Osteoimmunology: The Crosstalk between T Cells, B Cells, and Osteoclasts in Rheumatoid Arthritis
Abstract
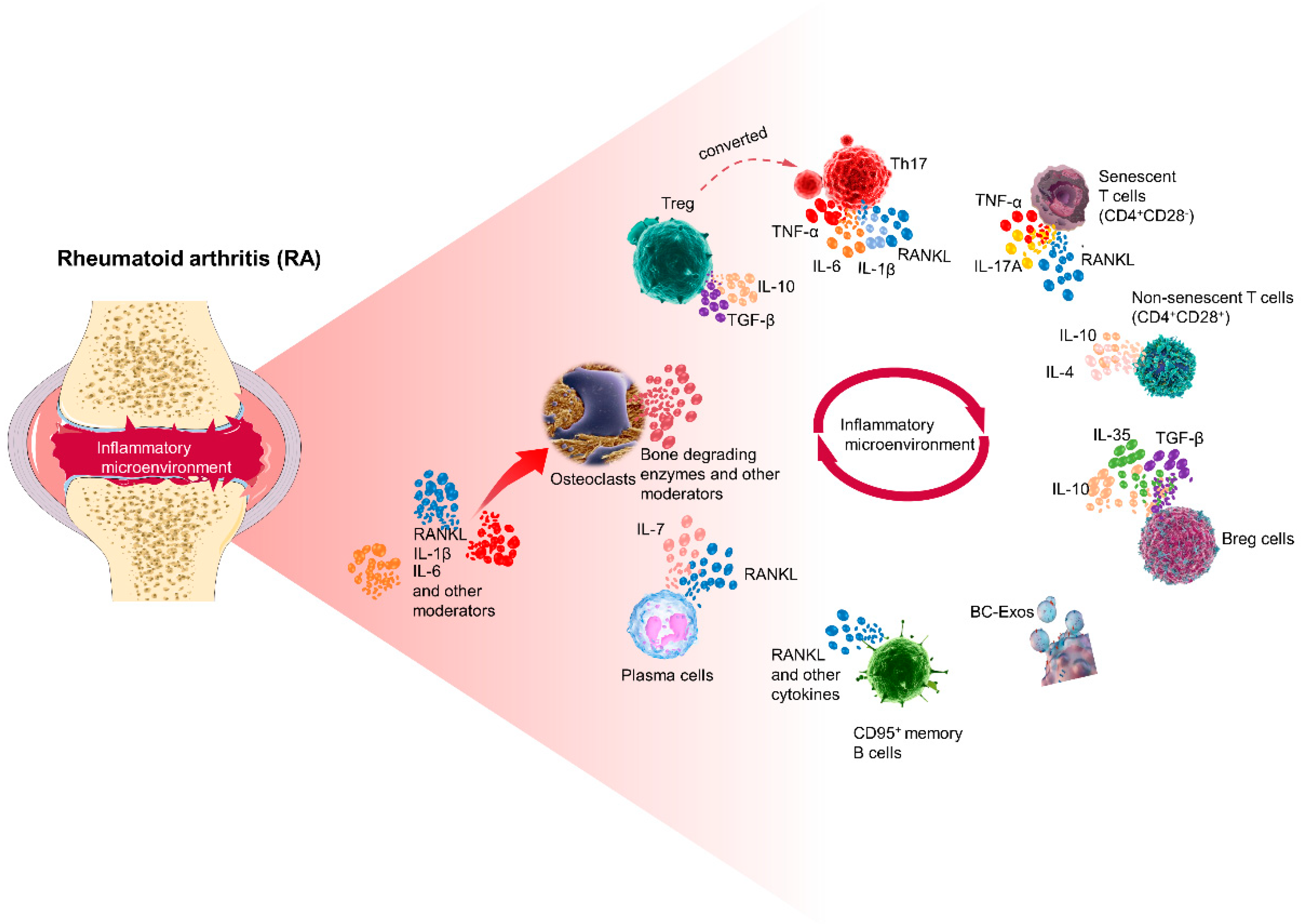
:1. Introduction
2. The Crosstalk between T Cells, B Cells, and Osteoclasts in the Immune Machinery of RA
3. Regulatory Signals for Osteoclast Differentiation and Maturation
3.1. Migration of Osteoclasts
3.2. Positive Regulation of Osteoclast Differentiation and Maturation
3.3. Negative Regulation of Osteoclast Differentiation and Maturation
4. Regulation of T Cells by RANKL
5. Regulation of B Cells by RANKL
6. Crosstalk between T Cells and Osteoclasts in RA
6.1. Crosstalk between Th17/Treg and Osteoclasts
6.2. Crosstalk between Young T Cells/Senescent T Cells and Osteoclasts
7. Crosstalk between B Cells and Osteoclasts in RA
7.1. Crosstalk between Memory B Cells and Osteoclasts
7.2. Crosstalk between Regulatory B Cells and Osteoclasts
7.3. Crosstalk between Plasma Cells and Osteoclasts
7.4. Crosstalk between B Cell Exosomes and Osteoclasts
8. Effect of Related Cytokines on Osteoclasts
8.1. Effects of TNF-α on Osteoclasts
8.2. Effects of IL-7 on Osteoclasts
8.3. Effects of IL-6 on Osteoclasts
8.4. Effects of IL-17 on Osteoclasts
9. New Biomarkers for RA
9.1. Anti-Malondialdehyde (MDA) and Anti-Malondialdehyde Acetaldehyde (MAA)
9.2. Anti-CII
9.3. Anti-Mutated Citrullinated Vimentin (MCV)
9.4. Other Biomarkers
10. Therapeutic Agents That Affect Osteoclasts or Alleviate Inflammation to Prevent Bone Erosion
10.1. Therapeutic Agents That Affect Osteoclasts
10.1.1. RANKL Monoclonal Antibody
10.1.2. Bisphosphonates
10.1.3. JAK Inhibitor
10.1.4. TNF-α Antagonist
10.1.5. Selective Estrogen Receptor Modulators/Hormone Replacement Therapy
10.1.6. Ctsk Inhibitor
10.2. Medications That Indirectly Suppress Osteoclast Activity by Reducing Inflammation
10.2.1. Methotrexate (MTX)
10.2.2. Biologic Response Modifiers
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, N.; Takayanagi, H. Mechanisms of joint destruction in rheumatoid arthritis—Immune cell-fibroblast-bone interactions. Nat. Rev. Rheumatol. 2022, 18, 415–429. [Google Scholar] [CrossRef]
- Rana, A.K.; Li, Y.; Dang, Q.; Yang, F. Monocytes in rheumatoid arthritis: Circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. Int. Immunopharmacol. 2018, 65, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Yoshida, K.; Nishizawa, T.; Otani, K.; Yamashita, Y.; Okabe, H.; Hadano, Y.; Kayama, T.; Kurosaka, D.; Saito, M. Inflammation and Bone Metabolism in Rheumatoid Arthritis: Molecular Mechanisms of Joint Destruction and Pharmacological Treatments. Int. J. Mol. Sci. 2022, 23, 2871. [Google Scholar] [CrossRef]
- Lucas, C.; Perdriger, A.; Amé, P. Definition of B cell helper T cells in rheumatoid arthritis and their behavior during treatment. Semin. Arthritis Rheum. 2020, 50, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef]
- Wang, W.; Shao, S.; Jiao, Z.; Guo, M.; Xu, H.; Wang, S. The Th17/Treg imbalance and cytokine environment in peripheral blood of patients with rheumatoid arthritis. Rheumatol. Int. 2012, 32, 887–893. [Google Scholar] [CrossRef]
- Lawson, C.A.; Brown, A.K.; Bejarano, V.; Douglas, S.H.; Burgoyne, C.H.; Greenstein, A.S.; Boylston, A.W.; Emery, P.; Ponchel, F.; Isaacs, J.D. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology 2006, 45, 1210–1217. [Google Scholar] [CrossRef]
- Komatsu, N.; Takayanagi, H. Inflammation and bone destruction in arthritis: Synergistic activity of immune and mesenchymal cells in joints. Front. Immunol. 2012, 3, 77. [Google Scholar] [CrossRef]
- Ambré, I.; Gaublomme, D.; Burssens, A.; Jacques, P.; Schryvers, N.; De Muynck, A.; Meuris, L.; Lambrecht, S.; Carter, S.; de Bleser, P.; et al. Mechanical strain determines the site-specific localization of inflammation and tissue damage in arthritis. Nat. Commun. 2018, 9, 4613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wei, K.; Slowikowski, K.; Fonseka, C.Y.; Rao, D.A.; Kelly, S.; Goodman, S.M.; Tabechian, D.; Hughes, L.B.; Salomon-Escoto, K.; et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 2019, 20, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Harre, U.; Georgess, D.; Bang, H.; Bozec, A.; Axmann, R.; Ossipova, E.; Jakobsson, P.J.; Baum, W.; Nimmerjahn, F.; Szarka, E.; et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Investig. 2012, 122, 1791–1802. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Toraldo, G.; Li, A.; Yang, X.; Zhang, H.; Qian, W.P.; Weitzmann, M.N. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 2007, 109, 3839–3848. [Google Scholar] [CrossRef] [PubMed]
- Meednu, N.; Zhang, H.; Owen, T.; Sun, W.; Wang, V.; Cistrone, C.; Rangel-Moreno, J.; Xing, L.; Anolik, J.H. Production of RANKL by Memory B Cells: A Link Between B Cells and Bone Erosion in Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Manabe, N.; Kawaguchi, H.; Chikuda, H.; Miyaura, C.; Inada, M.; Nagai, R.; Nabeshima, Y.; Nakamura, K.; Sinclair, A.M.; Scheuermann, R.H.; et al. Connection between B lymphocyte and osteoclast differentiation pathways. J. Immunol. 2001, 167, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Boumans, M.J.; Thurlings, R.M.; Yeo, L.; Scheel-Toellner, D.; Vos, K.; Gerlag, D.M.; Tak, P.P. Rituximab abrogates joint destruction in rheumatoid arthritis by inhibiting osteoclastogenesis. Ann. Rheum. Dis. 2012, 71, 108–113. [Google Scholar] [CrossRef]
