A growth selection system for the directed evolution of amine-forming or converting enzymes. Nat. Commun. 2022, 13, 7458. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wang, J.; Pang, Q.; Zhang, F.; Wang, J.; Wang, Q.; Qi, Q. Pathway optimization and key enzyme evolution of N-acetylneuraminate biosynthesis using an in vivo aptazyme-based biosensor. Metab. Eng. 2017, 43, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, P.; Cao, M.; Yu, T.; Lane, S.T.; Zhao, H. Directed Evolution: Methodologies and Applications. Chem. Rev. 2021, 121, 12384–12444. [Google Scholar] [CrossRef]
- Jha, R.K.; Kern, T.L.; Kim, Y.; Tesar, C.; Jedrzejczak, R.; Joachimiak, A.; Strauss, C.E. A microbial sensor for organophosphate hydrolysis exploiting an engineered specificity switch in a transcription factor. Nucleic Acids Res. 2016, 44, 8490–8500. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.; Chargaff, E. Massive incorporation of 5-fluorouracil into a bacterial ribonucleic acid. Nature 1959, 184, 1213–1215. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.X.; Cao, Y. CB-Dock2: Improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef]
- Fricker, P.C.; Gastreich, M.; Rarey, M. Automated drawing of structural molecular formulas under constraints. J. Chem. Inf. Comput. Sci. 2004, 44, 1065–1078. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.R., 3rd; McGee, T.D., Jr.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
- Kaczmarek, J.A.; Prather, K.L.J. Effective use of biosensors for high-throughput library screening for metabolite production. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab049. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef] [PubMed]
- The PyMOL Molecular Graphics System; Version 1.8; Schrodinger, LLC: New York, NY, USA, 2015.



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Liang, Y.; Li, J.; Yuan, X.; Tao, F.; Dong, C.; Shen, Z.; Sui, G.; Wang, P. Directed Evolution of 4-Hydroxyphenylpyruvate Biosensors Based on a Dual Selection System. Int. J. Mol. Sci. 2024, 25, 1533. https://doi.org/10.3390/ijms25031533
Du H, Liang Y, Li J, Yuan X, Tao F, Dong C, Shen Z, Sui G, Wang P. Directed Evolution of 4-Hydroxyphenylpyruvate Biosensors Based on a Dual Selection System. International Journal of Molecular Sciences. 2024; 25(3):1533. https://doi.org/10.3390/ijms25031533
Chicago/Turabian StyleDu, Hongxuan, Yaoyao Liang, Jianing Li, Xinyao Yuan, Fenglin Tao, Chengjie Dong, Zekai Shen, Guangchao Sui, and Pengchao Wang. 2024. "Directed Evolution of 4-Hydroxyphenylpyruvate Biosensors Based on a Dual Selection System" International Journal of Molecular Sciences 25, no. 3: 1533. https://doi.org/10.3390/ijms25031533




