Comparison of Tepotinib, Paclitaxel, or Ramucirumab Efficacy According to the Copy Number or Phosphorylation Status of the MET Gene: Doublet Treatment versus Single Agent Treatment
Abstract
:1. Introduction
2. Results
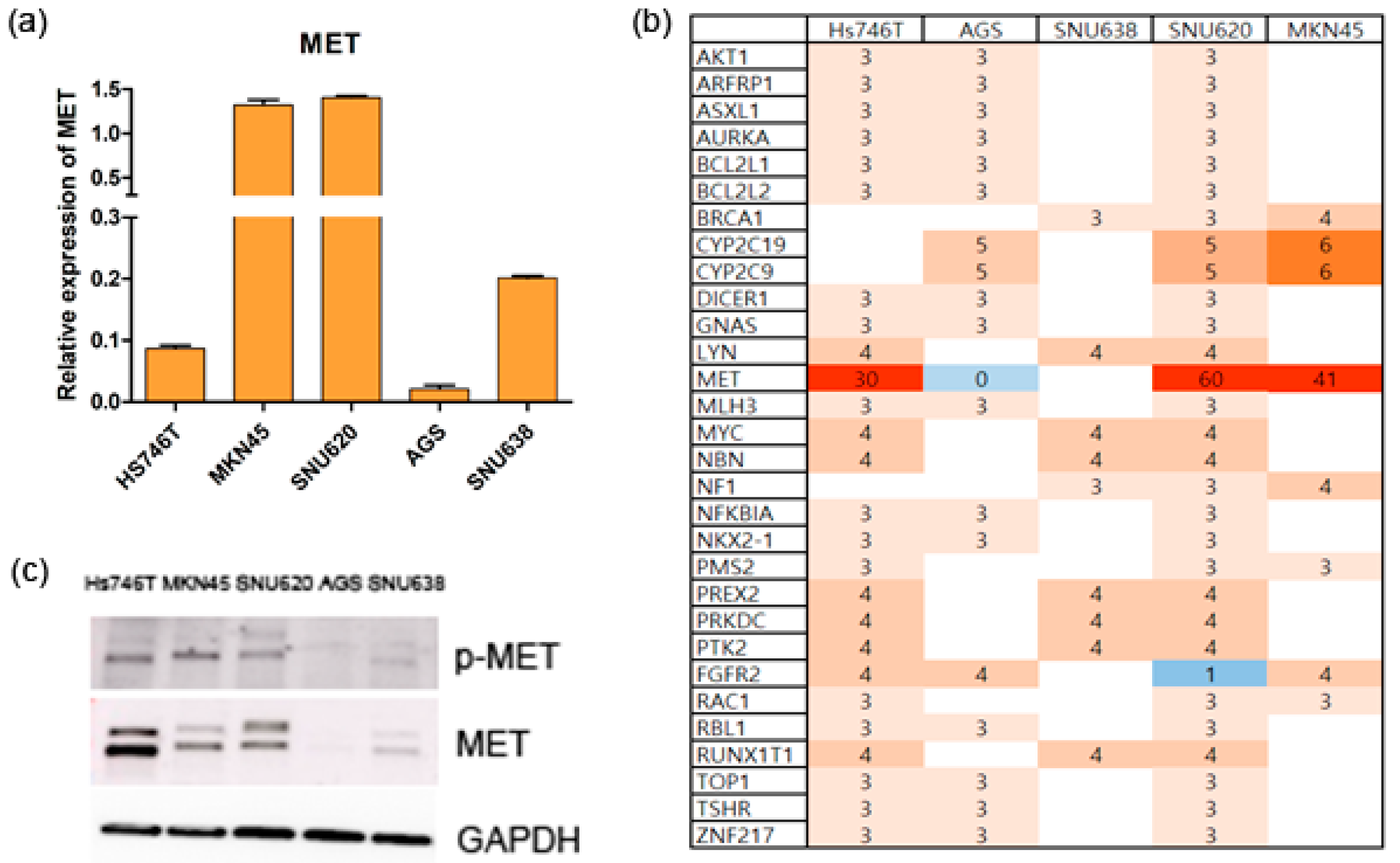
2.1. The Cell Lines
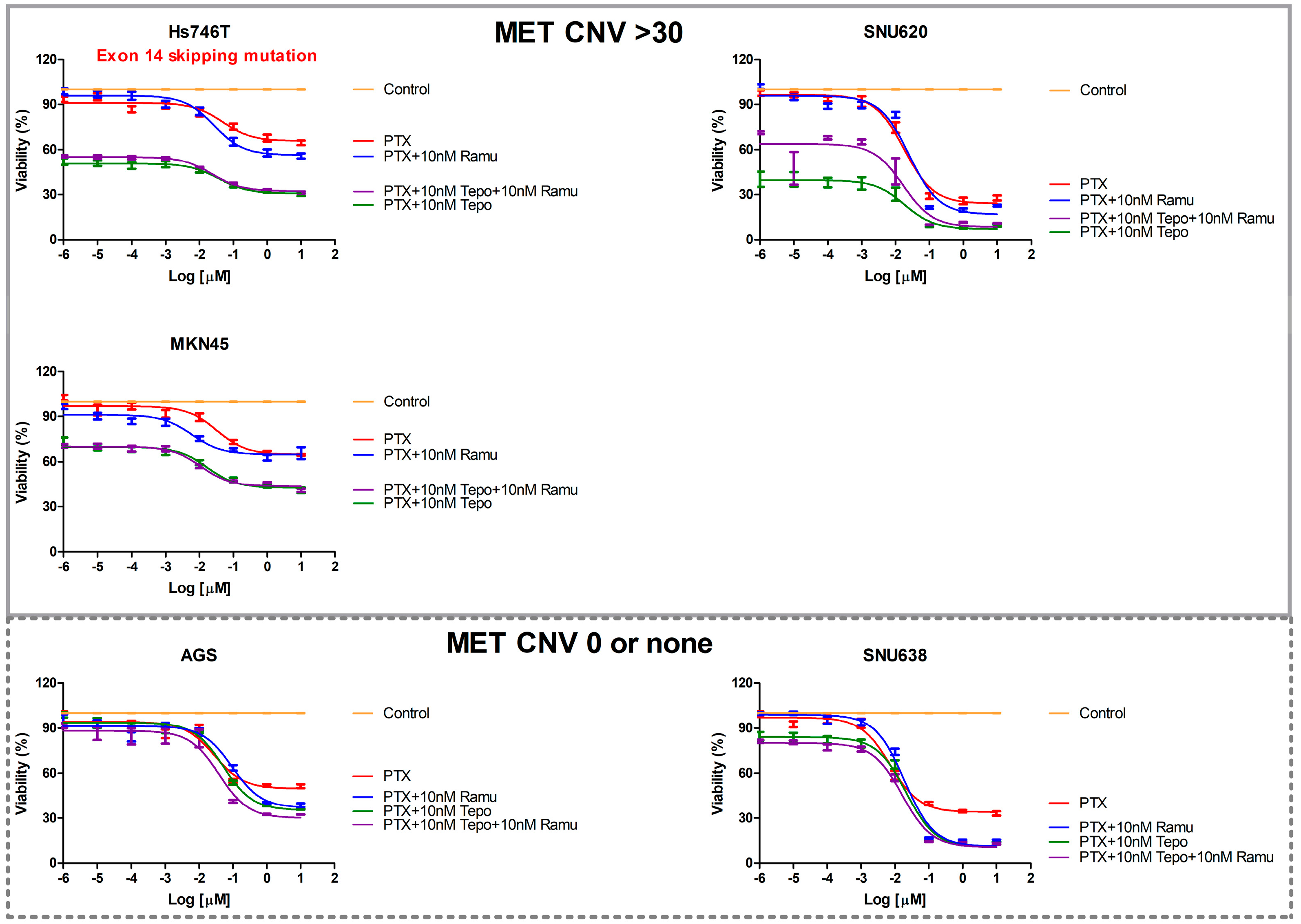
2.2. The Effects of Ramucirumab, Paclitaxel, and Tepotinib on the Viability of Cells According to MET Expression
2.3. Effects of Ramucirumab, Paclitaxel, and Tepotinib on the Death of GC Cells According to MET Expression
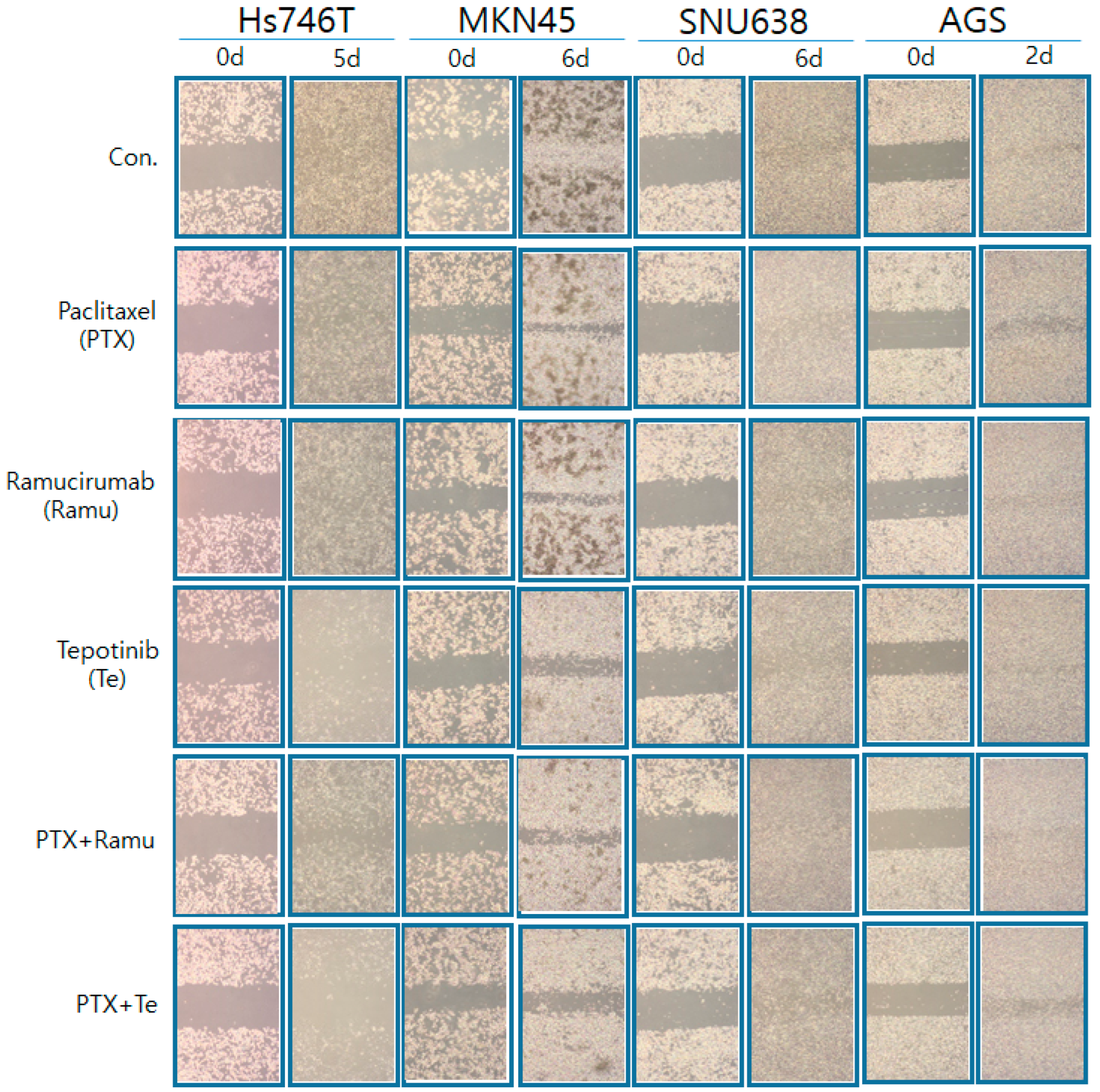
2.4. The Effects of Ramucirumab, Paclitaxel, and Tepotinib on the Migration of GC Lines According to MET Expression
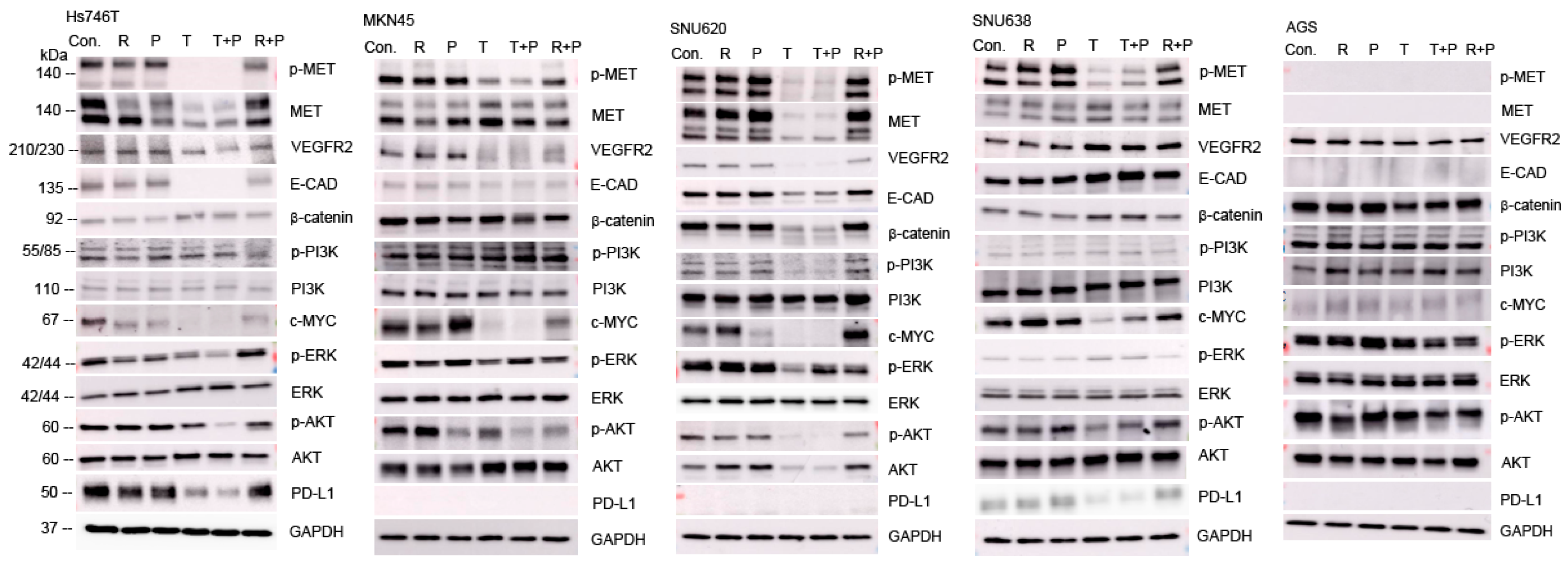
2.5. Effects of Ramucirumab, Paclitaxel, and Tepotinib on Protein Levels in GC Cell Lines According to MET Expression
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines and Cell Culture
4.3. Target Sequencing and Analysis
4.4. Real-Time RT-PCR Analysis
4.5. MTS Cell Proliferation Assay
4.6. Flow Cytometry
4.7. Migration Assay
4.8. Western Blotting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Muro, K.; Van Cutsem, E.; Narita, Y.; Pentheroudakis, G.; Baba, E.; Li, J.; Ryu, M.H.; Zamaniah, W.I.W.; Yong, W.P.; Yeh, K.H.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: A JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann. Oncol. 2019, 30, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 113–123. [Google Scholar] [CrossRef]
- Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean Practice Guideline for Gastric Cancer 2018: An Evidence-based, Multi-disciplinary Approach. J. Gastric Cancer 2019, 19, 372. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; Dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Barzi, A.; Lin, F.; Song, J.; Lam, C.; Nie, X.; Noman, A.; Kwong, W.J. Real-World Treatment Patterns and Economic Burden Following First-Line Trastuzumab in Patients with Metastatic Gastric Cancer in the USA. Drugs-Real. World Outcomes 2023, 10, 395–404. [Google Scholar] [CrossRef]
- Young, K.; Smyth, E.; Chau, I. Ramucirumab for advanced gastric cancer or gastro-oesophageal junction adenocarcinoma. Ther. Adv. Gastroenterol. 2015, 8, 373–383. [Google Scholar] [CrossRef]
- Birchmeier, C.; Birchmeier, W.; Gherardi, E.; Vande Woude, G.F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003, 4, 915–925. [Google Scholar] [CrossRef]
- Liu, X.; Yao, W.; Newton, R.C.; Scherle, P.A. Targeting the c-MET signaling pathway for cancer therapy. Expert. Opin. Investig. Drugs 2008, 17, 997–1011. [Google Scholar] [CrossRef]
- Sierra, J.R.; Tsao, M.S. c-MET as a potential therapeutic target and biomarker in cancer. Ther. Adv. Med. Oncol. 2011, 3, S21–S35. [Google Scholar] [CrossRef]
