Persistence of Chronic Lymphocytic Leukemia Stem-like Populations under Simultaneous In Vitro Treatment with Curcumin, Fludarabine, and Ibrutinib: Implications for Therapy Resistance
Abstract
:1. Introduction
2. Results
2.1. Limiting Dilution of the I83 Tetraploid Cell Line Reveals a Diploid Subpopulation That Can Spontaneously Regenerate the Tetraploid Main Population
2.2. Diploid and Tetraploid I83 Presented Similar Susceptibility to Curcumin and Fludarabine
2.3. ALP Activity Defines CLL Subpopulations with Higher Treatment Resistance
2.4. Curcumin, Ibrutinib, and Fludarabine Show Efficacy to Reduce Population of CLL Cells with Highest ALP Activity
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Patient Sample Analysis and Primary Cultures
4.3. Immunophenotyping
4.4. Karyotyping
4.5. Ploidy Analysis
4.6. Comparative Cell Membrane Integrity Analysis on I83
4.7. Alkaline Phosphatase Activity Analysis
4.8. Data Analysis
- CDI < 1 indicates synergism.
- CDI = 1 indicates additive effect.
- CDI > 1 indicates antagonism.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jordan, C.T.; Guzman, M.L.; Noble, M. Cancer stem cells. N. Engl. J. Med. 2006, 355, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Trumpp, A.; Haas, S. Cancer stem cells: The adventurous journey from hematopoietic to leukemic stem cells. Cell 2022, 185, 1266–1270. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Vanner, R.; Dirks, P.; Eaves, C.J. Cancer stem cells: An evolving concept. Nat. Rev. Cancer 2012, 12, 133–143. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-L.; Hu, E.Y.; Chang, L.; Labanowska, J.; Zapolnik, K.; Mo, X.; Shi, J.; Doong, T.-J.; Lozanski, A.; Yan, P.S.; et al. Leukemia-initiating HSCs in chronic lymphocytic leukemia reveal clonal leukemogenesis and differential drug sensitivity. Cell Rep. 2022, 40, 111115. [Google Scholar] [CrossRef]
- Kikushige, Y.; Ishikawa, F.; Miyamoto, T.; Shima, T.; Urata, S.; Yoshimoto, G.; Mori, Y.; Iino, T.; Yamauchi, T.; Eto, T.; et al. Self-renewing hematopoietic stem cell is the primary target in pathogenesis of human chronic lymphocytic leukemia. Cancer Cell 2011, 20, 246–259. [Google Scholar] [CrossRef]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise Review: Evidence for CD34 as a Common Marker for Diverse Progenitors. Stem Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Blau, H.; Brazelton, T.; Weimann, J. The evolving concept of a stem cell: Entity or function? Cell 2001, 105, 829–841. [Google Scholar] [CrossRef]
- Goodell, M.A.; Rosenzweig, M.; Kim, H.; Marks, D.F.; DeMaria, M.; Paradis, G.; Grupp, S.A.; Sieff, C.A.; Mulligan, R.C.; Johnson, R.P. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat. Med. 1997, 3, 1337–1345. [Google Scholar] [CrossRef]
- Nunez-Espinosa, C.; Garcia-Godoy, M.D.; Ferreira, I.; Rios-Kristjansson, J.G.; Rizo-Roca, D.; Rico, L.G.; Rubi-Sans, G.; Palacio, C.; Torrella, J.R.; Pages, T.; et al. Vybrant DyeCycle Violet Stain Discriminates Two Different Subsets of CD34+ Cells. Curr. Stem Cell Res. Ther. 2016, 11, 66–71. [Google Scholar] [CrossRef]
- Rico, L.G.; Juncà, J.; Ward, M.D.; Bradford, J.; Petriz, J. Is alkaline phosphatase the smoking gun for highly refractory primitive leukemic cells? Oncotarget 2016, 7, 72057–72066. [Google Scholar] [CrossRef]
- Rico, L.G.; Juncà, J.; Ward, M.D.; Bradford, J.A.; Petriz, J. Flow cytometric significance of cellular alkaline phosphatase activity in acute myeloid leukemia. Oncotarget 2019, 10, 6969–6980. [Google Scholar] [CrossRef]
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Niemann, C.; Kater, A.; Gregor, M.; Cymbalista, F.; Buske, C.; Hillmen, P.; et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 23–33. [Google Scholar] [CrossRef]
- Hus, I.; Roliński, J. Current concepts in diagnosis and treatment of chronic lymphocytic leukemia. Contemp. Oncol. 2015, 19, 361–367. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Kay, N.E. Maintenance therapy for B-chronic lymphocytic leukemia. Clin. Adv. Hematol. Oncol. 2011, 9, 22–31. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21326143 (accessed on 10 July 2023). [PubMed]
- Bellosillo, B.; Dalmau, M.; Colomer, D.; Gil, J. Involvement of CED-3/ICE proteases in the apoptosis of B-chronic lymphocytic leukemia cells. Blood 1997, 89, 3378–3384. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9129045 (accessed on 10 July 2023). [CrossRef] [PubMed]
- RGale, P.; Caligaris-Cappio, F.; Dighiero, G.; Keating, M.; Montserrat, E.; Rai, K. Recent progress in chronic lymphocytic leukemia. International Workshop on chronic Lymphocytic Leukemia. Leukemia 1994, 8, 1610–1614. Available online: http://www.ncbi.nlm.nih.gov/pubmed/7522296 (accessed on 10 July 2023).
