Renal Expression and Localization of the Receptor for (Pro)renin and Its Ligands in Rodent Models of Diabetes, Metabolic Syndrome, and Age-Dependent Focal and Segmental Glomerulosclerosis
Abstract
:1. Introduction
2. Results
2.1. Kidney Specimens and Renal Structural and Functional Features of the Experimental Rodent Models
2.2. Immunostaining for FL-(P)RR
2.3. Immunostaining for (Pro)renin (Total Renin)
2.4. Immunostaining for COX-2
2.5. mRNA Levels of (P)RR and Factors Involved in Its Intracellular Processing
2.6. Impact of Elevated Glucose Concentration on (P)RR Expression and Intracellular Processing in Podocytes
3. Discussion
4. Materials and Methods
4.1. Immunohistochemical and Morphometric Analysis of Renal Sections
4.2. RT-PCR
4.3. Cell Culture and Treatment
4.4. Immunofluorescence Staining for FL-(P)RR in Cultured Human Podocytes
4.5. Enzyme-Linked Immunosorbent Assay (ELISA) for s(P)RR
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Márquez, E.; Riera, M.; Pascual, J.; Soler, M.J. Renin-Angiotensin System within the Diabetic Podocyte. Am. J. Physiol.-Renal. Physiol. 2015, 308, F1–F10. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, N.; Kohan, D.E. The (pro)Renin Receptor: An Emerging Player in Hypertension and Metabolic Syndrome. Kidney Int. 2019, 95, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G. The (pro)Renin Receptor: Pathophysiological Roles in Cardiovascular and Renal Pathology. Curr. Opin. Nephrol. Hypertens. 2007, 16, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Kaneshiro, Y.; Ichihara, A.; Takemitsu, T.; Sakoda, M.; Suzuki, F.; Nakagawa, T.; Hayashi, M.; Inagami, T. Increased Expression of Cyclooxygenase-2 in the Renal Cortex of Human Prorenin Receptor Gene-Transgenic Rats. Kidney Int. 2006, 70, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Luetscher, J.A.; Kraemer, F.B.; Wilson, D.M.; Schwartz, H.C.; Bryer-Ash, M. Increased Plasma Inactive