- Ariyawutyakorn, W.; Saichaemchan, S.; Varella-Garcia, M. Understanding and Targeting MET Signaling in Solid Tumors—Are We There Yet? J. Cancer 2016, 7, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Kammula, U.S.; Kuntz, E.J.; Francone, T.D.; Zeng, Z.; Shia, J.; Landmann, R.G.; Paty, P.B.; Weiser, M.R. Molecular co-expression of the c-Met oncogene and hepatocyte growth factor in primary colon cancer predicts tumor stage and clinical outcome. Cancer Lett. 2007, 248, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, J.; Ogawa, M.; Yamashita, S.; Nomura, K.; Kuramoto, M.; Saishoji, T.; Shin, S. Immunoreactive hepatocyte growth factor is a strong and independent predictor of recurrence and survival in human breast cancer. Cancer Res. 1994, 54, 1630–1633. [Google Scholar] [PubMed]
- Wu, C.W.; Li, A.F.; Chi, C.W.; Chung, W.W.; Liu, T.Y.; Lui, W.Y.; P’Eng, F.K. Hepatocyte growth factor and Met/HGF receptors in patients with gastric adenocarcinoma. Oncol. Rep. 1998, 5, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research, N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Bladt, F.; Faden, B.; Friese-Hamim, M.; Knuehl, C.; Wilm, C.; Fittschen, C.; Gradler, U.; Meyring, M.; Dorsch, D.; Jaehrling, F.; et al. EMD 1214063 and EMD 1204831 constitute a new class of potent and highly selective c-Met inhibitors. Clin. Cancer Res. 2013, 19, 2941–2951. [Google Scholar] [CrossRef] [PubMed]
