Wet-Spun Chitosan–Sodium Caseinate Fibers for Biomedicine: From Spinning Process to Physical Properties
Abstract
:1. Introduction
2. Results
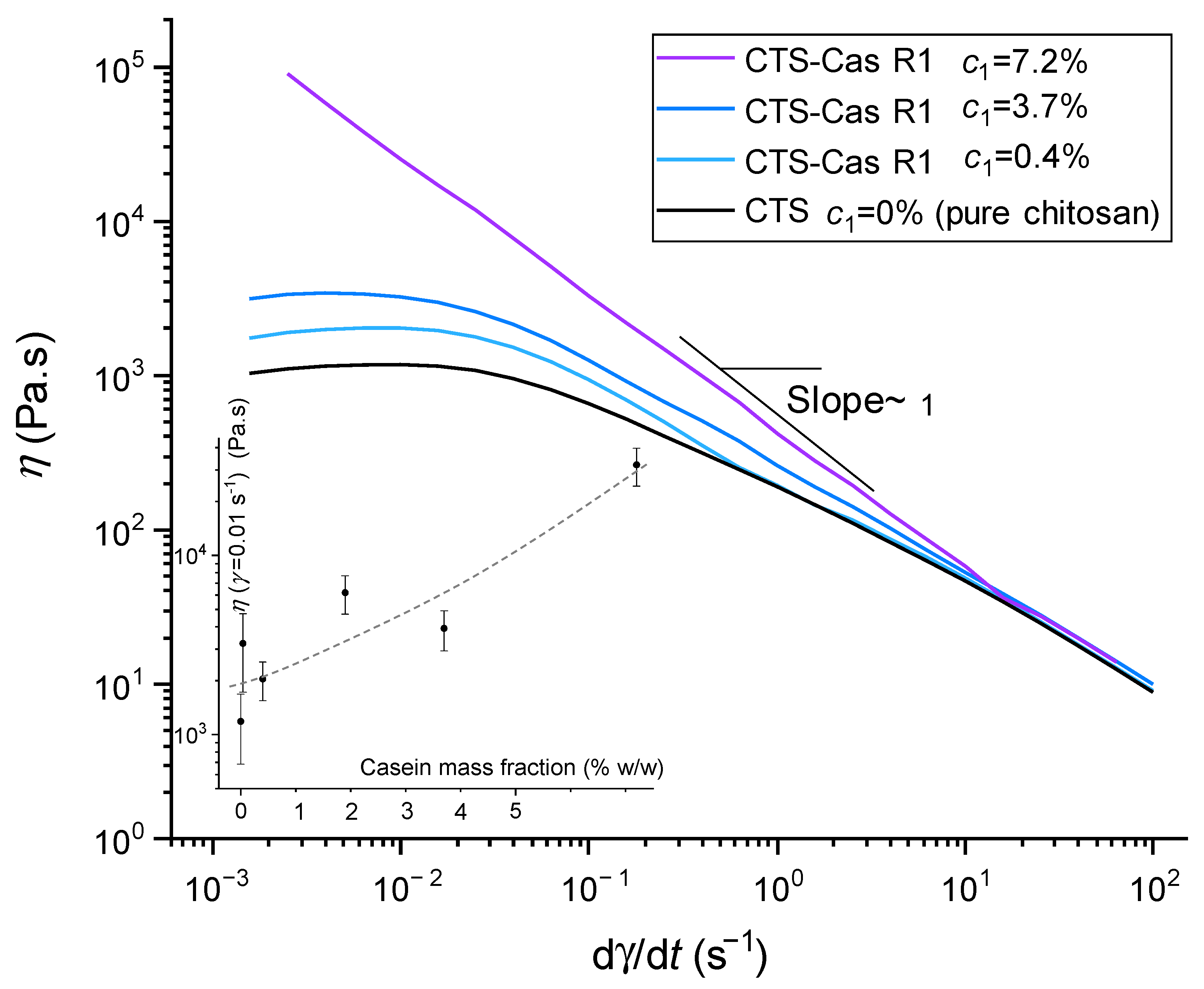
2.1. Rheology of Chitosan/Sodium Caseinate Collodions
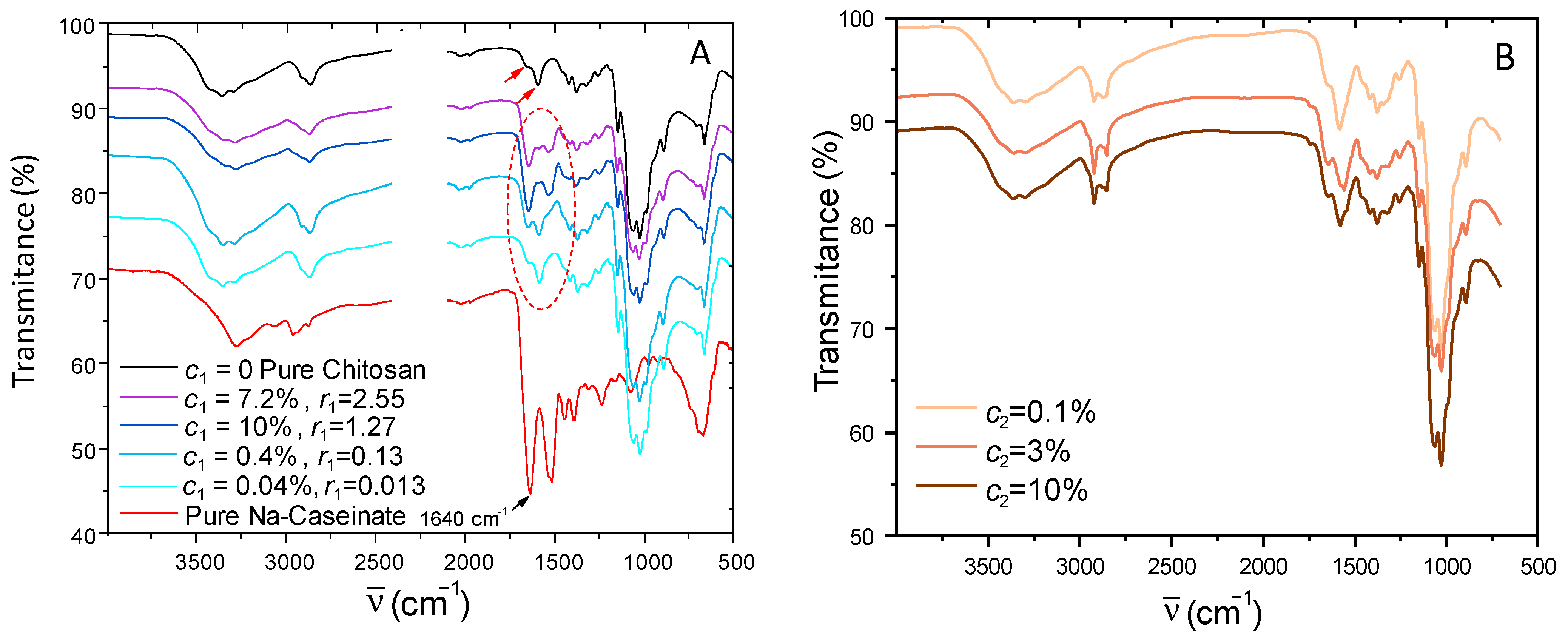
2.2. Evidence of Caseinate Incorporation Using ATR-FTIR Spectroscopy
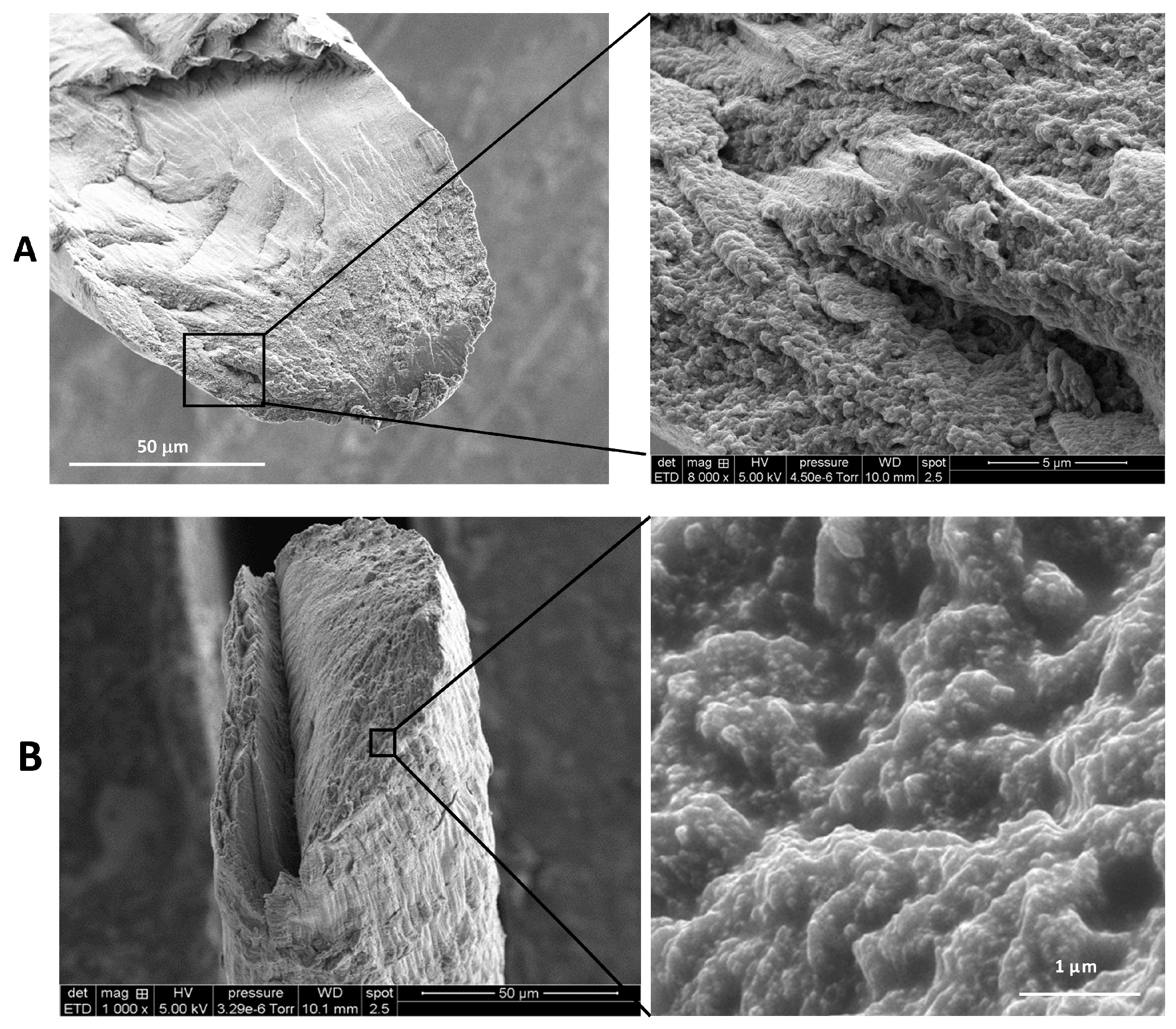
2.3. Fiber Microstructure Using SEM Analysis
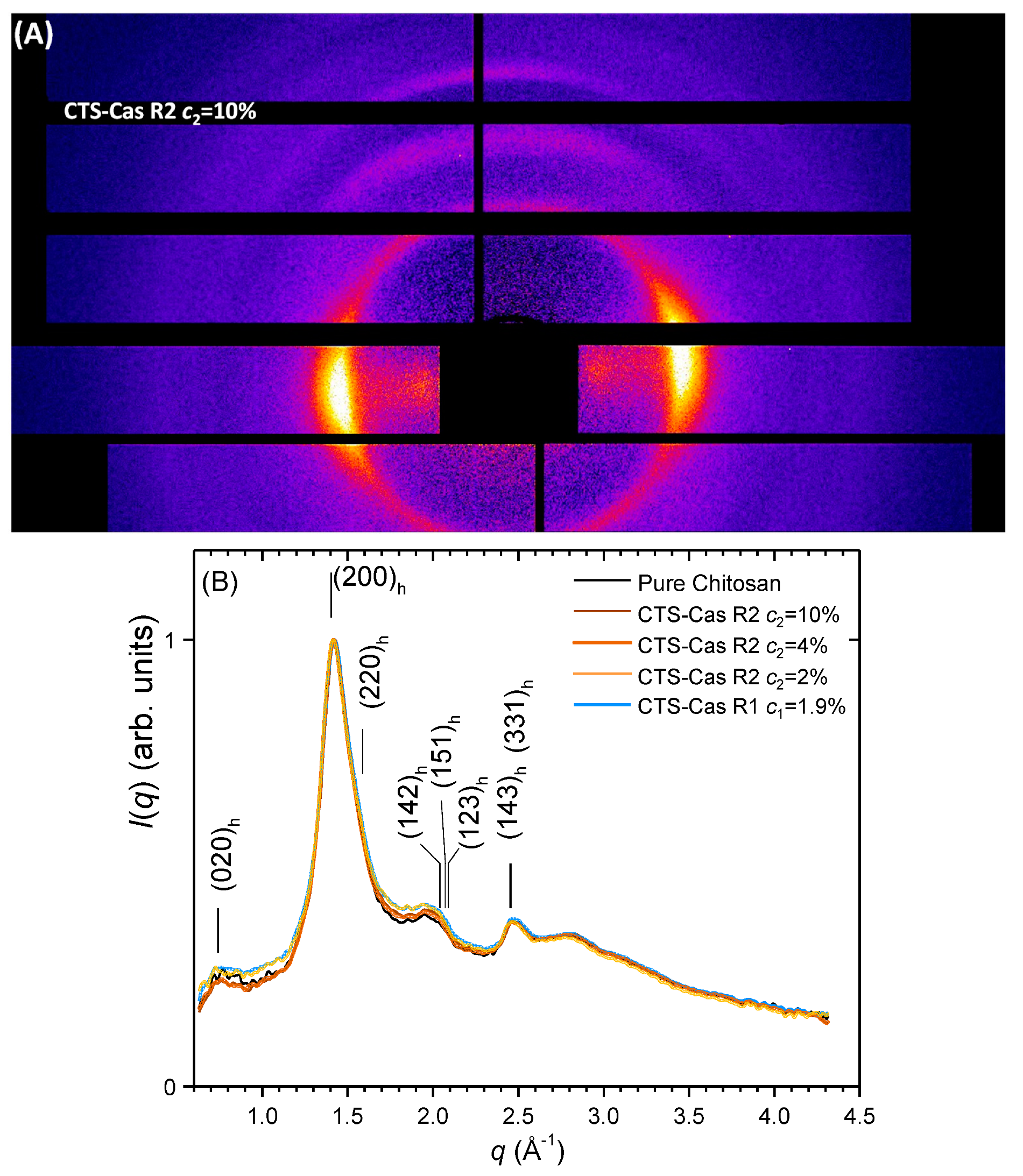
2.4. Crystalline Structural Analysis of Fibers using X-ray Diffraction
2.5. Mechanical Properties of Chitosan–Caseinate Fibers
3. Discussion
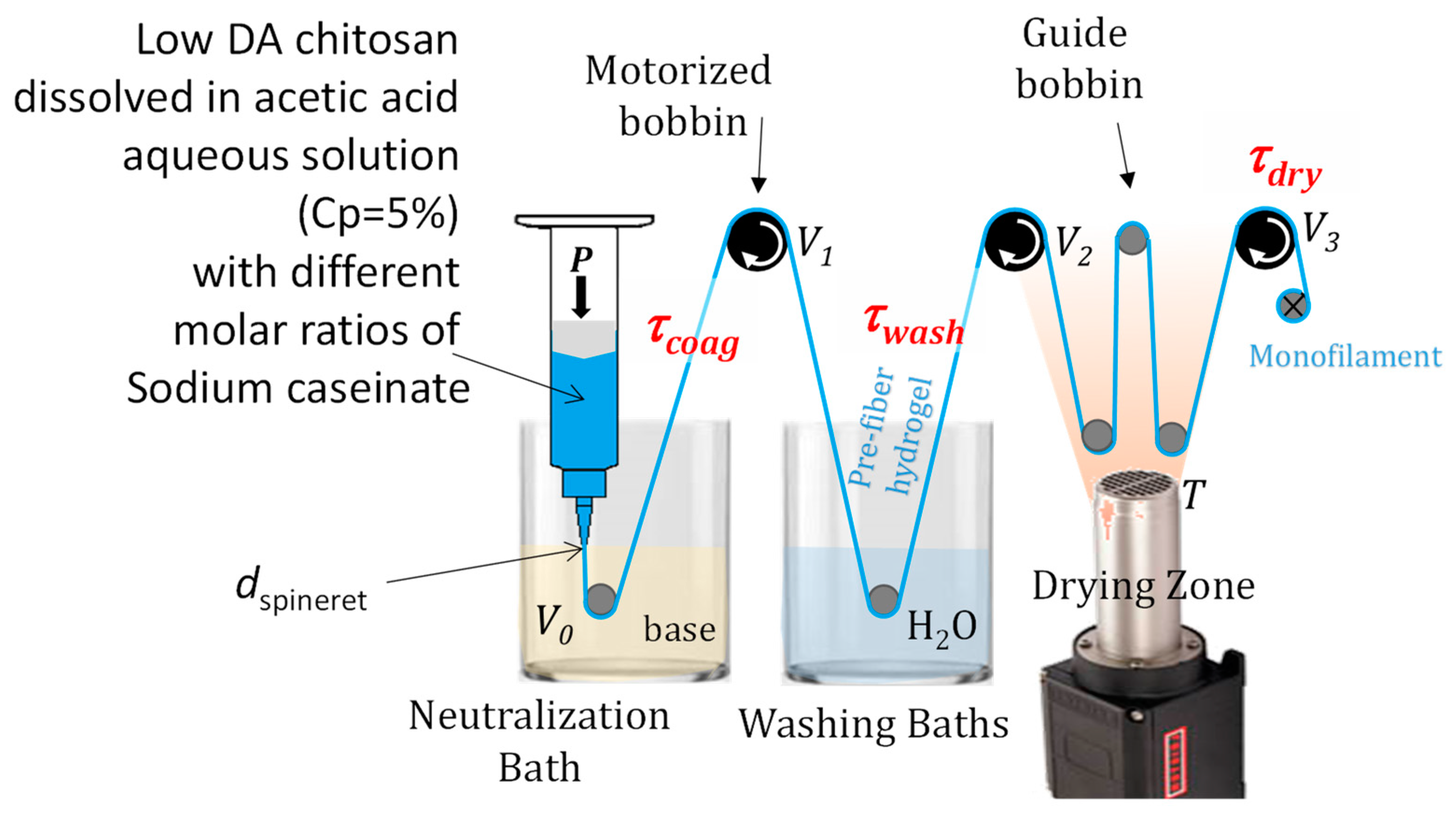
4. Materials and Methods
4.1. Materials
4.2. Preparations of Collodions and Coagulation Baths for Wet Spinning
4.3. Rheological Analysis on Collodions
4.4. Wet Spinning Process
4.5. X-ray Diffraction
4.6. Infrared Spectroscopy (ATR) on Films and Fibers
4.7. Uniaxial Tensile Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Temesgen, S.; Rennert, M.; Tesfaye, T.; Nase, M. Review on Spinning of Biopolymer Fibers from Starch. Polymers 2021, 13, 1121. [Google Scholar] [CrossRef]
- Miranda, C.S.; Ribeiro, A.R.M.; Homem, N.C.; Felgueiras, H.P. Spun Biotextiles in Tissue Engineering and Biomolecules Delivery Systems. Antibiotics 2020, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Potaś, J.; Szymańska, E.; Winnicka, K. Challenges in developing of chitosan—Based polyelectrolyte complexes as a platform for mucosal and skin drug delivery. Eur. Polym. J. 2020, 140, 110020. [Google Scholar] [CrossRef]
- Oliveira, P.N.; Montembault, A.; Sudre, G.; Alcouffe, P.; Marcon, L.; Gehan, H.; Lux, F.; Albespy, K.; Centis, V.; Campos, D.; et al. Self-crosslinked fibrous collagen/chitosan blends: Processing, properties evaluation and monitoring of degradation by bi-fluorescence imaging. Int. J. Biol. Macromol. 2019, 131, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Ucar, S.; Yilgor, P.; Hasirci, V.; Hasirci, N. Chitosan-based wet-spun scaffolds for bioactive agent delivery. J. Appl. Polym. Sci. 2013, 130, 3759–3769. [Google Scholar] [CrossRef]
- Herrera-Vázquez, S.E.; Dublán-García, O.; Arizmendi-Cotero, D.; Gómez-Oliván, L.M.; Islas-Flores, H.; Hernández-Navarro, M.D.; Ramírez-Durán, N. Optimization of the Physical, Optical and Mechanical Properties of Composite Edible Films of Gelatin, Whey Protein and Chitosan. Molecules 2022, 27, 869. [Google Scholar] [CrossRef]
- Becerra, J.; Sudre, G.; Royaud, I.; Montserret, R.; Verrier, B.; Rochas, C.; Delair, T.; David, L. Tuning the Hydrophilic/Hydrophobic Balance to Control the Structure of Chitosan Films and Their Protein Release Behavior. AAPS PharmSciTech 2017, 18, 1070–1083. [Google Scholar] [CrossRef]