Renin in Diabetes Mellitus. A Marker of Microvascular Complications. N. Engl. J. Med. 1985, 312, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Luetscher, J.A. Plasma Prorenin Activity and Complications in Children with Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1990, 323, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, F.; Pomilio, M.; De Luca, F.A.; Vecchiet, J.; Verrotti, A. Plasma Prorenin Levels May Predict Persistent Microalbuminuria in Children with Diabetes. Pediatr. Nephrol. 2001, 16, 116–120. [Google Scholar] [CrossRef]
- Deinum, J.; Rønn, B.; Mathiesen, E.; Derkx, F.H.M.; Hop, W.C.J.; Schalekamp, M.A.D.H. Increase in Serum Prorenin Precedes Onset of Microalbuminuria in Patients with Insulin-Dependent Diabetes Mellitus. Diabetologia 1999, 42, 1006–1010. [Google Scholar] [CrossRef]
- Kaneshiro, Y.; Ichihara, A.; Sakoda, M.; Takemitsu, T.; Nabi, A.H.M.N.; Uddin, M.N.; Nakagawa, T.; Nishiyama, A.; Suzuki, F.; Inagami, T.; et al. Slowly Progressive, Angiotensin II-Independent Glomerulosclerosis in Human (pro)Renin Receptor-Transgenic Rats. J. Am. Soc. Nephrol. 2007, 18, 1789–1795. [Google Scholar] [CrossRef]
- Feldt, S.; Maschke, U.; Dechend, R.; Luft, F.C.; Muller, D.N. The Putative (pro)Renin Receptor Blocker HRP Fails to Prevent (pro)Renin Signaling. J. Am. Soc. Nephrol. 2008, 19, 743–748. [Google Scholar] [CrossRef]
- Ramkumar, N.; Stuart, D.; Mironova, E.; Bugay, V.; Wang, S.; Abraham, N.; Ichihara, A.; Stockand, J.D.; Kohan, D.E. Renal Tubular Epithelial Cell Prorenin Receptor Regulates Blood Pressure and Sodium Transport. Am. J. Physiol.-Renal. Physiol. 2016, 311, F186–F194. [Google Scholar] [CrossRef] [PubMed]
- Trepiccione, F.; Gerber, S.D.; Grahammer, F.; López-Cayuqueo, K.I.; Baudrie, V.; Pǎunescu, T.G.; Capen, D.E.; Picard, N.; Alexander, R.T.; Huber, T.B.; et al. Renal Atp6ap2/(Pro)Renin Receptor Is Required for Normal Vacuolar H+-ATPase Function but Not for the Renin-Angiotensin System. J. Am. Soc. Nephrol. 2016, 27, 3320–3330. [Google Scholar] [CrossRef] [PubMed]