- Thompson, P.A.; Wierda, W.G. Eliminating minimal residual disease as a therapeutic end point: Working toward cure for patients with CLL. Blood 2016, 127, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, S.; Ritgen, M.; Fischer, K.; Stilgenbauer, S.; Busch, R.M.; Fingerle-Rowson, G.; Fink, A.M.; Bühler, A.; Zenz, T.; Wenger, M.K.; et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: A multivariate analysis from the randomized GCLLSG CLL8 trial. J. Clin. Oncol. 2012, 30, 980–988. [Google Scholar] [CrossRef]
- Ammon, H.P.T.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Medica 1991, 57, 1–7. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.H.; Kumar, A.P.; Sethi, G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Everett, P.C.; Meyers, J.A.; Makkinje, A.; Rabbi, M.; Lerner, A. Preclinical assessment of curcumin as a potential therapy for B-CLL. Am. J. Hematol. 2007, 82, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.; Mulero, L.; Pardo, C.; Morera, C.; Carrió, M.; Laricchia-Robbio, L.; Esteban, C.R.; Belmonte, J.C.I. Characterization of pluripotent stem cells. Nat. Protoc. 2013, 8, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Quintanilla, R.H.; Grecian, S.; Gee, K.R.; Rao, M.S.; Lakshmipathy, U. Novel Live Alkaline Phosphatase Substrate for Identification of Pluripotent Stem Cells. Stem Cell Rev. Rep. 2012, 8, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffa, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. (Eds.) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; lARC: Lyon, France, 2017. [Google Scholar]
- Myhrinder, A.L.; Hellqvist, E.; Bergh, A.-C.; Jansson, M.; Nilsson, K.; Hultman, P.; Jonasson, J.; Buhl, A.M.; Pedersen, L.B.; Jurlander, J.; et al. Molecular characterization of neoplastic and normal ‘sister’ lymphoblastoid B-cell lines from chronic lymphocytic leukemia. Leuk. Lymphoma 2013, 54, 1769–1779. [Google Scholar] [CrossRef]
- Wendel-Hansen, V.; Sällström, J.; De Campos-Lima, P.O.; Kjellström, G.; Sandlund, A.; Siegbahn, A.; Carlsson, M.; Nilsson, K.; Rosén, A. Epstein-Barr virus (EBV) can immortalize B-cll cells activated by cytokines. Leukemia 1994, 8, 476–484. [Google Scholar]
- Miller, C.R.; Ruppert, A.S.; Heerema, N.A.; Maddocks, K.J.; Labanowska, J.; Breidenbach, H.; Lozanski, G.; Zhao, W.; Gordon, A.L.; Jones, J.A.; et al. Near-tetraploidy is associated with Richter transformation in chronic lymphocytic leukemia patients receiving ibrutinib. Blood Adv. 2017, 1, 1584–1588. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Volak, L.P.; Ghirmai, S.; Cashman, J.R.; Court, M.H. Curcuminoids inhibit multiple human cytochromes P450, UDP-glucuronosyltransferase, and sulfotransferase enzymes, whereas piperine is a relatively selective CYP3A4 inhibitor. Drug Metab. Dispos. 2008, 36, 1594–1605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tan, T.M.C.; Lim, L.Y. Impact of curcumin-induced changes in P-glycoprotein and CYP3A expression on the pharmacokinetics of peroral celiprolol and midazolam in rats. Drug Metab. Dispos. 2007, 35, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Rodrigues, V.; Sousa, E.; Vasconcelos, M.H. Curcumin as a Modulator of P-Glycoprotein in Cancer: Challenges and Perspectives. Pharmaceuticals 2016, 9, 71. [Google Scholar] [CrossRef]
- Romiti, N.; Tongiani, R.; Cervelli, F.; Chieli, E. Effects of curcumin on P-glycoprotein in primary cultures of rat hepatocytes. Life Sci. 1998, 62, 2349–2358. [Google Scholar] [CrossRef]
- Huang, M.-T.; Lysz, T.; Ferrara, T.; Abidi, T.F.; Laskin, J.D.; Conney, A.H. Inhibitory Effects of Curcumin on In Vitro Lipoxygenase and Cyclooxygenase Activities in Mouse Epidermis. 1991. Available online: http://aacrjournals.org/cancerres/article-pdf/51/3/813/2446099/cr0510030813.pdf (accessed on 15 December 2023).