- Falchook, G.S.; Kurzrock, R.; Amin, H.M.; Xiong, W.; Fu, S.; Piha-Paul, S.A.; Janku, F.; Eskandari, G.; Catenacci, D.V.; Klevesath, M.; et al. First-in-Man Phase I Trial of the Selective MET Inhibitor Tepotinib in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2020, 26, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.H.; Sul, H.J.; Kim, B.; Kim, B.J.; Kim, H.S.; Zang, D.Y. Tepotinib Inhibits the Epithelial-Mesenchymal Transition and Tumor Growth of Gastric Cancers by Increasing GSK3beta, E-Cadherin, and Mucin 5AC and 6 Levels. Int. J. Mol. Sci. 2020, 21, 6027. [Google Scholar] [CrossRef]
- Sohn, S.H.; Sul, H.J.; Kim, B.J.; Zang, D.Y. Responses to the Tepotinib in Gastric Cancers with MET Amplification or MET Exon 14 Skipping Mutations and High Expression of Both PD-L1 and CD44. Cancers 2022, 14, 3444. [Google Scholar] [CrossRef]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Sunakawa, Y.; Lenz, H.J. Molecular classification of gastric adenocarcinoma: Translating new insights from the cancer genome atlas research network. Curr. Treat. Options Oncol. 2015, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Waddell, T.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. 6), vi57–vi63. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Q.; Yang, G.; Marando, C.; Koblish, H.K.; Hall, L.M.; Fridman, J.S.; Behshad, E.; Wynn, R.; Li, Y.; et al. A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin. Cancer Res. 2011, 17, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Malka, D.; Francois, E.; Penault-Llorca, F.; Castan, F.; Bouche, O.; Bennouna, J.; Ghiringhelli, F.; de la Fouchardiere, C.; Borg, C.; Samalin, E.; et al. FOLFOX alone or combined with rilotumumab or panitumumab as first-line treatment for patients with advanced gastroesophageal adenocarcinoma (PRODIGE 17-ACCORD 20-MEGA): A randomised, open-label, three-arm phase II trial. Eur. J. Cancer 2019, 115, 97–106. [Google Scholar] [CrossRef]
- Shah, M.A.; Bang, Y.J.; Lordick, F.; Alsina, M.; Chen, M.; Hack, S.P.; Bruey, J.M.; Smith, D.; McCaffery, I.; Shames, D.S.; et al. Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: The METGastric Randomized Clinical Trial. JAMA Oncol. 2017, 3, 620–627. [Google Scholar] [CrossRef]
- Sakai, D.; Chung, H.C.; Oh, D.Y.; Park, S.H.; Kadowaki, S.; Kim, Y.H.; Tsuji, A.; Komatsu, Y.; Kang, Y.K.; Uenaka, K.; et al. A non-randomized, open-label, single-arm, Phase 2 study of emibetuzumab in Asian patients with MET diagnostic positive, advanced gastric cancer. Cancer Chemother. Pharmacol. 2017, 80, 1197–1207. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Karaszewska, B.; Kang, Y.K.; Chung, H.C.; Shankaran, V.; Siena, S.; Go, N.F.; Yang, H.; Schupp, M.; Cunningham, D. A Multicenter Phase II Study of AMG 337 in Patients with MET-Amplified Gastric/Gastroesophageal Junction/Esophageal Adenocarcinoma and Other MET-Amplified Solid Tumors. Clin. Cancer Res. 2019, 25, 2414–2423. [Google Scholar] [CrossRef]