- Sun, W.; Meednu, N.; Rosenberg, A.; Rangel-Moreno, J.; Wang, V.; Glanzman, J.; Owen, T.; Zhou, X.; Zhang, H.; Boyce, B.F.; et al. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nat. Commun. 2018, 9, 5127. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Wright, L.M.; Maloney, W.; Yu, X.; Kindle, L.; Collin-Osdoby, P.; Osdoby, P. Stromal cell-derived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone 2005, 36, 840–853. [Google Scholar] [CrossRef]
- Kikuta, J.; Ishii, M. Osteoclast migration, differentiation and function: Novel therapeutic targets for rheumatic diseases. Rheumatology 2013, 52, 226–234. [Google Scholar] [CrossRef]
- Koizumi, K.; Saitoh, Y.; Minami, T.; Takeno, N.; Tsuneyama, K.; Miyahara, T.; Nakayama, T.; Sakurai, H.; Takano, Y.; Nishimura, M.; et al. Role of CX3CL1/fractalkine in osteoclast differentiation and bone resorption. J. Immunol. 2009, 183, 7825–7831. [Google Scholar] [CrossRef]
- Ishii, M.; Egen, J.G.; Klauschen, F.; Meier-Schellersheim, M.; Saeki, Y.; Vacher, J.; Proia, R.L.; Germain, R.N. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 2009, 458, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Kikuta, J.; Shimazu, Y.; Meier-Schellersheim, M.; Germain, R.N. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J. Exp. Med. 2010, 207, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef]
- Yasui, T.; Kadono, Y.; Nakamura, M.; Oshima, Y.; Matsumoto, T.; Masuda, H.; Hirose, J.; Omata, Y.; Yasuda, H.; Imamura, T.; et al. Regulation of RANKL-induced osteoclastogenesis by TGF-β through molecular interaction between Smad3 and Traf6. J. Bone Miner. Res. 2011, 26, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Troen, B.R. Molecular mechanisms underlying osteoclast formation and activation. Exp. Gerontol. 2003, 38, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Ionescu, A.M.; Sinal, C.J. At the Crossroads of the Adipocyte and Osteoclast Differentiation Programs: Future Therapeutic Perspectives. Int. J. Mol. Sci. 2020, 21, 2277. [Google Scholar] [CrossRef] [PubMed]
- Saad, S.; Dharmapatni, A.A.S.S.K.; Crotti, T.N.; Cantley, M.D.; Algate, K.; Findlay, D.M.; Atkins, G.J.; Haynes, D.R. Semaphorin-3a, neuropilin-1 and plexin-A1 in prosthetic-particle induced bone loss. Acta Biomater. 2016, 30, 311–318. [Google Scholar] [CrossRef]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Tsuji-Takechi, K.; Negishi-Koga, T.; Sumiya, E.; Kukita, A.; Kato, S.; Maeda, T.; Pandolfi, P.P.; Moriyama, K.; Takayanagi, H. Stage-specific functions of leukemia/lymphoma-related factor (LRF) in the transcriptional control of osteoclast development. Proc. Natl. Acad. Sci. USA 2012, 109, 2561–2566. [Google Scholar] [CrossRef]
- Maruyama, K.; Fukasaka, M.; Vandenbon, A.; Saitoh, T.; Kawasaki, T.; Kondo, T.; Yokoyama, K.K.; Kidoya, H.; Takakura, N.; Standley, D.; et al. The transcription factor Jdp2 controls bone homeostasis and antibacterial immunity by regulating osteoclast and neutrophil differentiation. Immunity 2012, 37, 1024–1036. [Google Scholar] [CrossRef]
- Baek, K.; Park, H.J.; Baek, J.H.; Kim, H.R. Isoproterenol Increases RANKL Expression in a ATF4/NFATc1-Dependent Manner in Mouse Osteoblastic Cells. Int. J. Mol. Sci. 2017, 18, 2204. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kim, S.; Lee, N.; Jeon, H.; Lee, J.; Takami, M.; Rho, J. Pax5 Negatively Regulates Osteoclastogenesis through Downregulation of Blimp1. Int. J. Mol. Sci. 2021, 22, 2097. [Google Scholar] [CrossRef]
- Negishi-Koga, T.; Gober, H.J.; Sumiya, E.; Komatsu, N.; Okamoto, K.; Sawa, S.; Suematsu, A.; Suda, T.; Sato, K.; Takai, T.; et al. Immune complexes regulate bone metabolism through FcRγ signalling. Nat. Commun. 2015, 6, 6637. [Google Scholar] [CrossRef] [PubMed]
- Krzeszinski, J.Y.; Wei, W.; Huynh, H.; Jin, Z.; Wang, X.; Chang, T.C.; Xie, X.J.; He, L.; Mangala, L.S.; Lopez-Berestein, G.; et al. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature 2020, 582, 134. [Google Scholar] [CrossRef]
- Luo, J.; Yang, Z.; Ma, Y.; Yue, Z.; Lin, H.; Qu, G.; Huang, J.; Dai, W.; Li, C.; Zheng, C.; et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat. Med. 2016, 22, 539–546. [Google Scholar] [CrossRef]
- Okamoto, K.; Takayanagi, H. Osteoimmunology. Cold Spring Harb. Perspect. Med. 2019, 9, a031245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jimi, E.; Bothwell, A.L. Receptor activator of NF-κB ligand stimulates recruitment of SHP-1 to the complex containing TNFR-associated factor 6 that regulates osteoclastogenesis. J. Immunol. 2003, 171, 3620–3626. [Google Scholar] [CrossRef]
- Teufel, S.; Grötsch, B.; Luther, J.; Derer, A.; Schinke, T.; Amling, M.; Schett, G.; Mielenz, D.; David, J.P. Inhibition of bone remodeling in young mice by bisphosphonate displaces the plasma cell niche into the spleen. J. Immunol. 2014, 193, 223–233. [Google Scholar] [CrossRef]
- Mansour, A.; Abou-Ezzi, G.; Sitnicka, E.; Derer, A.; Schinke, T.; Amling, M.; Schett, G.; Mielenz, D.; David, J.P. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J. Exp. Med. 2012, 209, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wong, P.; Li, J.; Lv, Z.; Xu, L.; Zhu, G.; He, M.; Luo, Y. Osteoimmunology: The correlation between osteoclasts and the Th17/Treg balance in osteoporosis. J. Cell Mol. Med. 2022, 26, 3591–3597. [Google Scholar] [CrossRef]