- Dhandapani, K.M.; Mahesh, V.B.; Brann, D.W. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J. Neurochem. 2007, 102, 522–538. [Google Scholar] [CrossRef]
- Shishodia, S.; Amin, H.M.; Lai, R.; Aggarwal, B.B. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem. Pharmacol. 2005, 70, 700–713. [Google Scholar] [CrossRef]
- Chuang, S.-E.; Yeh, P.-Y.; Lu, Y.-S.; Lai, G.-M.; Liao, C.-M.; Gao, M.; Cheng, A.-L. Basal levels and patterns of anticancer drug-induced activation of nuclear factor-κB (NF-κB), and its attenuation by tamoxifen, dexamethasone, and curcumin in carcinoma cells. Biochem. Pharmacol. 2002, 63, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Lin, S.J.; Lin, J.K. Inhibitory effects of curcumin on protein kinase C activity induced by 12-O-tetradecanoyl-phorbol-13-acetate in NIH 3T3 cells. Carcinogenesis 1993, 14, 857–861. [Google Scholar] [CrossRef]
- Huang, T.S.; Lee, S.C.; Lin, J.K. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc. Natl. Acad. Sci. USA 1991, 88, 5292–5296. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Zhu, D.-J.; Chen, X.-W.; Chen, Q.-K.; Luo, Z.-T.; Liu, C.-C.; Wang, G.-X.; Zhang, W.-J.; Liao, N.-Z. Curcumin enhances the effects of irinotecan on colorectal cancer cells through the generation of reactive oxygen species and activation of the endoplasmic reticulum stress pathway. Oncotarget 2017, 8, 40264–40275. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Dadhaniya, P.; Patel, C.; Muchhara, J.; Bhadja, N.; Mathuria, N.; Vachhani, K.; Soni, M.G. Safety assessment of a solid lipid curcumin particle preparation: Acute and subchronic toxicity studies. Food Chem. Toxicol. 2011, 49, 1834–1842. [Google Scholar] [CrossRef]
- Ganiger, S.; Malleshappa, H.N.; Krishnappa, H.; Rajashekhar, G.; Rao, V.R.; Sullivan, F. A two generation reproductive toxicity study with curcumin, turmeric yellow, in Wistar rats. Food Chem. Toxicol. 2007, 45, 64–69. [Google Scholar] [CrossRef]
- Land, B.V.; Blijlevens, N.M.A.; Marteijn, J.; Timal, S.; Donnelly, J.P.; de Witte, T.J.M.; M’Rabet, L. Role of curcumin and the inhibition of NF-ΚB in the onset of chemotherapy-induced mucosal barrier injury. Leukemia 2004, 18, 276–284. [Google Scholar] [CrossRef]
- Chen, C.; Tang, Q.; Zhang, Y.; Yu, M.; Jing, W.; Tian, W. Physioxia: A more effective approach for culturing human adipose-derived stem cells for cell transplantation. Stem Cell Res. Ther. 2018, 9, 148. [Google Scholar] [CrossRef]
- Sakulterdkiat, T.; Srisomsap, C.; Udomsangpetch, R.; Svasti, J.; Lirdprapamongkol, K. Curcumin Resistance Induced by Hypoxia in HepG2 Cells Is Mediated by Multidrug-resistance-associated Proteins. Anticancer Res. 2012, 32, 5337–5342. [Google Scholar] [PubMed]