- Aparicio, T.; Cozic, N.; de la Fouchardiere, C.; Meriaux, E.; Plaza, J.; Mineur, L.; Guimbaud, R.; Samalin, E.; Mary, F.; Lecomte, T.; et al. The Activity of Crizotinib in Chemo-Refractory MET-Amplified Esophageal and Gastric Adenocarcinomas: Results from the AcSe-Crizotinib Program. Target. Oncol. 2021, 16, 381–388. [Google Scholar] [CrossRef]
- Shah, M.A.; Wainberg, Z.A.; Catenacci, D.V.; Hochster, H.S.; Ford, J.; Kunz, P.; Lee, F.C.; Kallender, H.; Cecchi, F.; Rabe, D.C.; et al. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS ONE 2013, 8, e54014. [Google Scholar] [CrossRef]
- Kang, Y.K.; Muro, K.; Ryu, M.H.; Yasui, H.; Nishina, T.; Ryoo, B.Y.; Kamiya, Y.; Akinaga, S.; Boku, N. A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Investig. New Drugs 2014, 32, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Patel, M.; Kurkjian, C.; Hemphill, B.; Flores, M.; Thompson, D.; Bendell, J. A Phase II Study of the c-Met Inhibitor Tivantinib in Combination with FOLFOX for the Treatment of Patients with Previously Untreated Metastatic Adenocarcinoma of the Distal Esophagus, Gastroesophageal Junction, or Stomach. Cancer Investig. 2017, 35, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Smyth, E.C.; Chau, I. Ramucirumab: Successfully targeting angiogenesis in gastric cancer. Clin. Cancer Res. 2014, 20, 5875–5881. [Google Scholar] [CrossRef]