- Bekale, L.; Agudelo, D.; Tajmir-Riahi, H. Effect of polymer molecular weight on chitosan–protein interaction. Colloids Surf. B Biointerfaces 2015, 125, 309–317. [Google Scholar] [CrossRef]
- Barbosa, F.C.; Silva, M.C.; Silva, H.N.; Albuquerque, D.; Gomes, A.A.; Silva, S.M.; Fook, M.V. Progress in the Development of Chitosan Based Insulin Delivery Systems: A Systematic Literature Review. Polymers 2020, 12, 2499. [Google Scholar] [CrossRef]
- Semenova, M.G.; Zelikina, D.V.; Antipova, A.S.; Martirosova, E.I.; Palmina, N.P.; Chebotarev, S.A.; Samuseva, Y.V.; Bogdanova, N.G.; Kasparov, V.V. Impact of the character of the associative interactions between chitosan and whey protein isolate on the structure, thermodynamic parameters, and functionality of their complexes with essential lipids. Food Hydrocoll. 2020, 105, 105803. [Google Scholar] [CrossRef]
- Bayomi, M.A.; Al-Suwayeh, S.A.; El-Helw, A.M.; Mesnad, A.F. Preparation of casein–chitosan microspheres containing diltiazem hydrochloride by an aqueous coacervation technique. Pharm. Acta Helv. 1998, 73, 187–192. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Y.; Ju, X.; Udenigwe, C.C.; He, R. Polyelectrolyte Complex Nanoparticles from Chitosan and Acylated Rapeseed Cruciferin Protein for Curcumin Delivery. J. Agric. Food Chem. 2018, 66, 2685–2693. [Google Scholar] [CrossRef]
- Xu, W.; Tang, Y.; Yang, Y.; Wang, G.; Zhou, S. Establishment of a stable complex formed from whey protein isolate and chitosan and its stability under environmental stresses. Int. J. Biol. Macromol. 2020, 165, 2823–2833. [Google Scholar] [CrossRef]
- Kurukji, D.; Norton, I.; Spyropoulos, F. Fabrication of sub-micron protein-chitosan electrostatic complexes for encapsulation and pH-Modulated delivery of model hydrophilic active compounds. Food Hydrocoll. 2016, 53, 249–260. [Google Scholar] [CrossRef]
- Doench, I.; Torres-Ramos, M.E.W.; Montembault, A.; Nunes de Oliveira, P.; Halimi, C.; Viguier, E.; Heux, L.; Siadous, R.; Thiré, R.M.S.M.; Osorio-Madrazo, A. Injectable and Gellable Chitosan Formulations Filled with Cellulose Nanofibers for Intervertebral Disc Tissue Engineering. Polymers 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Kamdem Tamo, A.; Doench, I.; Walter, L.; Montembault, A.; Sudre, G.; David, L.; Morales-Helguera, A.; Selig, M.; Rolauffs, B.; Bernstein, A.; et al. Development of Bioinspired Functional Chitosan/Cellulose Nanofiber 3D Hydrogel Constructs by 3D Printing for Application in the Engineering of Mechanically Demanding Tissues. Polymers 2021, 13, 1663. [Google Scholar] [CrossRef]