- Rosén, A.; Bergh, A.-C.; Gogok, P.; Evaldsson, C.; Myhrinder, A.L.; Hellqvist, E.; Rasul, A.; Björkholm, M.; Jansson, M.; Mansouri, L.; et al. Lymphoblastoid cell line with B1 cell characteristics established from a chronic lymphocytic leukemia clone by in vitro EBV infection. Oncoimmunology 2012, 1, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, L.G.; Slovak, M.; Campbell, L.J. ISCN 2013: An International System for Human Cytogenetic Nomenclature; S. Karger AG: Basel, Switzerland, 2013. [Google Scholar]
- Rico, L.G.; Salvia, R.; Ward, M.D.; Bradford, J.A.; Petriz, J. Flow-cytometry-based protocols for human blood/marrow immunophenotyping with minimal sample perturbation. STAR Protoc. 2021, 2, 100883. [Google Scholar] [CrossRef] [PubMed]
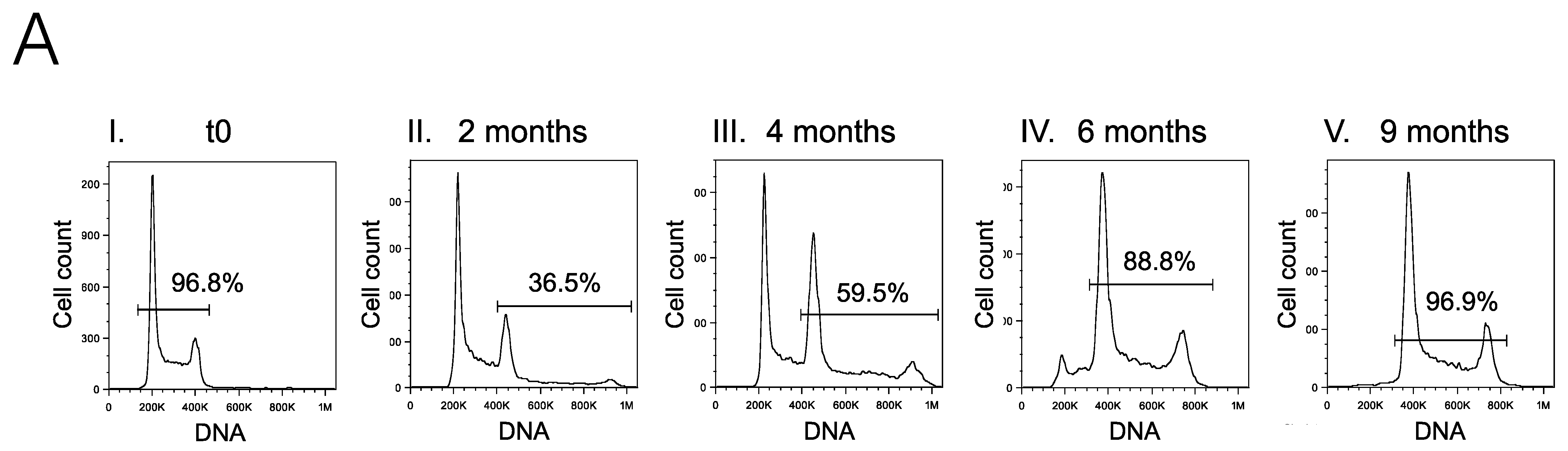
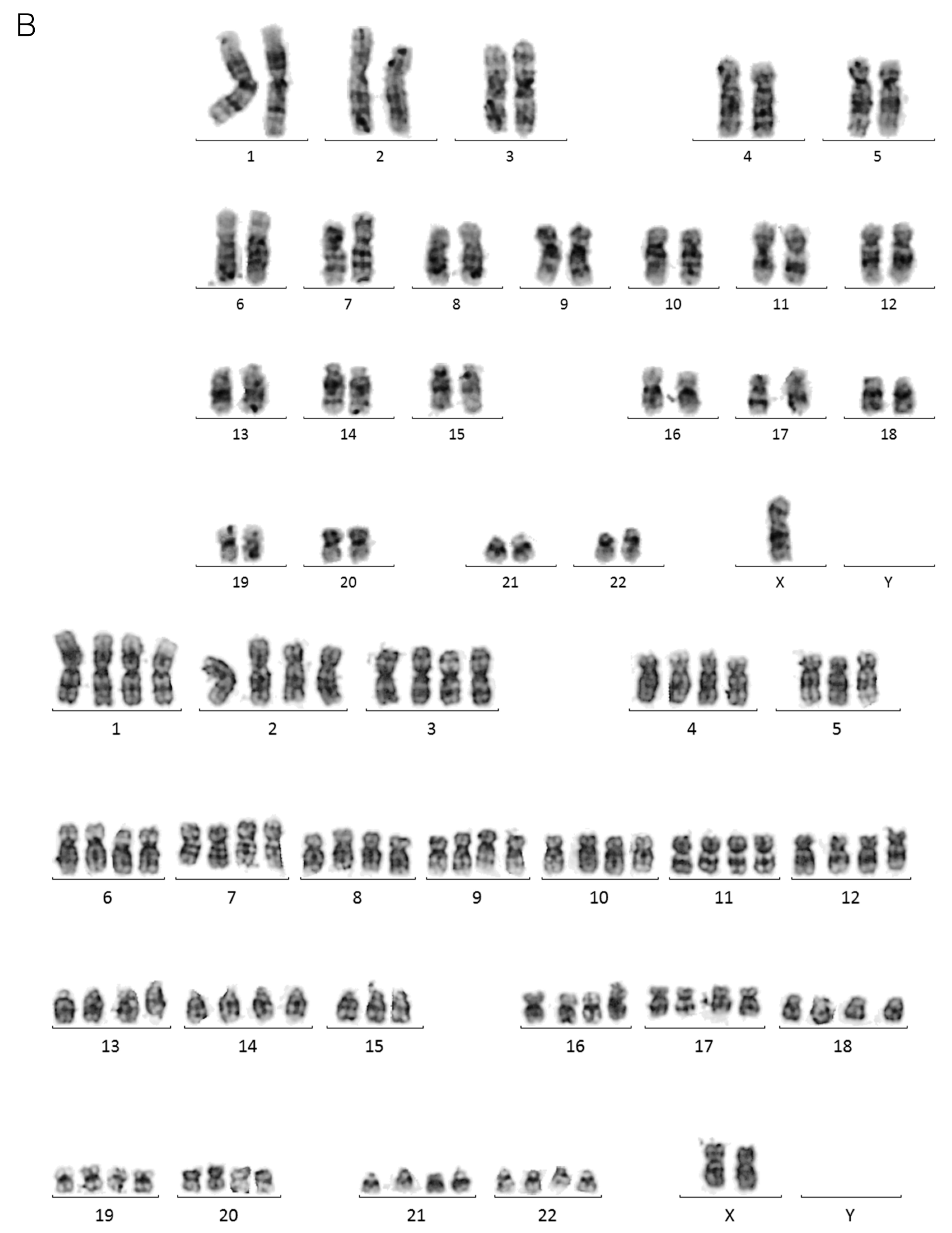
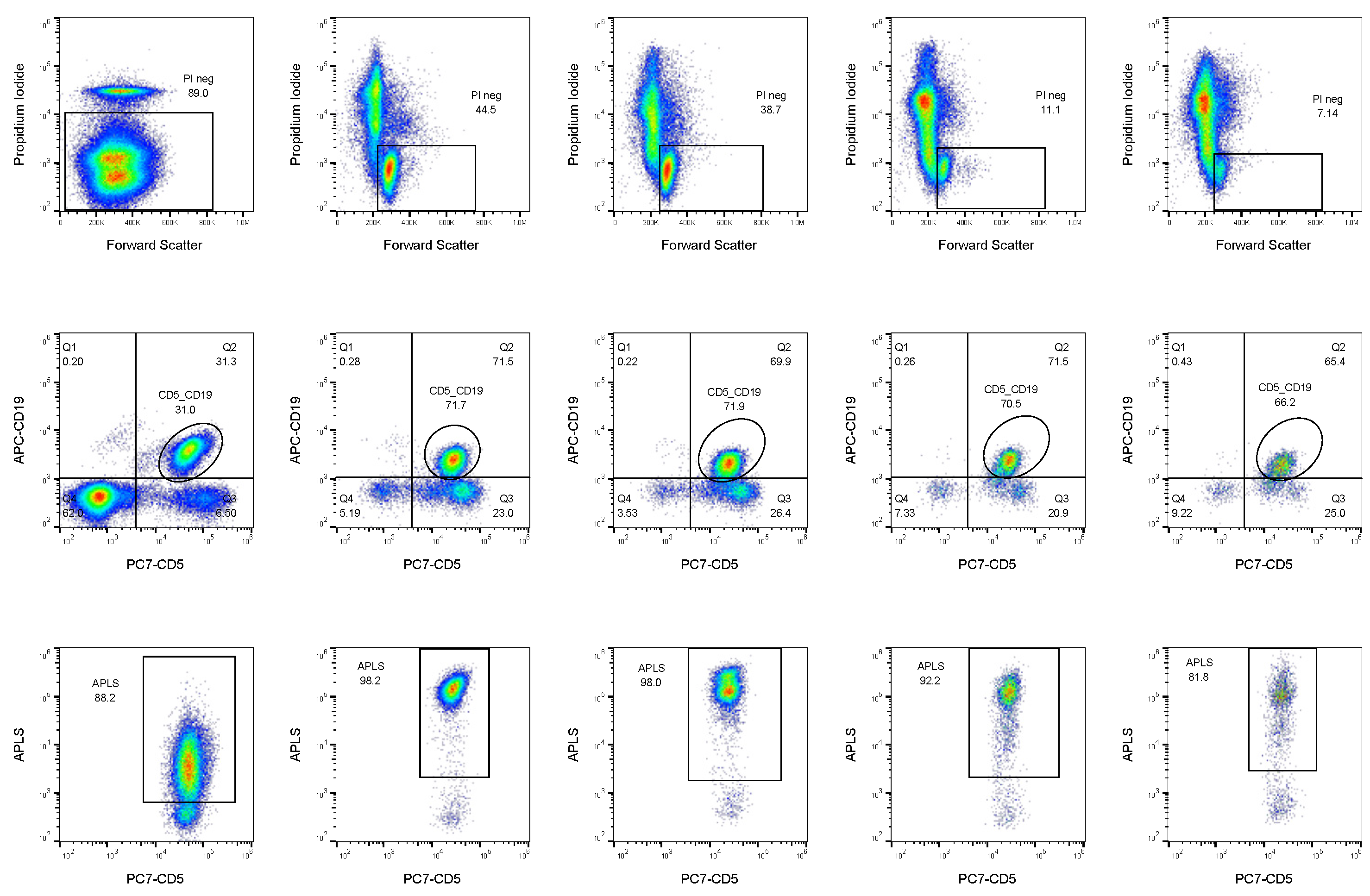
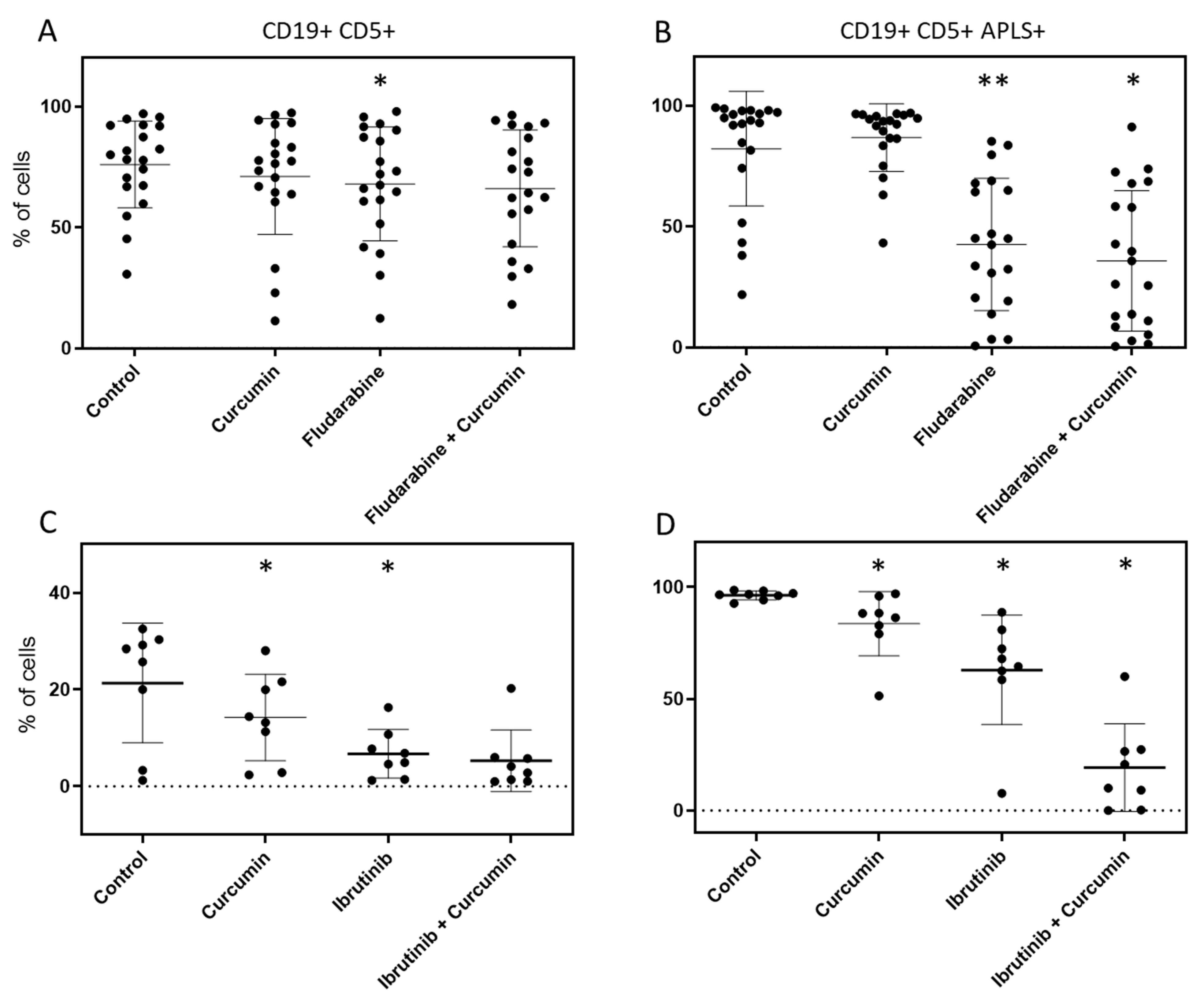
| Timepoint | Leukocyte Count (×106/L) | Age | Sex | % CD19 + CD5+ | % APLS + (over CD19+ CD5+) | Status | Treatment | Prognostic Factors | |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 5.3 | 72 | M | 0.776 | 11.673 | Partial response, MRD 0.03% | Ibrutinib + Ofatumumab | 86% of nuclei with del(13)(q14) (DLEU) and del(11)(q22) (ATM) | |
| P2 | 1.9 | 71 | M | 0.02 | 25 | Complete remission | Rituximab-Bendamustine | Del(13q), mutated IGHV | |
| P3 | 12.5 | 63 | M | 28.042 | 0.804 | Leukocytosis since 2012, CLL features present | R-CHOP (6 cycles) | Cytogenetics:+13; t(14;19). Transforming CLL, no Richter criteria | |
| P4 | 1 | 7.6 | 65 | M | 9.917 | 6.319 | Partial response, MRD 35% | Ibrutinib, alone and combined with Ofatumumab | Del(13)(q14), del(11q) (ATM), ZAP70+. BinetB, Rai1 |