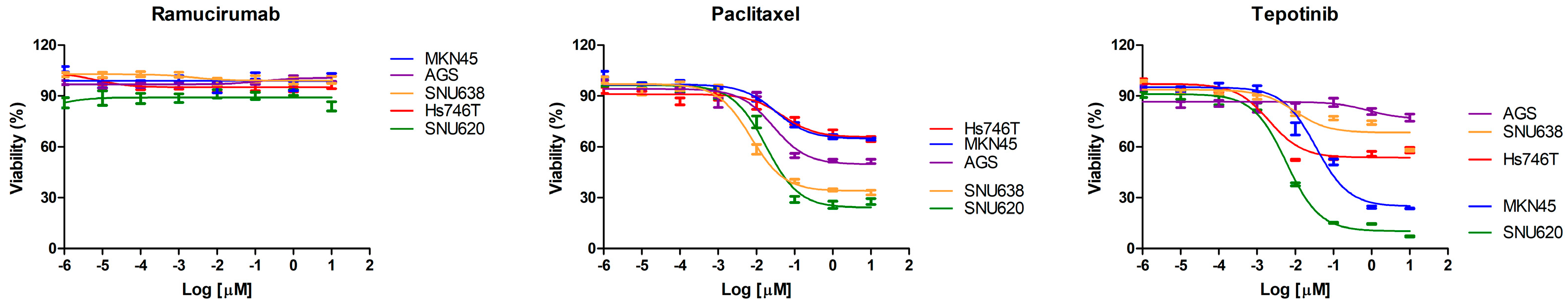

| Drug | IC50 (nM) | ||||
|---|---|---|---|---|---|
| Hs746T | SNU620 | AGS | SNU638 | MKN45 | |
| Ramucirumab | - | - | - | - | - |
| Paclitaxel | - | 18.02 | 28.16 | 7.272 | - |
| Tepotinib | 2.083 | 5.898 | - | - | 34.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohn, S.-H.; Sul, H.J.; Kim, B.J.; Zang, D.Y. Comparison of Tepotinib, Paclitaxel, or Ramucirumab Efficacy According to the Copy Number or Phosphorylation Status of the MET Gene: Doublet Treatment versus Single Agent Treatment. Int. J. Mol. Sci. 2024, 25, 1769. https://doi.org/10.3390/ijms25031769
Sohn S-H, Sul HJ, Kim BJ, Zang DY. Comparison of Tepotinib, Paclitaxel, or Ramucirumab Efficacy According to the Copy Number or Phosphorylation Status of the MET Gene: Doublet Treatment versus Single Agent Treatment. International Journal of Molecular Sciences. 2024; 25(3):1769. https://doi.org/10.3390/ijms25031769
Chicago/Turabian StyleSohn, Sung-Hwa, Hee Jung Sul, Bum Jun Kim, and Dae Young Zang. 2024. "Comparison of Tepotinib, Paclitaxel, or Ramucirumab Efficacy According to the Copy Number or Phosphorylation Status of the MET Gene: Doublet Treatment versus Single Agent Treatment" International Journal of Molecular Sciences 25, no. 3: 1769. https://doi.org/10.3390/ijms25031769