- Wong, B.R.; Rho, J.; Arron, J.; Robinson, E.; Orlinick, J.; Chao, M.; Kalachikov, S.; Cayani, E.; Bartlett, F.S., III; Frankel, W.N.; et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J. Biol. Chem. 1997, 272, 25190–25194. [Google Scholar] [CrossRef]
- Penninger, J.M.; Crabtree, G.R. The actin cytoskeleton and lymphocyte activation. Cell 1999, 96, 9–12. [Google Scholar] [CrossRef]
- Ashcroft, A.J.; Cruickshank, S.M.; Croucher, P.I.; Perry, M.J.; Rollinson, S.; Lippitt, J.M.; Child, J.A.; Dunstan, C.; Felsburg, P.J.; Morgan, G.J.; et al. Colonic dendritic cells, intestinal inflammation, and T cell-mediated bone destruction are modulated by recombinant osteoprotegerin. Immunity 2003, 19, 849–861. [Google Scholar] [CrossRef]
- Green, E.A.; Choi, Y.; Flavell, R.A. Pancreatic lymph node-derived CD4(+)CD25(+) Treg cells: Highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity 2002, 16, 183–191. [Google Scholar] [CrossRef]
- Francisconi, C.F.; Vieira, A.E.; Azevedo, M.C.S.; Tabanez, A.P.; Fonseca, A.C.; Trombone, A.P.F.; Letra, A.; Silva, R.M.; Sfeir, C.S.; Little, S.R.; et al. RANKL Triggers Treg-Mediated Immunoregulation in Inflammatory Osteolysis. J. Dent. Res. 2018, 97, 917–927. [Google Scholar] [CrossRef]
- Kumar, G.; Roger, P.M. From Crosstalk between Immune and Bone Cells to Bone Erosion in Infection. Int. J. Mol. Sci. 2019, 20, 5154. [Google Scholar] [CrossRef]
- Perlot, T.; Penninger, J.M. Development and function of murine B cells lacking RANK. J. Immunol. 2012, 188, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 2022, 123, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, I.E.; Chao, C.C.; Geissler, R.; Laface, D.; Blumenschein, W.; Iwakura, Y.; McClanahan, T.; Bowman, E.P. Interleukin-17A upregulates receptor activator of NF-κB on osteoclast precursors. Arthritis Res. Ther. 2010, 12, R29. [Google Scholar] [CrossRef]
- Sato, K.; Suematsu, A.; Okamoto, K.; Yamaguchi, A.; Morishita, Y.; Kadono, Y.; Tanaka, S.; Kodama, T.; Akira, S.; Iwakura, Y.; et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006, 203, 2673–2682. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Feige, U.; Sarosi, I.; Bolon, B.; Tafuri, A.; Morony, S.; Capparelli, C.; Li, J.; Elliott, R.; McCabe, S.; et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 1999, 402, 304–309. [Google Scholar] [CrossRef]
- Takayanagi, H.; Ogasawara, K.; Hida, S.; Chiba, T.; Murata, S.; Sato, K.; Takaoka, A.; Yokochi, T.; Oda, H.; Tanaka, K.; et al. T cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature 2000, 408, 600–605. [Google Scholar] [CrossRef]
- Zhu, L.; Hua, F.; Ding, W.; Ding, K.; Zhang, Y.; Xu, C. The correlation between the Th17/Treg cell balance and bone health. Immun. Ageing 2020, 17, 30. [Google Scholar] [CrossRef]
- Grötsch, B.; Lux, A.; Rombouts, Y.; Hoffmann, A.C.; Andreev, D.; Nimmerjahn, F.; Xiang, W.; Scherer, H.U.; Schett, G.; Bozec, A. Fra1 Controls Rheumatoid Factor Autoantibody Production by Bone Marrow Plasma Cells and the Development of Autoimmune Bone Loss. J. Bone Miner. Res. 2019, 34, 1352–1365. [Google Scholar] [CrossRef]
- Yuan, F.L.; Li, X.; Lu, W.G.; Xu, R.S.; Zhao, Y.Q.; Li, C.W.; Li, J.P.; Chen, F.H. Regulatory T cells as a potent target for controlling bone loss. Biochem. Biophys. Res. Commun. 2010, 402, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.; Herkner, C.; Kitte, R.; Dohnke, S.; Riewaldt, J.; Kretschmer, K.; Garbe, A.I. Foxp3+ Regulatory T Cells in Bone and Hematopoietic Homeostasis. Front. Endocrinol. 2019, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Dar, H.Y.; Azam, Z.; Anupam, R.; Mondal, R.K.; Srivastava, R.K. Osteoimmunology: The Nexus between bone and immune system. Front. Biosci. (Landmark Ed.) 2018, 23, 464–492. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, M.; Giro, G.; Taubman, M.A.; Han, X.; Mayer, M.P.; Kawai, T. Role of periodontal pathogenic bacteria in RANKL-mediated bone destruction in periodontal disease. J. Oral Microbiol. 2010, 2, 5532. [Google Scholar] [CrossRef] [PubMed]
- Bhadricha, H.; Patel, V.; Singh, A.K.; Savardekar, L.; Patil, A.; Surve, S.; Desai, M. Increased frequency of Th17 cells and IL-17 levels are associated with low bone mineral density in postmenopausal women. Sci. Rep. 2021, 11, 16155. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-hora, M.; Kodama, T.; Tanaka, S.; Bluestone, J.A.; Takayanagi, H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 2014, 20, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M.; Komatsu, N.; Nagashima, K.; Nitta, T.; Pluemsakunthai, W.; Shukunami, C.; Iwakura, Y.; Nakashima, T.; Okamoto, K.; Takayanagi, H. Host defense against oral microbiota by bone-damaging T cells. Nat. Commun. 2018, 9, 701. [Google Scholar] [CrossRef] [PubMed]
- Kornete, M.; Mason, E.; Istomine, R.; Piccirillo, C.A. KLRG1 expression identifies short-lived Foxp3+ Treg effector cells with functional plasticity in islets of NOD mice. Autoimmunity 2017, 50, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.O.; Nurieva, R.; Martinez, G.J.; Kang, H.S.; Chung, Y.; Pappu, B.P.; Shah, B.; Chang, S.H.; Schluns, K.S.; Watowich, S.S.; et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 2008, 29, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Deknuydt, F.; Bioley, G.; Valmori, D.; Ayyoub, M. IL-1beta and IL-2 convert human Treg into T(H)17 cells. Clin. Immunol. 2009, 131, 298–307. [Google Scholar] [CrossRef] [PubMed]