| 2 | 7.8 | 65 | M | 8.647 | 2.453 | Partial response, MRD 33% | |||
| 3 | 7.4 | 65 | M | 14.616 | 6.495 | Partial response, MRD 29% | |||
| 4 | 8.6 | 65 | M | 1.597 | 0.158 | Partial response, MRD 28% | |||
| P5 | 13 | 70 | F | 55.234 | 85.899 | MRD 55%. | Del(11)(q21q25) (ATM), del(13) (q14q21) (DLEU) | ||
| P6 | 1 | 11.8 | 79 | M | 46.745 | 0.343 | Lymphocytosis, 86%; lymphocytes with CLL pheotype, narrowly suprassing 5000/µL; follow-up needed to confirm CLL | None | |
| 2 | 16.7 | 79 | M | 17.287 | 0.052 | Binet-RaiA1. MRD 87%, CLL confirmation | |||
| P7 | 128 | 83 | F | 82.265 | 93.923 | None | |||
| P8 | 13 | 80 | F | 31.113 | 87.753 | Diagnostic | None | ||
| P9 | 26.4 | 80 | F | 73.914 | 70.039 | Progressive lymphocytosis; 84% lymphocytes with CLL phenotype. Binet III, Rai3 (lymphocytosis + anemia), relapse-progression | Tx1: Obinutuzumab + Chlorambucil. Tx2: Ibrutinib (intolerant). Tx3: R-idealisib (intolerant). Pending of Venetoclax | t(12;13)(q14;15), del(13) | |
| P10 | 5.7 | 74 | M | 1.3 | 5.221 | Partial response, 68% of B lymphocytes with CLL phenotype. RaiIV Binet C. Almost complete remission | Ibrutinib | Unmutated IGHV | |
| P11 | 7.1 | 74 | F | 26.492 | 5.236 | Incipient relapse, 48% of CLL lymphocytes in peripheral blood. Brother with CLL | Tx1: R-CHOPx6 and TITx6, radiotherapy. AutoTPH | Del(13q) | |
| P12 | 10 | 66 | M | 44.491 | 1.911 | 69% CD19/CD5 | None | ||