- Marquez-Bravo, S.; Doench, I.; Molina, P.; Bentley, F.E.; Tamo, A.K.; Passieux, R.; Lossada, F.; David, L.; Osorio-Madrazo, A. Functional Bionanocomposite Fibers of Chitosan Filled with Cellulose Nanofibers Obtained by Gel Spinning. Polymers 2021, 13, 1563. [Google Scholar] [CrossRef] [PubMed]
- Tamo, A.K.; Tran, T.A.; Doench, I.; Jahangir, S.; Lall, A.; David, L.; Peniche-Covas, C.; Walther, A.; Osorio-Madrazo, A. 3D Printing of Cellulase-Laden Cellulose Nanofiber/Chitosan Hydrogel Composites: Towards Tissue Engineering Functional Biomaterials with Enzyme-Mediated Biodegradation. Materials 2022, 15, 6039. [Google Scholar] [CrossRef]
- Doench, I.; Ahn Tran, T.; David, L.; Montembault, A.; Viguier, E.; Gorzelanny, C.; Sudre, G.; Cachon, T.; Louback-Mohamed, M.; Horbelt, N.; et al. Cellulose Nanofiber-Reinforced Chitosan Hydrogel Composites for Intervertebral Disc Tissue Repair. Biomimetics 2019, 4, 19. [Google Scholar] [CrossRef]
- Desorme, M.; Montembault, A.; Lucas, J.-M.; Rochas, C.; Bouet, T.; David, L. Spinning of hydroalcoholic chitosan solutions. Carbohydr. Polym. 2013, 98, 50–63. [Google Scholar] [CrossRef]
- Notin, L.; Viton, C.; David, L.; Alcouffe, P.; Rochas, C.; Domard, A. Morphology and mechanical properties of chitosan fibers obtained by gel-spinning: Influence of the dry-jet-stretching step and ageing. Acta Biomater. 2006, 2, 387–402. [Google Scholar] [CrossRef]
- Desorme, M.; Montembault, A.; Tamet, T.; Maleysson, P.; Bouet, T.; David, L. Spinning of hydroalcoholic chitosan solutions: Mechanical behavior and multiscale microstructure of resulting fibers. J. Appl. Polym. Sci. 2019, 136, 47130. [Google Scholar] [CrossRef]
- Passieux, R.; Sudre, G.; Montembault, A.; Renard, M.; Hagege, A.; Alcouffe, P.; Haddane, A.; Vandesteene, M.; Boucard, N.; Bordenave, L.; et al. Cytocompatibility/Antibacterial Activity Trade-off for Knittable Wet-Spun Chitosan Monofilaments Functionalized by the In Situ Incorporation of Cu2+ and Zn2+. ACS Biomater. Sci. Eng. 2022, 8, 1735–1748. [Google Scholar] [CrossRef]
- Amine, S.; Montembault, A.; Fumagalli, M.; Osorio-Madrazo, A.; David, L. Controlled Polyelectrolyte Association of Chitosan and Carboxylated Nano-Fibrillated Cellulose by Desalting. Polymers 2021, 13, 2023. [Google Scholar] [CrossRef]
- Yim, E.K.; Wan, A.C.; Le Visage, C.; Liao, I.-C.; Leong, K.W. Proliferation and differentiation of human mesenchymal stem cell encapsulated in polyelectrolyte complexation fibrous scaffold. Biomaterials 2006, 27, 6111–6122. [Google Scholar] [CrossRef]