- González-Osuna, L.; Sierra-Cristancho, A.; Rojas, C.; Cafferata, E.A.; Melgar-Rodríguez, S.; Cárdenas, A.M.; Vernal, R. Premature Senescence of T cells Favors Bone Loss During Osteolytic Diseases. A New Concern in the Osteoimmunology Arena. Aging Dis. 2021, 12, 1150–1161. [Google Scholar] [CrossRef]
- Vallejo, A.N. CD28 extinction in human T cells: Altered functions and the program of T cell senescence. Immunol. Rev. 2005, 205, 158–169. [Google Scholar] [CrossRef]
- Kumar, G.; Roger, P.M.; Ticchioni, M.; Trojani, C.; Bernard de Dompsur, R.; Bronsard, N.; Carles, M.; Bernard, E. T cells from chronic bone infection show reduced proliferation and a high proportion of CD28− CD4 T cells. Clin. Exp. Immunol. 2014, 176, 49–57. [Google Scholar] [CrossRef]
- Dapunt, U.; Giese, T.; Prior, B.; Gaida, M.M.; Hänsch, G.M. Infectious versus non-infectious loosening of implants: Activation of T lymphocytes differentiates between the two entities. Int. Orthop. 2014, 38, 1291–1296. [Google Scholar] [CrossRef]
- Fessler, J.; Husic, R.; Schwetz, V.; Lerchbaum, E.; Aberer, F.; Fasching, P.; Ficjan, A.; Obermayer-Pietsch, B.; Duftner, C.; Graninger, W.; et al. Senescent T cells Promote Bone Loss in Rheumatoid Arthritis. Front. Immunol. 2018, 9, 95. [Google Scholar] [CrossRef]
- Reinke, S.; Geissler, S.; Taylor, W.R.; Schmidt-Bleek, K.; Juelke, K.; Schwachmeyer, V.; Dahne, M.; Hartwig, T.; Akyüz, L.; Meisel, C.; et al. Terminally differentiated CD8⁺ T cells negatively affect bone regeneration in humans. Sci. Transl. Med. 2013, 5, 177ra36. [Google Scholar] [CrossRef]
- Alvarez, C.; Suliman, S.; Almarhoumi, R.; Vega, M.E.; Rojas, C.; Monasterio, G.; Galindo, M.; Vernal, R.; Kantarci, A. Regulatory T cell phenotype and anti-osteoclastogenic function in experimental periodontitis. Sci. Rep. 2020, 10, 19018. [Google Scholar] [CrossRef]
- Alvarez, C.; Rojas, C.; Rojas, L.; Cafferata, E.A.; Monasterio, G.; Vernal, R. Regulatory T Lymphocytes in Periodontitis: A Translational View. Mediators Inflamm. 2018, 2018, 7806912. [Google Scholar] [CrossRef]
- Soligo, M.; Camperio, C.; Caristi, S.; Scottà, C.; Del Porto, P.; Costanzo, A.; Mantel, P.Y.; Schmidt-Weber, C.B.; Piccolella, E. CD28 costimulation regulates FOXP3 in a RelA/NF-κB-dependent mechanism. Eur. J. Immunol. 2011, 41, 503–513. [Google Scholar] [CrossRef]
- Tang, Q.; Henriksen, K.J.; Boden, E.K.; Tooley, A.J.; Ye, J.; Subudhi, S.K.; Zheng, X.X.; Strom, T.B.; Bluestone, J.A. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J. Immunol. 2003, 171, 3348–3352. [Google Scholar] [CrossRef] [PubMed]
- Adlowitz, D.G.; Barnard, J.; Biear, J.N.; Cistrone, C.; Owen, T.; Wang, W.; Palanichamy, A.; Ezealah, E.; Campbell, D.; Wei, C.; et al. Expansion of Activated Peripheral Blood Memory B Cells in Rheumatoid Arthritis, Impact of B Cell Depletion Therapy, and Biomarkers of Response. PLoS ONE 2015, 10, e0128269. [Google Scholar] [CrossRef] [PubMed]
- LeBien, T.W.; Tedder, T.F. B lymphocytes: How they develop and function. Blood 2008, 112, 1570–1580. [Google Scholar] [CrossRef]
- Ran, Z.; Yue-Bei, L.; Qiu-Ming, Z.; Huan, Y. Regulatory B Cells and Its Role in Central Nervous System Inflammatory Demyelinating Diseases. Front. Immunol. 2020, 11, 1884. [Google Scholar] [CrossRef] [PubMed]
- Sapra, L.; Bhardwaj, A.; Mishra, P.K.; Garg, B.; Verma, B.; Mishra, G.C.; Srivastava, R.K. Regulatory B Cells (Bregs) Inhibit Osteoclastogenesis and Play a Potential Role in Ameliorating Ovariectomy-Induced Bone Loss. Front. Immunol. 2021, 12, 691081. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, X.; Lin, J.; Hu, Y.; Zhao, Q.; Kawai, T.; Taubman, M.A.; Han, X. B10 Cells Alleviate Periodontal Bone Loss in Experimental Periodontitis. Infect. Immun. 2017, 85, e00335-17. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Rosser, E.C.; Piper, C.J.M.; Matei, D.E.; Blair, P.A.; Rendeiro, A.F.; Orford, M.; Alber, D.G.; Krausgruber, T.; Catalan, D.; Klein, N.; et al. Microbiota-Derived Metabolites Suppress Arthritis by Amplifying Aryl-Hydrocarbon Receptor Activation in Regulatory B Cells. Cell Metab. 2020, 31, 837–851.e10. [Google Scholar] [CrossRef]
- Rosser, E.C.; Oleinika, K.; Tonon, S.; Doyle, R.; Bosma, A.; Carter, N.A.; Harris, K.A.; Jones, S.A.; Klein, N.; Mauri, C. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat. Med. 2014, 20, 1334–1339. [Google Scholar] [CrossRef]
- Komatsu, N.; Win, S.; Yan, M.; Huynh, N.C.; Sawa, S.; Tsukasaki, M.; Terashima, A.; Pluemsakunthai, W.; Kollias, G.; Nakashima, T.; et al. Plasma cells promote osteoclastogenesis and periarticular bone loss in autoimmune arthritis. J. Clin. Investig. 2021, 131, e143060. [Google Scholar] [CrossRef]
- Giuliani, N.; Colla, S.; Sala, R.; Moroni, M.; Lazzaretti, M.; La Monica, S.; Bonomini, S.; Hojden, M.; Sammarelli, G.; Barillè, S.; et al. Human myeloma cells stimulate the receptor activator of nuclear factor-κ B ligand (RANKL) in T lymphocytes: A potential role in multiple myeloma bone disease. Blood 2002, 100, 4615–4621. [Google Scholar] [CrossRef]