- Brandis, A.; Bianchi, G.; Reale, E.; Helmchen, U.; Kühn, K. Age-Dependent Glomerulosclerosis and Proteinuria Occurring in Rats of the Milan Normotensive Strain and Not in Rats of the Milan Hypertensive Strain. Lab Investig. 1986, 55, 234–243. [Google Scholar]
- Menini, S.; Ricci, C.; Iacobini, C.; Bianchi, G.; Pugliese, G.; Pesce, C. Glomerular Number and Size in Milan Hypertensive and Normotensive Rats: Their Relationship to Susceptibility and Resistance to Hypertension and Renal Disease. J. Hypertens. 2004, 22, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Ricci, C.; Iacobini, C.; Menini, S.; Fioretto, P.; Ferrandi, M.; Giardino, L.A.; Armelloni, S.; Mattinzoli, D.; Rastaldi, M.P.; et al. Glomerular Barrier Dysfunction in Glomerulosclerosis- Resistant Milan Rats with Experimental Diabetes: The Role of Renal Haemodynamics. J. Pathol. 2007, 213, 210–218. [Google Scholar] [CrossRef]
- Menini, S.; Amadio, L.; Oddi, G.; Ricci, C.; Pesce, C.; Pugliese, F.; Giorgio, M.; Migliaccio, E.; Pelicci, P.G.; Iacobini, C.; et al. Deletion of P66Shc Longevity Gene Protects against Experimental Diabetic Glomerulopathy by Preventing Diabetes-Induced Oxidative Stress. Diabetes 2006, 55, 1642–1650. [Google Scholar] [CrossRef]
- Solini, A.; Menini, S.; Rossi, C.; Ricci, C.; Santini, E.; Blasetti Fantauzzi, C.; Iacobini, C.; Pugliese, G. The Purinergic 2X7 Receptor Participates in Renal Inflammation and Injury Induced by High-Fat Diet: Possible Role of NLRP3 Inflammasome Activation. J. Pathol. 2013, 231, 342–353. [Google Scholar] [CrossRef]
- Floege, J.; Hackmann, B.; Kliem, V.; Kriz, W.; Alpers, C.E.; Johnson, R.J.; Kühn, K.W.; Koch, K.M.; Brunkhorst, R. Age-Related Glomerulosclerosis and Interstitial Fibrosis in Milan Normotensive Rats: A Podocyte Disease. Kidney Int 1997, 51, 230–243. [Google Scholar] [CrossRef]