- Peniche, H.; Osorio, A.; Acosta, N.; de la Campa, A.; Peniche, C. Preparation and characterization of superparamagnetic chitosan microspheres: Application as a support for the immobilization of tyrosinase. J. Appl. Polym. Sci. 2005, 98, 651–657. [Google Scholar] [CrossRef]
- Chen, J.-P.; Chen, S.-H.; Lai, G.-J. Preparation and characterization of biomimetic silk fibroin/chitosan composite nanofibers by electrospinning for osteoblasts culture. Nanoscale Res. Lett. 2012, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Yu, Z.J.; Zuo, B.Q. Structure and Antibacterial Activity of Silk Fibroin/chitosan Nanofibrous Mats Using an Electrospinning Technique. Adv. Mater. Res. 2011, 332–334, 967–972. [Google Scholar] [CrossRef]
- Won Ho, P.; Lim, J.; Dong Il, Y.; Sam, H. Effect of chitosan on morphology and conformation of electrospun silk fibroin nanofibers. Polymer 2004, 45, 7151–7157. [Google Scholar] [CrossRef]
- Xiao, J.; Li, L.; You, H.; Zhou, S.; Feng, Y.; You, R. Silk nanofibrils/chitosan composite fibers with enhanced mechanical properties. Polym. Eng. Sci. 2023, 63, 379–386. [Google Scholar] [CrossRef]
- Siridamrong, P.; Phrotjanatharee, P.; Thamronganaskul, N. Chemical Crosslinking of Silk Fibroin, Chitosan and Gelatin Blend Nanofiber Mats. Key Eng. Mater. 2016, 695, 273–277. [Google Scholar] [CrossRef]
- Zou, F.; Li, R.; Jiang, J.; Mo, X.; Gu, G.; Guo, Z.; Chen, Z. Mechanical enhancement and in vitro biocompatibility of nanofibrous collagen-chitosan scaffolds for tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 2255–2270. [Google Scholar] [CrossRef]
- Liu, J.; Cui, T.; Xu, X.; Du, Y.; Wang, L.; Chen, S.; Pang, J. Robust Alcohol Soluble Polyurethane/Chitosan/Silk Sericin (APU/CS/SS) Nanofiber Scaffolds Toward Artificial Skin Extracellular Matrices via Microfluidic Blow-Spinning. Adv. Fiber Mater. 2023, 5, 349–361. [Google Scholar] [CrossRef]
- Chen, J.; Guo, J.; Zhao, M.; Zhang, R.; Guan, F. Hydrogen bonding in chitosan/Antarctic krill protein composite system: Study on construction and enhancement mechanism. Int. J. Biol. Macromol. 2020, 142, 513–520. [Google Scholar] [CrossRef]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef]
- Peniche Agüero, H.; David, L.; Peniche Covas, C.; Osorio-Madrazo, A. Bioinspired chitosan-BSA fibres for applications in intervertebral disc annulus fibrosus tissue engineering. Rev. Cuba. Investig. Bioméd. 2017, 36, 1–11. [Google Scholar]
- Elzoghby, A.O.; El-Fotoh, W.S.A.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Control. Release 2011, 153, 206–216. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Holt, C. Casein Micelle Structure, Functions and Interactions. In Advanced Dairy Chemistry—1 Proteins; Springer: Boston, MA, USA, 2003; pp. 233–276. [Google Scholar] [CrossRef]