- Giuliani, N.; Colla, S.; Morandi, F.; Lazzaretti, M.; Sala, R.; Bonomini, S.; Grano, M.; Colucci, S.; Svaldi, M.; Rizzoli, V. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood 2005, 106, 2472–2483. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Wang, G.; Zhang, B.; Wang, C.; Li, D.; Ding, C.; Wei, Q.; Fan, Z.; Tang, H.; et al. Single-Cell RNA Sequencing Reveals B Cells Are Important Regulators in Fracture Healing. Front. Endocrinol. 2021, 12, 666140. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Investig. 2000, 106, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Al-Bogami, M.; Bystrom, J.; Clanchy, F.; Taher, T.E.; Mangat, P.; Williams, R.O.; Jawad, A.S.; Mageed, R.A. TNFα inhibitors reduce bone loss in rheumatoid arthritis independent of clinical response by reducing osteoclast precursors and IL-20. Rheumatology 2021, 60, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Middleton, S.; Bolon, B.; Stolina, M.; Brown, H.; Zhu, L.; Pretorius, J.; Zack, D.J.; Kostenuik, P.; Feige, U. Additive bone-protective effects of anabolic treatment when used in conjunction with RANKL and tumor necrosis factor inhibition in two rat arthritis models. Arthritis Rheum. 2005, 52, 1604–1611. [Google Scholar] [CrossRef]
- Redlich, K.; Hayer, S.; Ricci, R.; David, J.P.; Tohidast-Akrad, M.; Kollias, G.; Steiner, G.; Smolen, J.S.; Wagner, E.F.; Schett, G. Osteoclasts are essential for TNF-α-mediated joint destruction. J. Clin. Investig. 2002, 110, 1419–1427. [Google Scholar] [CrossRef]
- Alzabin, S.; Abraham, S.M.; Taher, T.E.; Palfreeman, A.; Hull, D.; McNamee, K.; Jawad, A.; Pathan, E.; Kinderlerer, A.; Taylor, P.C.; et al. Incomplete response of inflammatory arthritis to TNFα blockade is associated with the Th17 pathway. Ann. Rheum. Dis. 2012, 71, 1741–1748. [Google Scholar] [CrossRef]
- Gravallese, E.M.; Walsh, N.C. Rheumatoid arthritis: Repair of erosion in RA—Shifting the balance to formation. Nat. Rev. Rheumatol. 2011, 7, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Schneeweiss, S.; Liu, J.; Daniel, G.W.; Chang, C.L.; Garneau, K.; Solomon, D.H. Risk of osteoporotic fracture in a large population-based cohort of patients with rheumatoid arthritis. Arthritis Res. Ther. 2010, 12, R154. [Google Scholar] [CrossRef] [PubMed]
- Güler-Yüksel, M.; Bijsterbosch, J.; Goekoop-Ruiterman, Y.P.; Breedveld, F.C.; Allaart, C.F.; de Vries-Bouwstra, J.K.; Dijkmans, B.A.C.; Lems, W.F.; Ronday, H.K.; Peeters, A.J.; et al. Bone mineral density in patients with recently diagnosed, active rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Keystone, E.C.; Kavanaugh, A.F.; Sharp, J.T.; Tannenbaum, H.; Hua, Y.; Teoh, L.S.; Fischkoff, S.A.; Chartash, E.K. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: A randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004, 50, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Corfe, S.A.; Paige, C.J. The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. Semin. Immunol. 2012, 24, 198–208. [Google Scholar] [CrossRef]
- Peschon, J.J.; Morrissey, P.J.; Grabstein, K.H.; Ramsdell, F.J.; Maraskovsky, E.; Gliniak, B.C.; Park, L.S.; Ziegler, S.F.; Williams, D.E.; Ware, C.B.; et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 1994, 180, 1955–1960. [Google Scholar] [CrossRef]
- Horowitz, M.C.; Fretz, J.A.; Lorenzo, J.A. How B cells influence bone biology in health and disease. Bone 2010, 47, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Sim, J.H.; Lee, S.; Seol, M.A.; Ye, S.K.; Shin, H.M.; Lee, E.B.; Lee, Y.J.; Choi, Y.J.; Yoo, W.H.; et al. Interleukin-7 Induces Osteoclast Formation via STAT5, Independent of Receptor Activator of NF-κB Ligand. Front. Immunol. 2017, 8, 1376. [Google Scholar] [CrossRef]
- Titanji, K.; Ofotokun, I.; Weitzmann, M.N. Immature/transitional B cell expansion is associated with bone loss in HIV-infected individuals with severe CD4+ T cell lymphopenia. AIDS 2020, 34, 1475–1483. [Google Scholar] [CrossRef]
- Salopek, D.; Grcević, D.; Katavić, V.; Kovacić, N.; Lukić, I.K.; Marusić, A. Increased bone resorption and osteopenia are a part of the lymphoproliferative phenotype of mice with systemic over-expression of interleukin-7 gene driven by MHC class II promoter. Immunol. Lett. 2008, 121, 134–139. [Google Scholar] [CrossRef]
- Lee, S.K.; Kalinowski, J.F.; Jastrzebski, S.L.; Puddington, L.; Lorenzo, J.A. Interleukin-7 is a direct inhibitor of in vitro osteoclastogenesis. Endocrinology 2003, 144, 3524–3531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hilton, M.J.; Anolik, J.H.; Welle, S.L.; Zhao, C.; Yao, Z.; Li, X.; Wang, Z.; Boyce, B.F.; Xing, L. NOTCH inhibits osteoblast formation in inflammatory arthritis via noncanonical, NF-κB. J. Clin. Investig 2014, 124, 3200–3214. [Google Scholar] [CrossRef]
- Sanchez, C.; Gabay, O.; Salvat, C.; Henrotin, Y.E.; Berenbaum, F. Mechanical loading highly increases IL-6 production and decreases OPG expression by osteoblasts. Osteoarthr. Cartil. 2009, 17, 473–481. [Google Scholar] [CrossRef]
- Kaneshiro, S.; Ebina, K.; Shi, K.; Higuchi, C.; Hirao, M.; Okamoto, M.; Koizumi, K.; Morimoto, T.; Yoshikawa, H.; Hashimoto, J. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J. Bone Miner. Metab. 2014, 32, 378–392. [Google Scholar] [CrossRef]