- Remuzzi, G.; FitzGerald, G.A.; Patrono, C. Thromboxane Synthesis and Action within the Kidney. Kidney Int. 1992, 41, 1483–1493. [Google Scholar] [CrossRef]
- Salvati, P.; Ferti, C.; Ferrario, R.G.; Lamberti, E.; Duzzi, L.; Bianchi, G.; Remuzzi, G.; Perico, N.; Benigni, A.; Braidotti, P.; et al. Role of Enhanced Glomerular Synthesis of Thromboxane A2 in Progressive Kidney Disease. Kidney Int. 1990, 38, 447–458. [Google Scholar] [CrossRef]
- Campbell, D.J.; Nussberger, J.; Stowasser, M.; Danser, A.H.J.; Morganti, A.; Frandsen, E.; Ménard, J. Activity Assays and Immunoassays for Plasma Renin and Prorenin: Information Provided and Precautions Necessary for Accurate Measurement. Clin. Chem. 2009, 55, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.A.; Luffman, C.; Bourgeois, C.R.T.; Vio, C.P.; Prieto, M.C. Angiotensin II-Independent Upregulation of Cyclooxygenase-2 by Activation of the (Pro)Renin Receptor in Rat Renal Inner Medullary Cells. Hypertension 2013, 61, 443–449. [Google Scholar] [CrossRef]
- Huang, J.; Siragy, H.M. Glucose Promotes the Production of Interleukine-1beta and Cyclooxygenase-2 in Mesangial Cells via Enhanced (Pro)Renin Receptor Expression. Endocrinology 2009, 150, 5557–5565. [Google Scholar] [CrossRef]
- Cousin, C.; Bracquart, D.; Contrepas, A.; Corvol, P.; Muller, L.; Nguyen, G. Soluble Form of the (pro)Renin Receptor Generated by Intracellular Cleavage by Furin Is Secreted in Plasma. Hypertension 2009, 53, 1077–1082. [Google Scholar] [CrossRef]
- Wang, F.; Xu, C.; Luo, R.; Peng, K.; Ramkumar, N.; Xie, S.; Lu, X.; Zhao, L.; Zuo, C.J.; Kohan, D.E.; et al. Site-1 Protease-Derived Soluble (pro)Renin Receptor Targets Vasopressin Receptor 2 to Enhance Urine Concentrating Capability. JCI Insight 2019, 4, e124174. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, Y.; Wang, S.; Chi, C. Interaction of Mint3 with Furin Regulates the Localization of Furin in the Trans-Golgi Network. J. Cell Sci. 2008, 121, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Kinouchi, K.; Ichihara, A.; Sakoda, M.; Kurauchi-Mito, A.; Bokuda, K.; Narita, T.; Kurosawa, H.; Sun-Wada, G.H.; Wada, Y.; et al. Prorenin Receptor Is Essential for Normal Podocyte Structure and Function. J. Am. Soc. Nephrol. 2011, 22, 2203–2212. [Google Scholar] [CrossRef]
- Li, C.; Siragy, H.M. High Glucose Induces Podocyte Injury via Enhanced (pro)Renin Receptor-Wnt-β-Catenin-Snail Signaling Pathway. PLoS ONE 2014, 9, e89233. [Google Scholar] [CrossRef]
- Leung, J.C.K.; Chan, L.Y.Y.; Saleem, M.A.; Mathieson, P.W.; Tang, S.C.W.; Lai, K.N. Combined Blockade of Angiotensin II and Prorenin Receptors Ameliorates Podocytic Apoptosis Induced by IgA-Activated Mesangial Cells. Apoptosis 2015, 20, 907–920. [Google Scholar] [CrossRef]
- Li, C.; Siragy, H.M. (Pro)Renin Receptor Regulates Autophagy and Apoptosis in Podocytes Exposed to High Glucose. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E302–E310. [Google Scholar] [CrossRef]
- Gogulamudi, V.R.; Arita, D.Y.; Bourgeois, C.R.T.; Jorgensen, J.; He, J.; Wimley, W.C.; Satou, R.; Gonzalez, A.A.; Prieto, M.C. High glucose induces trafficking of prorenin receptor and stimulates profibrotic factors in the collecting duct. Sci. Rep. 2021, 5, 13815. [Google Scholar] [CrossRef]
- Bahreini, E.; Rezaei-Chianeh, Y.; Nabi-Afjadi, M. Molecular Mechanisms Involved in Intrarenal Renin-Angiotensin and Alternative Pathways in Diabetic Nephropathy—A Review. Rev. Diabet. Stud. 2021, 17, 1–10. [Google Scholar] [CrossRef]
- Rüster, C.; Wolf, G. The role of the renin-angiotensin-aldosterone system in obesity-related renal diseases. Semin. Nephrol. 2013, 33, 44–53. [Google Scholar] [CrossRef]