- Corredig, M.; Sharafbafi, N.; Kristo, E. Polysaccharide–protein interactions in dairy matrices, control and design of structures. Food Hydrocoll. 2011, 25, 1833–1841. [Google Scholar] [CrossRef]
- Kethireddipalli, P.; Hill, A.R.; Dalgleish, D.G. Interaction between casein micelles and whey pro-tein/κ-casein complexes during renneting of heat-treated reconstituted skim milk powder and casein micelle/serum mixtures. J. Agric. Food Chem. 2011, 59, 1442–1448. [Google Scholar] [CrossRef]
- Huppertz, T.; Vaia, B.; Smiddy, M.A. Reformation of casein particles from alkaline-disrupted casein micelles. J. Dairy Res. 2008, 75, 44–47. [Google Scholar] [CrossRef]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Q.; Liu, T.; Long, Z.; Kong, J.; Zhao, M. Sodium caseinate/xanthan gum interactions in aqueous solution: Effect on protein adsorption at the oil–water interface. Food Hydrocoll. 2012, 27, 339–346. [Google Scholar] [CrossRef]
- Elmer, C.; Karaca, A.C.; Low, N.H.; Nickerson, M.T. Complex coacervation in pea protein isolate–chitosan mixtures. Food Res. Int. 2011, 44, 1441–1446. [Google Scholar] [CrossRef]
- Kayitmazer, A.B.; Shaw, D.; Dubin, P.L. Role of Polyelectrolyte Persistence Length in the Binding of Oppositely Charged Micelles, Dendrimers, and Protein to Chitosan and Poly(dimethyldiallyammonium chloride). Macromolecules 2005, 38, 5198–5204. [Google Scholar] [CrossRef]
- Kayitmazer, A.B.; Strand, S.P.; Tribet, C.; Jaeger, W.; Dubin, P.L. Effect of polyelectrolyte structure on protein-polyelectrolyte coacervates: Coacervates of bovine serum albumin with poly(diallyldimethylammonium chloride) versus chitosan. Biomacromolecules 2007, 8, 3568–3577. [Google Scholar] [CrossRef]
- Koo, S.Y.; Mok, I.K.; Pan, C.H.; Kim, S.M. Preparation of Fucoxanthin-Loaded Nanoparticles Composed of Casein and Chitosan with Improved Fucoxanthin Bioavailability. J. Agric. Food Chem. 2016, 64, 9428–9435. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, S.; Fleming, E.; Lee, J.Y.; Luo, Y. Chitosan-caseinate-dextran ternary complex nanoparticles for potential oral delivery of astaxanthin with significantly improved bioactivity. Int. J. Biol. Macromol. 2020, 151, 747–756. [Google Scholar] [CrossRef]
- Razmi, M.; Divsalar, A.; Saboury, A.A.; Izadi, Z.; Haertlé, T.; Mansuri-Torshizi, H. Beta-casein and its complexes with chitosan as nanovehicles for delivery of a platinum anticancer drug. Colloids Surf. B Biointerfaces 2013, 112, 362–367. [Google Scholar] [CrossRef]