- O’Brien, W.; Fissel, B.M.; Maeda, Y.; Yan, J.; Ge, X.; Gravallese, E.M.; Aliprantis, A.O.; Charles, J.F. RANK-Independent Osteoclast Formation and Bone Erosion in Inflammatory Arthritis. Arthritis Rheumatol. 2016, 68, 2889–2900. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Sato, K.; Miyazaki, T.; Kitaura, H.; Kayama, H.; Miyoshi, F.; Araki, Y.; Akiyama, Y.; Takeda, K.; Mimura, T. Combination of tumor necrosis factor α and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheumatol. 2014, 66, 121–129. [Google Scholar] [CrossRef]
- Gough, A.K.; Lilley, J.; Eyre, S.; Holder, R.L.; Emery, P. Generalised bone loss in patients with early rheumatoid arthritis. Lancet 1994, 344, 23–27. [Google Scholar] [CrossRef] [PubMed]
- De Benedetti, F.; Rucci, N.; Del Fattore, A.; Peruzzi, B.; Paro, R.; Longo, M.; Vivarelli, M.; Muratori, F.; Berni, S.; Ballanti, P.; et al. Impaired skeletal development in interleukin-6-transgenic mice: A model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum. 2006, 54, 3551–3563. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, L.; Xie, Y.; Chen, X.; Tian, L.; Liang, Y.; Li, H.; Zhang, J.; Liu, Y.; Yu, X. Interleukin-6 Knockout Inhibits Senescence of Bone Mesenchymal Stem Cells in High-Fat Diet-Induced Bone Loss. Front. Endocrinol. 2021, 11, 622950. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Suzuki, M.; Tanaka, K.; Takeda, S.; Yogo, K.; Matsumoto, Y. Anti-interleukin-6 receptor antibody prevents loss of bone structure and bone strength in collagen-induced arthritis mice. Scand. J. Rheumatol. 2018, 47, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Effects of inflammatory and anti-inflammatory cytokines on the bone. Eur. J. Clin. Investig. 2011, 41, 1361–1366. [Google Scholar] [CrossRef]
- Okamoto, K.; Takayanagi, H. Osteoclasts in arthritis and Th17 cell development. Int. Immunopharmacol. 2011, 11, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Feng, W.; Yimin; Cui, J.; Lv, S.; Hasegawa, T.; Sun, B.; Li, J.; Oda, K.; Amizuka, N. Histological Evidence of Increased Osteoclast Cell Number and Asymmetric Bone Resorption Activity in the Tibiae of Interleukin-6-Deficient Mice. J. Histochem. Cytochem. 2014, 62, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Lubberts, E.; van den Bersselaar, L.; Oppers-Walgreen, B.; Schwarzenberger, P.; Coenen-de Roo, C.J.; Kolls, J.K.; Joosten, L.A.; van den Berg, W.B. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-κB ligand/osteoprotegerin balance. J. Immunol. 2003, 170, 2655–2662. [Google Scholar] [CrossRef]
- Adamopoulos, I.E.; Suzuki, E.; Chao, C.C.; Gorman, D.; Adda, S.; Maverakis, E.; Zarbalis, K.; Geissler, R.; Asio, A.; Blumenschein, W.M.; et al. IL-17A gene transfer induces bone loss and epidermal hyperplasia associated with psoriatic arthritis. Ann. Rheum. Dis. 2015, 74, 1284–1292. [Google Scholar] [CrossRef]
- Uluçkan, Ö.; Jimenez, M.; Karbach, S.; Jeschke, A.; Graña, O.; Keller, J.; Busse, B.; Croxford, A.L.; Finzel, S.; Koenders, M.; et al. Chronic skin inflammation leads to bone loss by IL-17-mediated inhibition of Wnt signaling in osteoblasts. Sci. Transl. Med. 2016, 8, 330ra37. [Google Scholar] [CrossRef]
- Kampylafka, E.; d’Oliveira, I.; Linz, C.; Lerchen, V.; Stemmler, F.; Simon, D.; Englbrecht, M.; Sticherling, M.; Rech, J.; Kleyer, A.; et al. Resolution of synovitis and arrest of catabolic and anabolic bone changes in patients with psoriatic arthritis by IL-17A blockade with secukinumab: Results from the prospective PSARTROS study. Arthritis Res. Ther. 2018, 20, 153. [Google Scholar] [CrossRef]
- van der Heijde, D.; Gladman, D.D.; Kishimoto, M.; Okada, M.; Rathmann, S.S.; Moriarty, S.R.; Shuler, C.L.; Carlier, H.; Benichou, O.; Mease, P.J. Efficacy and Safety of Ixekizumab in Patients with Active Psoriatic Arthritis: 52-week Results from a Phase III Study (SPIRIT-P1). J. Rheumatol. 2018, 45, 367–377. [Google Scholar] [CrossRef]
- Blanco, F.J.; Möricke, R.; Dokoupilova, E.; Codding, C.; Neal, J.; Andersson, M.; Rohrer, S.; Richards, H. Secukinumab in Active Rheumatoid Arthritis: A Phase III Randomized, Double-Blind, Active Comparator- and Placebo-Controlled Study. Arthritis Rheumatol. 2017, 69, 1144–1153. [Google Scholar] [CrossRef]
- Yu, M.; Malik Tyagi, A.; Li, J.Y.; Adams, J.; Denning, T.L.; Weitzmann, M.N.; Jones, R.M.; Pacifici, R. PTH induces bone loss via microbial-dependent expansion of intestinal TNF+ T cells and Th17 cells. Nat. Commun. 2020, 11, 468. [Google Scholar] [CrossRef]
- Li, J.Y.; D’Amelio, P.; Robinson, J.; Walker, L.D.; Vaccaro, C.; Luo, T.; Tyagi, A.M.; Yu, M.; Reott, M.; Sassi, F.; et al. IL-17A Is Increased in Humans with Primary Hyperparathyroidism and Mediates PTH-Induced Bone Loss in Mice. Cell Metab. 2015, 22, 799–810. [Google Scholar] [CrossRef]
- Ono, T.; Okamoto, K.; Nakashima, T.; Nitta, T.; Hori, S.; Iwakura, Y.; Takayanagi, H. IL-17-producing γδ T cells enhance bone regeneration. Nat. Commun. 2016, 7, 10928. [Google Scholar] [CrossRef] [PubMed]
- Mangashetti, L.S.; Khapli, S.M.; Wani, M.R. IL-4 inhibits bone-resorbing activity of mature osteoclasts by affecting NF-κB and Ca2+ signaling. J. Immunol. 2005, 175, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Wu, Z.F.; Yu, Y.H.; Wang, L.; Cheng, L. Effects of interleukin-7/interleukin-7 receptor on RANKL-mediated osteoclast differentiation and ovariectomy-induced bone loss by regulating c-Fos/c-Jun pathway. J. Cell Physiol. 2018, 233, 7182–7194. [Google Scholar] [CrossRef]