- Piani, F.; Reinicke, T.; Lytvyn, Y.; Melena, I.; Lovblom, L.E.; Lai, V.; Tse, J.; Cham, L.; Orszag, A.; Perkins, B.A.; et al. Vasopressin associated with renal vascular resistance in adults with longstanding type 1 diabetes with and without diabetic kidney disease. J. Diabetes Complicat. 2021, 35, 107807. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.; Fitzgerald, G.A. Cellular Activation by Thromboxane A2 and Other Eicosanoids. Eur. Heart J. 1993, 14 (Suppl. K), 88–93. [Google Scholar] [PubMed]
- Schefe, J.H.; Menk, M.; Reinemund, J.; Effertz, K.; Hobbs, R.M.; Pandolfi, P.P.; Ruiz, P.; Unger, T.; Funke-Kaiser, H. A Novel Signal Transduction Cascade Involving Direct Physical Interaction of the Renin/Prorenin Receptor with the Transcription Factor Promyelocytic Zinc Finger Protein. Circ. Res. 2006, 99, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Suzuki-Nakagawa, C.; Watanabe, A.; Asami, E.; Matsumoto, M.; Nakano, M.; Ebihara, A.; Uddin, M.N.; Suzuki, F. Site-1 Protease Is Required for the Generation of Soluble (pro)Renin Receptor. J. Biochem. 2017, 161, 369–379. [Google Scholar] [CrossRef]
- Hamada, K.; Taniguchi, Y.; Shimamura, Y.; Inoue, K.; Ogata, K.; Ishihara, M.; Horino, T.; Fujimoto, S.; Ohguro, T.; Yoshimoto, Y.; et al. Serum Level of Soluble (pro)Renin Receptor Is Modulated in Chronic Kidney Disease. Clin. Exp. Nephrol. 2013, 17, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Luo, R.; Zou, C.J.; Xie, S.; Peng, K.; Zhao, L.; Yang, K.T.; Xu, C.; Yang, T. Soluble (pro)Renin Receptor Treats Metabolic Syndrome in Mice with Diet-Induced Obesity via Interaction with PPARγ. JCI Insight 2020, 5, e128061. [Google Scholar] [CrossRef]
- Sakamoto, T.; Seiki, M. Mint3 Enhances the Activity of Hypoxia-Inducible Factor-1 (HIF-1) in Macrophages by Suppressing the Activity of Factor Inhibiting HIF-1. J. Biol. Chem. 2009, 284, 30350–30359. [Google Scholar] [CrossRef]