- Razi, M.A.; Wakabayashi, R.; Goto, M.; Kamiya, N. Formation and Characterization of Caseinate–Chitosan Nanocomplexes for Encapsulation of Curcumin. J. Chem. Eng. Jpn. 2018, 51, 445–453. [Google Scholar] [CrossRef]
- Razi, M.A.; Wakabayashi, R.; Tahara, Y.; Goto, M.; Kamiya, N. Genipin-stabilized caseinate-chitosan nanoparticles for enhanced stability and anti-cancer activity of curcumin. Colloids Surf. B Biointerfaces 2018, 164, 308–315. [Google Scholar] [CrossRef]
- Zhou, N.; Pan, F.; Ai, X.; Tuersuntuoheti, T.; Zhao, L.; Zhao, L.; Wang, Y. Preparation, characterization and antioxidant activity of sinapic acid grafted chitosan and its application with casein as a nanoscale delivery system for black rice anthocyanins. Int. J. Biol. Macromol. 2022, 210, 33–43. [Google Scholar] [CrossRef]
- Zhang, X.; Lyu, X.; Tong, Y.; Wang, J.; Ye, J.; Yang, R. Chitosan/casein based microparticles with a bilayer shell–core structure for oral delivery of nattokinase. Food Funct. 2020, 11, 10799–10816. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Hu, Q.; Sun, J.; Pang, X.; Li, X.; Lu, Y. Effect of oxidized dextran on the stability of gallic acid-modified chitosan-sodium caseinate nanoparticles. Int. J. Biol. Macromol. 2021, 192, 360–368. [Google Scholar] [CrossRef]
- Ding, L.; Huang, Y.; Cai, X.; Wang, S. Impact of pH, ionic strength and chitosan charge density on chitosan/casein complexation and phase behavior. Carbohydr. Polym. 2018, 208, 133–141. [Google Scholar] [CrossRef]
- Lall, A.; Kamdem Tamo, A.; Doench, I.; David, L.; Nunes de Oliveira, P.; Gorzelanny, C.; Osorio-Madrazo, A. Nanoparticles and Colloidal Hydrogels of Chitosan–Caseinate Polyelectrolyte Complexes for Drug-Controlled Release Applications. Int. J. Mol. Sci. 2020, 21, 5602. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Z.; Cao, Z.; Zhuang, L.; Xu, Y.; Liu, X.; Xu, Y.; Gong, Y. Enhancing proliferation and osteogenic differentiation of HMSCs on casein/chitosan multilayer films. Colloids Surf. B Biointerfaces 2016, 141, 397–407. [Google Scholar] [CrossRef]
- Szyk-Warszyńska, L.; Raszka, K.; Warszyński, P. Interactions of Casein and Polypeptides in Mul-tilayer Films Studied by FTIR and Molecular Dynamics. Polymers 2019, 11, 920. [Google Scholar] [CrossRef]
- Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Characterization of Chitosan/Caseinate Films. J. Appl. Polym. Sci. 2008, 107, 1080–1090. [Google Scholar] [CrossRef]
- Farrer, D.; Lips, A. On the self-assembly of sodium caseinate. Int. Dairy J. 1999, 9, 281–286. [Google Scholar] [CrossRef]