| P13 | 13.6 | 67 | M | 27.716 | 34.293 | CD19/CD5 51%. Recent diagnostic | None, control follow-up | ||
| P14 | 7.9 | 56 | M | 21.358 | 85.838 | Relapse, stage IV, 25% MRD in peripheral blood | Tx1: R-FC (2010), Tx2: R-idealisib (2016), Tx3: ibrutinib (2016), starting venetoclax | ZAP70+, del(13)(q14) (DLEU). Altered karyotype, del TP53 | |
| P15 | 154 | 77 | M | 92.646 | 88.506 | Progression. CD19/CD5 lymphocytes 91% | FISH: del(13)(q14)(DLEU), del(17)(p13)(TP53) in 23% nuclei | ||
| P16 | 13,800 * | 81 | M | 54.798 | 96.777 | Relapse. MRD: 90% in peripheral blood | Del(11)(q22) (ATM gene) | ||
| P17 | 10.9 | 71 | M | 53.99 | 2.516 | MRD: 85%. Incipient relapse | R-FC | Del(13)(q14)(D13S319) | |
| P18 | 89.5 | 79 | M | 94.154 | 0.247 | Progression. CD19/CD5 lymphocytes 95% | Del(13)(q14)(DLEU) | ||
| P19 | 13.2 | 71 | F | 69.002 | 82.096 | Diagnostic, 75% CD19/CD5 BinetA, Rai1 | None | ||
| P20 | 24,600 * | 53 | F | 64.385 | 0.028 | Progression. MRD: 75% in peripheral blood | FISH: 92% of biallelic del(13)(q14) (DLEU) | ||
| P21 | 114 | 88 | M | 86.879 | 0.192 | Trisomy 12. | |||
| P22 | 16.3 | 79 | M | 51.03 | 0.034 | Diagnostic, 75% CD19/CD5 | |||
| P23 | 18.2 | 73 | M | 55.723 | 0.069 | Diagnostic, 74% CD19/CD5 A1 Stage | None | Trisomy 12, del(13)(q14) (DLEU) | |
| P24 | 10.5 | 81 | F | 3.887 | 0 | Diagnosed on 2011. Progression in 2017. Lymphocytes with CLL phenotype: 40% | Tx1: Obinutuzumab + Chlorambucil. Tx2: Ibrutinib (2018) | ZAP70+, trisomy 12. 4.3 beta 2 microglobulin (September 2019) | |