- Ten, T.; Nagatoishi, S.; Maeda, R.; Hoshino, M.; Nakayama, Y.; Seiki, M.; Sakamoto, T.; Tsumoto, K. Structural and Thermodynamical Insights into the Binding and Inhibition of FIH-1 by the N-Terminal Disordered Region of Mint3. J. Biol. Chem. 2021, 297, 101304. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Vitale, M.; Pugliese, G.; Menini, S. Normalizing HIF-1α Signaling Improves Cellular Glucose Metabolism and Blocks the Pathological Pathways of Hyperglycemic Damage. Biomedicines 2021, 9, 1139. [Google Scholar] [CrossRef] [PubMed]
- Menini, S.; Iacobini, C.; Ricci, C.; Fantauzzi, C.B.; Pugliese, G. Protection from Diabetes-Induced Atherosclerosis and Renal Disease by D-Carnosine-Octylester: Effects of Early vs Late Inhibition of Advanced Glycation End-Products in Apoe-Null Mice. Diabetologia 2015, 58, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Doronzo, G.; Russo, I.; Mattiello, L.; Riganti, C.; Anfossi, G.; Trovati, M. Insulin Activates Hypoxia-Inducible Factor-1alpha in Human and Rat Vascular Smooth Muscle Cells via Phosphatidylinositol-3 Kinase and Mitogen-Activated Protein Kinase Pathways: Impairment in Insulin Resistance Owing to Defects in Insulin Signalling. Diabetologia 2006, 49, 1049–1063. [Google Scholar] [CrossRef]
- Matoba, K.; Kawanami, D.; Okada, R.; Tsukamoto, M.; Kinoshita, J.; Ito, T.; Ishizawa, S.; Kanazawa, Y.; Yokota, T.; Murai, N.; et al. Rho-Kinase Inhibition Prevents the Progression of Diabetic Nephropathy by Downregulating Hypoxia-Inducible Factor 1α. Kidney Int. 2013, 84, 545–554. [Google Scholar] [CrossRef]
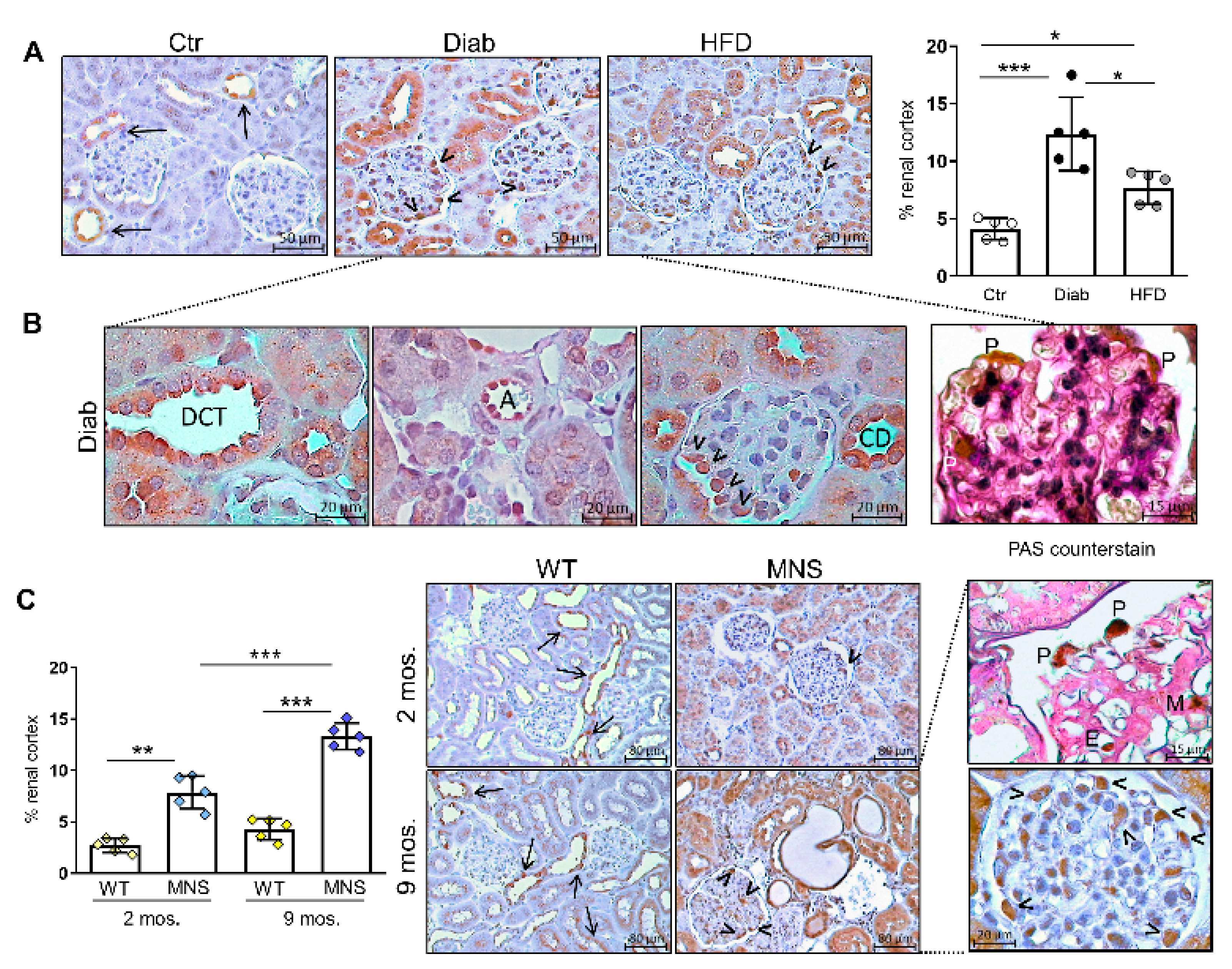

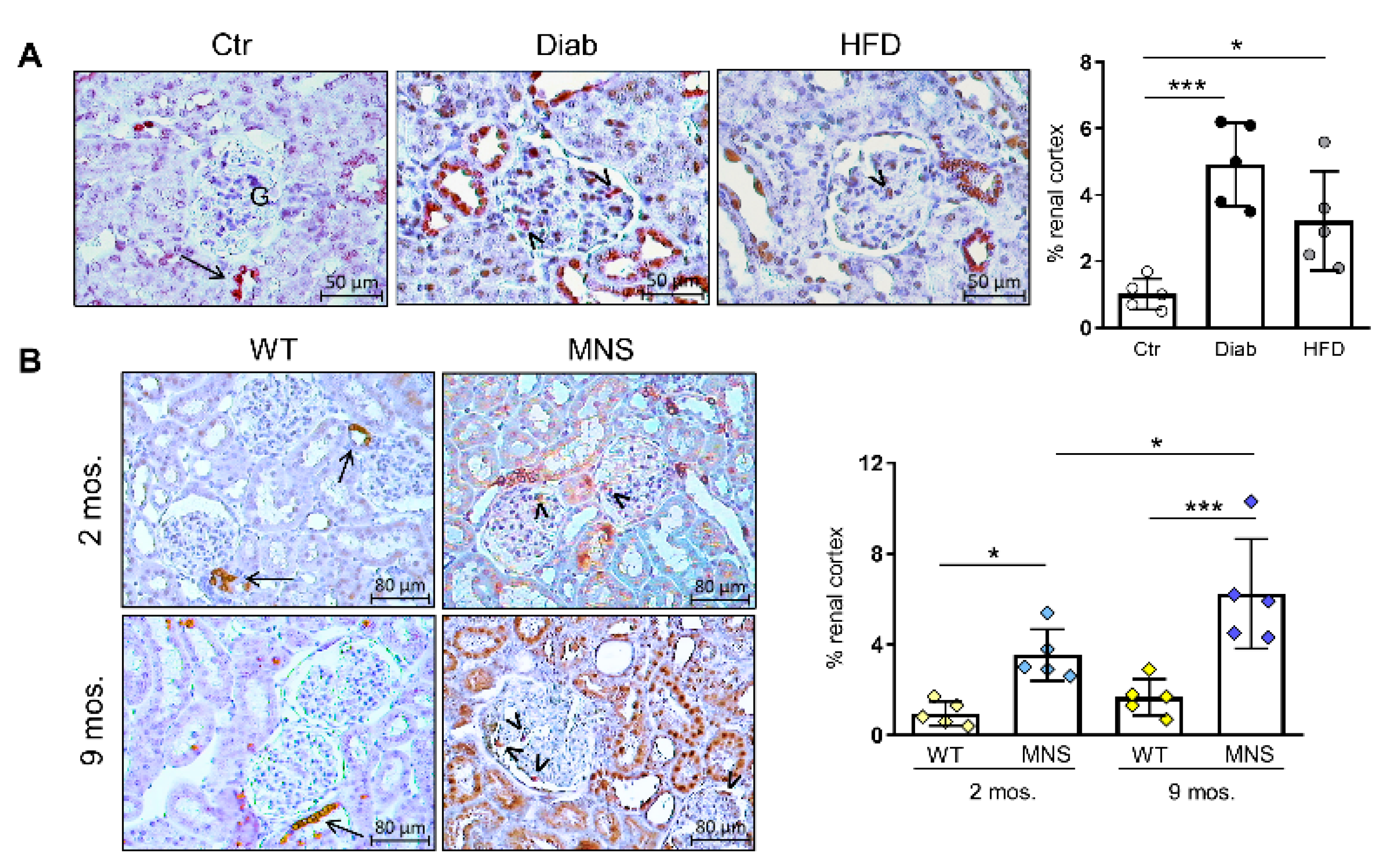
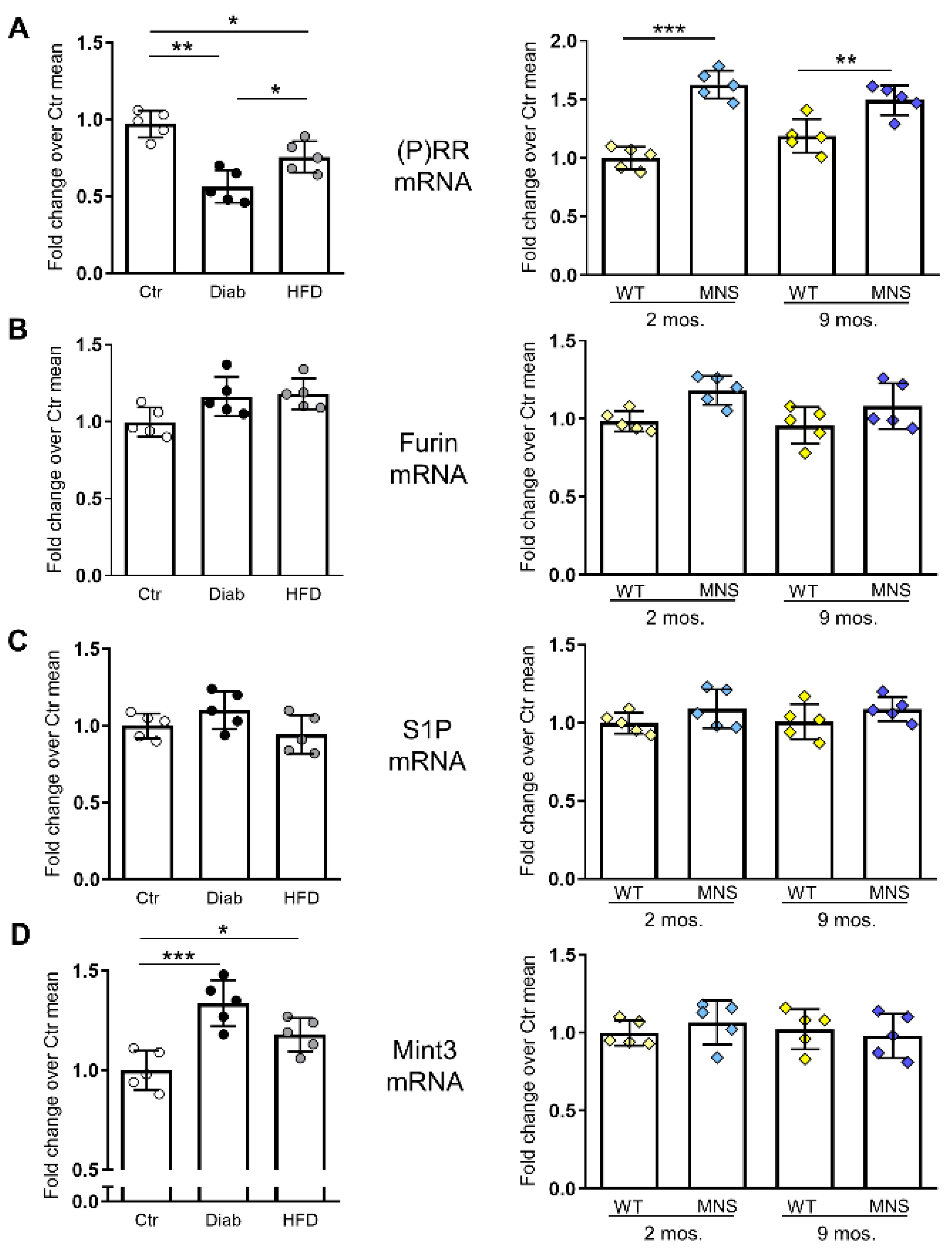

| Diab Mice (4 Months of Diabetes) [16] | HFD-Fed Mice (4 Months of Fatty Diet) [17] | MNS Rats (9 Months Old) [14] | |
|---|---|---|---|
| Metabolic phenotype | severe hyperglycemia, insulinopenia (type 1 diabetes), body weight reduction, normal blood pressure | mild hyperglycemia, overweight, hyperinsulinemia, dyslipidemia, normal blood pressure | normal blood glucose, normal body weight, normal blood pressure |