- Panouillé, M.; Durand, D.; Nicolai, T.; Larquet, E.; Boisset, N. Aggregation and gelation of micellar casein particles. J. Colloid Interface Sci. 2005, 287, 85–93. [Google Scholar] [CrossRef]
- Sadiq, U.; Gill, H.; Chandrapala, J. Casein Micelles as an Emerging Delivery System for Bioactive Food Components. Foods 2021, 10, 1965. [Google Scholar] [CrossRef]
- Zahariev, N.; Marudova, M.; Milenkova, S.; Uzunova, Y.; Pilicheva, B. Casein Micelles as Nanocarriers for Benzydamine Delivery. Polymers 2021, 13, 4357. [Google Scholar] [CrossRef]
- Sorlier, P.; Denuzière, A.; Viton, C.; Domard, A. Relation between the Degree of Acetylation and the Electrostatic Properties of Chitin and Chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef]
- Sorlier, P.; Viton, C.; Domard, A. Relation between Solution Properties and Degree of Acetylation of Chitosan: Role of Aging. Biomacromolecules 2002, 3, 1336–1342. [Google Scholar] [CrossRef]
- Okuyama, K.; Noguchi, K.; Miyazawa, T.; Yui, T.; Ogawa, K. Molecular and Crystal Structure of Hydrated Chitosan. Macromolecules 1997, 30, 5849–5855. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Peniche-Covas, C.; Domard, A. Kinetics Study of the Solid-State Acid Hydrolysis of Chitosan: Evolution of the Crystallinity and Macromolecular Structure. Biomacromolecules 2010, 11, 1376–1386. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Peniche-Covas, C.; Domard, A. Highly crystalline chitosan produced by multi-steps acid hydrolysis in the solid-state. Carbohydr. Polym. 2011, 83, 1730–1739. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Peniche-Covas, C.; Rochas, C.; Putaux, J.-L.; Trombotto, S.; Alcouffe, P.; Domard, A. Fine microstructure of processed chitosan nanofibril networks preserving directional packing and high molecular weight. Carbohydr. Polym. 2015, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.B.; Quinn, G.D. A practical and systematic review of Weibull statistics for reporting strengths of dental materials. Dent. Mater. 2010, 26, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Bentley, F.E.; Passieux, R.; David, L.; Osorio-Madrazo, A. Pure Chitosan Biomedical Textile Fibers from Mixtures of Low- and High-Molecular Weight Bidisperse Polymer Solutions: Processing and Understanding of Microstructure–Mechanical Properties’ Relationship. Int. J. Mol. Sci. 2022, 23, 4767. [Google Scholar] [CrossRef] [PubMed]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- ISO 579:1995 (en); Textile Fibres—Determination of Breaking Force and Elongation at Break of Individual Fibres. ISO International Organization for Standardization: Geneva, Switzerland, 1995.