- Evans, K.E.; Fox, S.W. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. 2007, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yao, S.; Wise, G.E. Effect of interleukin-10 on gene expression of osteoclastogenic regulatory molecules in the rat dental follicle. Eur. J. Oral Sci. 2006, 114, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, L.; Castañeda, S. Osteoimmunology: The study of the relationship between the immune system and bone tissue. Reumatol. Clin. 2013, 9, 303–315, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- Tarbell, K.V.; Yamazaki, S.; Steinman, R.M. The interactions of dendritic cells with antigen-specific, regulatory T cells that suppress autoimmunity. Semin. Immunol. 2006, 18, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Yago, T.; Nanke, Y.; Kawamoto, M.; Kobashigawa, T.; Yamanaka, H.; Kotake, S. IL-35 inhibits human osteoclastogenesis from monocytes induced by receptor-activator of NF-κB ligand. Cent. Eur. J. Immunol. 2018, 43, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kohara, H.; Kitaura, H.; Fujimura, Y.; Yoshimatsu, M.; Morita, Y.; Eguchi, T.; Masuyama, R.; Yoshida, N. IFN-γ directly inhibits TNF-α-induced osteoclastogenesis in vitro and in vivo and induces apoptosis mediated by Fas/Fas ligand interactions. Immunol. Lett. 2011, 137, 53–61. [Google Scholar] [CrossRef]
- Conigliaro, P.; Chimenti, M.S.; Triggianese, P.; Sunzini, F.; Novelli, L.; Perricone, C.; Perricone, R. Autoantibodies in inflammatory arthritis. Autoimmun. Rev. 2016, 15, 673–683. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Thiele, G.M.; Duryee, M.J.; Anderson, D.R.; Klassen, L.W.; Mohring, S.M.; Young, K.A.; Benissan-Messan, D.; Sayles, H.; Dusad, A.; Hunter, C.D.; et al. Malondialdehyde-acetaldehyde adducts and anti-malondialdehyde-acetaldehyde antibodies in rheumatoid arthritis. Arthritis Rheumatol. 2015, 67, 645–655. [Google Scholar] [CrossRef]
- Vehkala, L.; Ukkola, O.; Kesäniemi, Y.A.; Kähönen, M.; Nieminen, M.S.; Salomaa, V.; Jula, A.; Hörkkö, S. Plasma IgA antibody levels to malondialdehyde acetaldehyde-adducts are associated with inflammatory mediators, obesity and type 2 diabetes. Ann. Med. 2013, 45, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Rolla, R.; Vay, D.; Mottaran, E.; Parodi, M.; Traverso, N.; Aricó, S.; Sartori, M.; Bellomo, G.; Klassen, L.W.; Thiele, G.M.; et al. Detection of circulating antibodies against malondialdehyde-acetaldehyde adducts in patients with alcohol-induced liver disease. Hepatology 2000, 31, 878–884. [Google Scholar] [CrossRef]
- Rowley, M.J.; Williamson, D.J.; Mackay, I.R. Evidence for local synthesis of antibodies to denatured collagen in the synovium in rheumatoid arthritis. Arthritis Rheum. 1987, 30, 1420–1425. [Google Scholar] [CrossRef]
- Tarkowski, A.; Klareskog, L.; Carlsten, H.; Herberts, P.; Koopman, W.J. Secretion of antibodies to types I and II collagen by synovial tissue cells in patients with rheumatoid arthritis. Arthritis Rheum. 1989, 32, 1087–1092. [Google Scholar] [CrossRef]
- Rojanasantikul, P.; Pattrapornpisut, P.; Anuruckparadorn, K.; Katchamart, W. The performance of a point of care test for detection of anti-mutated citrullinated vimentin and rheumatoid factor in early rheumatoid arthritis. Clin. Rheumatol. 2014, 33, 919–923. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S.C.; Song, G.G. Diagnostic accuracy of anti-MCV and anti-CCP antibodies in rheumatoid arthritis: A meta-analysis. Z. Rheumatol. 2015, 74, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Degboé, Y.; Constantin, A.; Nigon, D.; Tobon, G.; Cornillet, M.; Schaeverbeke, T.; Chiocchia, G.; Nicaise-Roland, P.; Nogueira, L.; Serre, G.; et al. Predictive value of autoantibodies from anti-CCP2, anti-MCV and anti-human citrullinated fibrinogen tests, in early rheumatoid arthritis patients with rapid radiographic progression at 1 year: Results from the ESPOIR cohort. RMD Open 2015, 1, e000180. [Google Scholar] [CrossRef] [PubMed]
- Svärd, A.; Kastbom, A.; Söderlin, M.K.; Reckner-Olsson, Å.; Skogh, T. A comparison between IgG- and IgA-class antibodies to cyclic citrullinated peptides and to modified citrullinated vimentin in early rheumatoid arthritis and very early arthritis. J. Rheumatol. 2011, 38, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Bläss, S.; Union, A.; Raymackers, J.; Schumann, F.; Ungethüm, U.; Müller-Steinbach, S.; De Keyser, F.; Engel, J.M.; Burmester, G.R. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis Rheum. 2001, 44, 761–771. [Google Scholar] [CrossRef]
- Shoda, H.; Fujio, K.; Shibuya, M.; Okamura, T.; Sumitomo, S.; Okamoto, A.; Sawada, T.; Yamamoto, K. Detection of autoantibodies to citrullinated BiP in rheumatoid arthritis patients and pro-inflammatory role of citrullinated BiP in collagen-induced arthritis. Arthritis Res. Ther. 2011, 13, R191. [Google Scholar] [CrossRef]
- Bodman-Smith, M.D.; Corrigall, V.M.; Berglin, E.; Cornell, H.R.; Tzioufas, A.G.; Mavragani, C.P.; Chan, C.; Rantapää-Dahlqvist, S.; Panayi, G.S. Antibody response to the human stress protein BiP in rheumatoid arthritis. Rheumatology 2004, 43, 1283–1287. [Google Scholar] [CrossRef]