| Renal lesions | moderate diffuse glomerulosclerosis, increased GSI (↑↑), no tubular damage | mild diffuse glomerulosclerosis, increased GSI (↑), no tubular damage | severe segmental or global glomerulosclerosis, interstitial fibrosis, and tubular damage |
| Proteinuria | 5 times than the control | 1.7 times than the control | 30 times than the control |
| Target | Assay | ||
|---|---|---|---|
| Human | Mouse | Rat | |
| ATP6AP2 | Hs00997145_m1 | Mm00510396_m1 | Rn01430719_m1 |
| Furin | Hs00159829_m1 | Mm00440646_m1 | Rn00570970_m1 |
| MBTPS1 | Hs00921626_m1 | Mm00490600_m1 | Rn00585707_m1 |
| Mint3 | Hs01114376_m1 | Mm00444450_m1 | Rn00582358_m1 |
| ACTB | Hs99999903_m1 | Mm02619580_m1 | Rn00667869_m1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacobini, C.; Vitale, M.; Sentinelli, F.; Haxhi, J.; Pugliese, G.; Menini, S. Renal Expression and Localization of the Receptor for (Pro)renin and Its Ligands in Rodent Models of Diabetes, Metabolic Syndrome, and Age-Dependent Focal and Segmental Glomerulosclerosis. Int. J. Mol. Sci. 2024, 25, 2217. https://doi.org/10.3390/ijms25042217
Iacobini C, Vitale M, Sentinelli F, Haxhi J, Pugliese G, Menini S. Renal Expression and Localization of the Receptor for (Pro)renin and Its Ligands in Rodent Models of Diabetes, Metabolic Syndrome, and Age-Dependent Focal and Segmental Glomerulosclerosis. International Journal of Molecular Sciences. 2024; 25(4):2217. https://doi.org/10.3390/ijms25042217
Chicago/Turabian StyleIacobini, Carla, Martina Vitale, Federica Sentinelli, Jonida Haxhi, Giuseppe Pugliese, and Stefano Menini. 2024. "Renal Expression and Localization of the Receptor for (Pro)renin and Its Ligands in Rodent Models of Diabetes, Metabolic Syndrome, and Age-Dependent Focal and Segmental Glomerulosclerosis" International Journal of Molecular Sciences 25, no. 4: 2217. https://doi.org/10.3390/ijms25042217