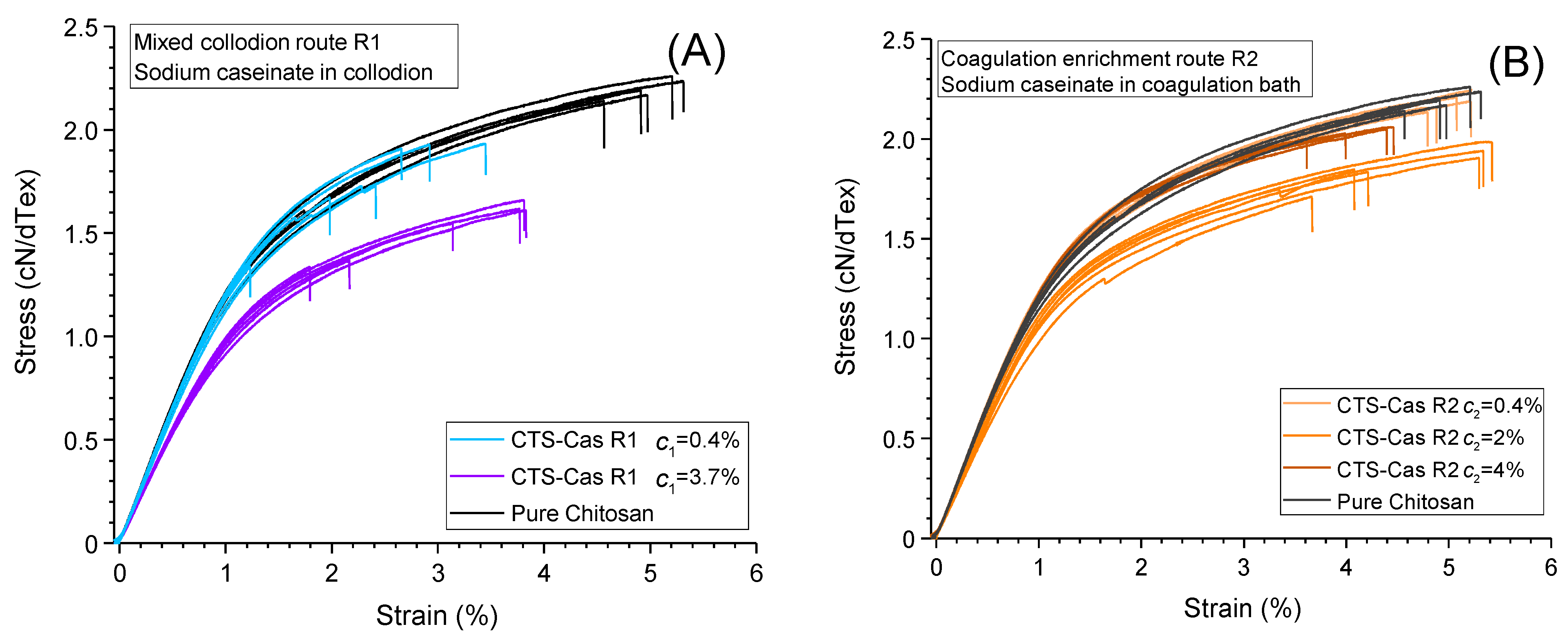
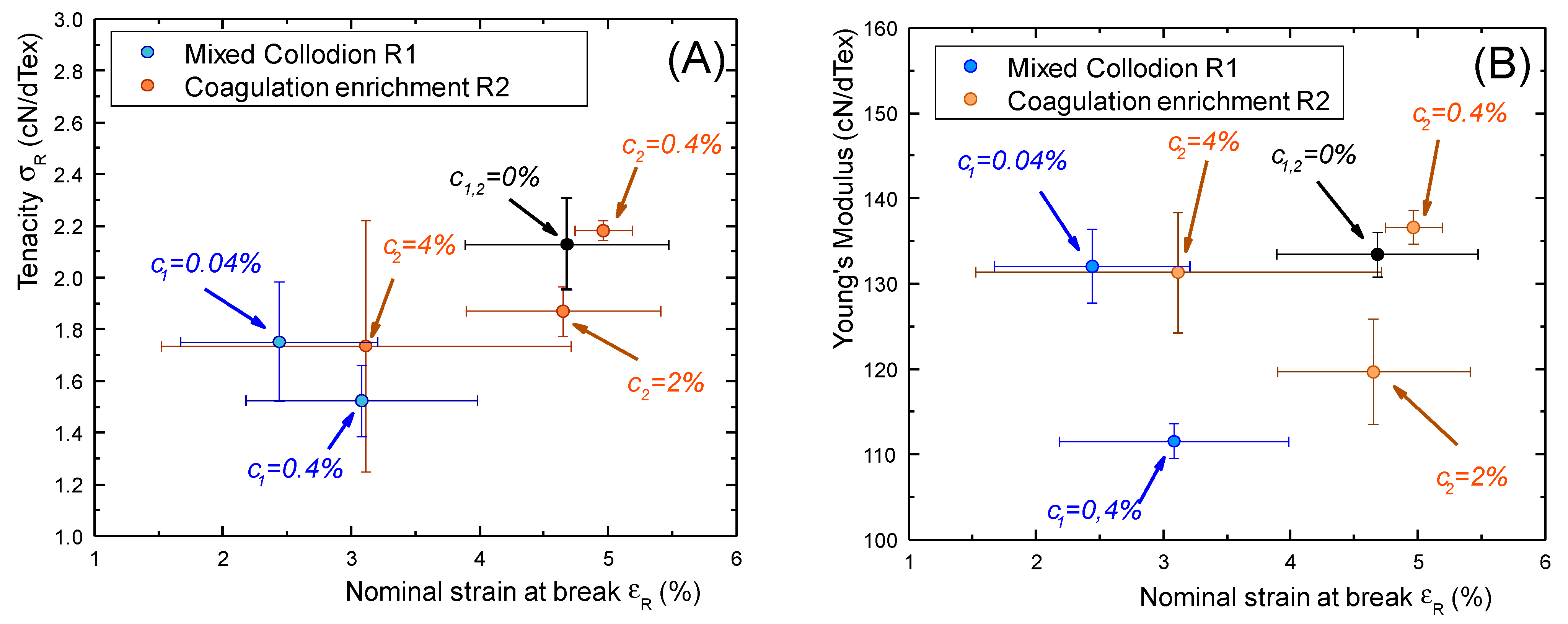
| Sample | Tenacity (cN/dtex) ± std | Young’s Modulus (cN/dtex) ± std | Strain-at-Break (%) ± std | Weibull’s Modulus m±5 | Titer (Tex) ±2 Tex | Diameter (μm) from Titer and Density (1.4 g/cm3) |
|---|---|---|---|---|---|---|
| c1 = 0 (Pure Chitosan) | 2.13 ± 0.18 | 133 ± 3 | 4.6 ± 0.79 | 35 | 50 | 70 |
| Mixed collodion route R1 (Caseinate incorporated in the chitosan dope) | ||||||
| c1 = 0.1% | 1.76 ± 0.23 | 132 ± 4 | 2.4 ± 0.77 | 7 | 58 | 81.2 |
| c1 = 1% | 1.52 ± 0.14 | 112 ± 2 | 3.1 ± 0.90 | 11 | 54 | 75.6 |
| Coagulation enrichment route R2 (Caseinate incorporated in the neutralization bath) | ||||||
| c2 = 0.4% | 2.18 ± 0.04 | 137 ± 2 | 5.0 ± 0.22 | 60 | 50 | 70.0 |
| c2 = 2% | 1.87 ± 0.1 | 120 ± 6 | 4.7 ± 0.76 | 20 | 63 | 88.2 |
| c2 = 4% | 1.74 ± 0.49 | 131 ± 7 | 3.1 ± 1.59 | 45 | 48 | 67.2 |
| Mixed Collodion Route R1 (Caseinate Incorporated in the Chitosan Dope) | Coagulation Enrichment Route R2 (Caseinate Incorporated in the Coagulation Bath) | ||||
|---|---|---|---|---|---|
| Sample | c1 (% w/w) (Caseinate Mass Fraction in Dope) | Total Draw τtot | Sample | c2 (% w/w) (Caseinate Mass Fraction in Coagulation Bath) | Total Draw τtot |
| CTS-R1 Cas 0.04% | 0.04% | 1.67 | CTS-R2 Cas 0.4% | 0. 4% | 1.67 |
| CTS-R1 Cas 0.2% | 0.2% | 1.67 | CTS-R2 Cas 2% | 2% | 1.67 |
| CTS-R1 Cas 0.4% | 0.4% | 1.66 | CTS-R2 Cas 3% | 3% | |
| CTS-R1 Cas 1.9% | 1.9% | 1.62 | CTS-R2 Cas 4% | 4% | 1.67 |
| CTS-R1 Cas 3.7% | 3.7% | 1.67 | CTS-R2 Cas 10% | 10% | |
| CTS-R1 Cas 7.2% | 7.2% | ||||
| Pure CTS | 0 | 1.67 | Pure CTS | 0 | 1.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peniche, H.; Razonado, I.A.; Alcouffe, P.; Sudre, G.; Peniche, C.; Osorio-Madrazo, A.; David, L. Wet-Spun Chitosan–Sodium Caseinate Fibers for Biomedicine: From Spinning Process to Physical Properties. Int. J. Mol. Sci. 2024, 25, 1768. https://doi.org/10.3390/ijms25031768
Peniche H, Razonado IA, Alcouffe P, Sudre G, Peniche C, Osorio-Madrazo A, David L. Wet-Spun Chitosan–Sodium Caseinate Fibers for Biomedicine: From Spinning Process to Physical Properties. International Journal of Molecular Sciences. 2024; 25(3):1768. https://doi.org/10.3390/ijms25031768
Chicago/Turabian StylePeniche, Hazel, Ivy Ann Razonado, Pierre Alcouffe, Guillaume Sudre, Carlos Peniche, Anayancy Osorio-Madrazo, and Laurent David. 2024. "Wet-Spun Chitosan–Sodium Caseinate Fibers for Biomedicine: From Spinning Process to Physical Properties" International Journal of Molecular Sciences 25, no. 3: 1768. https://doi.org/10.3390/ijms25031768