| P25 | 78 | 62 | M | 54.284 | 0.016 | Diagnostic. MRD: 85%in peripheral blood |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bistué-Rovira, À.; Rico, L.G.; Bardina, J.; Juncà, J.; Granada, I.; Bradford, J.A.; Ward, M.D.; Salvia, R.; Solé, F.; Petriz, J. Persistence of Chronic Lymphocytic Leukemia Stem-like Populations under Simultaneous In Vitro Treatment with Curcumin, Fludarabine, and Ibrutinib: Implications for Therapy Resistance. Int. J. Mol. Sci. 2024, 25, 1994. https://doi.org/10.3390/ijms25041994
Bistué-Rovira À, Rico LG, Bardina J, Juncà J, Granada I, Bradford JA, Ward MD, Salvia R, Solé F, Petriz J. Persistence of Chronic Lymphocytic Leukemia Stem-like Populations under Simultaneous In Vitro Treatment with Curcumin, Fludarabine, and Ibrutinib: Implications for Therapy Resistance. International Journal of Molecular Sciences. 2024; 25(4):1994. https://doi.org/10.3390/ijms25041994
Chicago/Turabian StyleBistué-Rovira, Àngel, Laura G. Rico, Jorge Bardina, Jordi Juncà, Isabel Granada, Jolene A. Bradford, Michael D. Ward, Roser Salvia, Francesc Solé, and Jordi Petriz. 2024. "Persistence of Chronic Lymphocytic Leukemia Stem-like Populations under Simultaneous In Vitro Treatment with Curcumin, Fludarabine, and Ibrutinib: Implications for Therapy Resistance" International Journal of Molecular Sciences 25, no. 4: 1994. https://doi.org/10.3390/ijms25041994