- Shoda, H.; Fujio, K.; Sakurai, K.; Ishigaki, K.; Nagafuchi, Y.; Shibuya, M.; Sumitomo, S.; Okamura, T.; Yamamoto, K. Autoantigen BiP-Derived HLA-DR4 Epitopes Differentially Recognized by Effector and Regulatory T Cells in Rheumatoid Arthritis. Arthritis Rheumatol. 2015, 67, 1171–1181. [Google Scholar] [CrossRef]
- Jiang, X.; Trouw, L.A.; van Wesemael, T.J.; Shi, J.; Bengtsson, C.; Källberg, H.; Malmström, V.; Israelsson, L.; Hreggvidsdottir, H.; Verduijn, W.; et al. Anti-CarP antibodies in two large cohorts of patients with rheumatoid arthritis and their relationship to genetic risk factors, cigarette smoking and other autoantibodies. Ann. Rheum. Dis. 2014, 73, 1761–1768. [Google Scholar] [CrossRef]
- Shi, J.; Knevel, R.; Suwannalai, P.; van der Linden, M.P.; Janssen, G.M.; van Veelen, P.A.; Levarht, N.E.; van der Helm-van Mil, A.H.; Cerami, A.; Huizinga, T.W.; et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc. Natl. Acad. Sci. USA 2011, 108, 17372–17377. [Google Scholar] [CrossRef]
- Shi, J.; van Steenbergen, H.W.; van Nies, J.A.; Levarht, E.W.; Huizinga, T.W.; van der Helm-van Mil, A.H.; Toes, R.E.; Trouw, L.A. The specificity of anti-carbamylated protein antibodies for rheumatoid arthritis in a setting of early arthritis. Arthritis Res. Ther. 2015, 17, 339. [Google Scholar] [CrossRef]
- Brink, M.; Verheul, M.K.; Rönnelid, J.; Berglin, E.; Holmdahl, R.; Toes, R.E.; Klareskog, L.; Trouw, L.A.; Rantapää-Dahlqvist, S. Anti-carbamylated protein antibodies in the pre-symptomatic phase of rheumatoid arthritis, their relationship with multiple anti-citrulline peptide antibodies and association with radiological damage. Arthritis Res. Ther. 2015, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.W.; Trouw, L.A.; Shi, J.; Toes, R.E.; Huizinga, T.W.; Demoruelle, M.K.; Kolfenbach, J.R.; Zerbe, G.O.; Deane, K.D.; Edison, J.D.; et al. Anti-carbamylated protein antibodies are present prior to rheumatoid arthritis and are associated with its future diagnosis. J. Rheumatol. 2015, 42, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Billington, E.O. Drug therapy for osteoporosis in older adults. Lancet 2022, 399, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; San Martin, J.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Leder, B.Z. An Essential Warning. J. Bone Miner. Res. 2018, 33, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Tutaworn, T.; Nieves, J.W.; Wang, Z.; Levin, J.E.; Yoo, J.E.; Lane, J.M. Bone loss after denosumab discontinuation is prevented by alendronate and zoledronic acid but not risedronate: A retrospective study. Osteoporos. Int. 2023, 34, 573–584. [Google Scholar] [CrossRef]
- Leder, B.Z.; Tsai, J.N.; Uihlein, A.V.; Wallace, P.M.; Lee, H.; Neer, R.M.; Burnett-Bowie, S.A. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): Extension of a randomised controlled trial. Lancet 2015, 386, 1147–1155. [Google Scholar] [CrossRef]
- Yue, J.; Griffith, J.F.; Xiao, F.; Shi, L.; Wang, D.; Shen, J.; Wong, P.; Li, E.K.; Li, M.; Li, T.K.; et al. Repair of Bone Erosion in Rheumatoid Arthritis by Denosumab: A High-Resolution Peripheral Quantitative Computed Tomography Study. Arthritis Care Res. 2017, 69, 1156–1163. [Google Scholar] [CrossRef]
- Takeuchi, T.; Tanaka, Y.; Ishiguro, N.; Yamanaka, H.; Yoneda, T.; Ohira, T.; Okubo, N.; Genant, H.K.; van der Heijde, D. Effect of denosumab on Japanese patients with rheumatoid arthritis: A dose-response study of AMG 162 (Denosumab) in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)-a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann. Rheum. Dis. 2016, 75, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Kaneko, Y.; Izumi, K.; Takeuchi, T. Efficacy of denosumab combined with bDMARDs on radiographic progression in rheumatoid arthritis. Jt. Bone Spine 2017, 84, 379–380. [Google Scholar] [CrossRef]
- Padhi, D.; Jang, G.; Stouch, B.; Fang, L.; Posvar, E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J. Bone Miner. Res. 2011, 26, 19–26. [Google Scholar] [CrossRef]
- Molina-Collada, J.; Castrejón, I.; Rivera, J.; Martínez-Barrio, J.; Nieto-González, J.C.; López, K.; Montero, F.; Trives, L.; González, C.; Álvaro-Gracia, J.M. The role of ultrasound and FDG-PET/CT to detect extracranial artery involvement in patients with suspected large vessel vasculitis. Mod. Rheumatol. 2023, 33, 549–556. [Google Scholar] [CrossRef]
- Russell, R.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M.J. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef]
- Recker, R.R.; Weinstein, R.S.; Chesnut, C.H., III; Schimmer, R.C.; Mahoney, P.; Hughes, C.; Bonvoisin, B.; Meunier, P.J. Histomorphometric evaluation of daily and intermittent oral ibandronate in women with postmenopausal osteoporosis: Results from the BONE study. Osteoporos. Int. 2004, 15, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Recker, R.R.; Delmas, P.D.; Halse, J.; Reid, I.R.; Boonen, S.; García-Hernandez, P.A.; Supronik, J.; Lewiecki, E.M.; Ochoa, L.; Miller, P.; et al. Effects of intravenous zoledronic acid once yearly on bone remodeling and bone structure. J. Bone Miner. Res. 2008, 23, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Torres, M.; Reyes-García, R.; Mezquita-Raya, P.; Fernández-García, D.; Alonso, G.; de Dios Luna, J.; Ruiz-Requena, M.E.; Escobar-Jiménez, F. Serum cathepsin K as a marker of bone metabolism in postmenopausal women treated with alendronate. Maturitas 2009, 64, 188–192. [Google Scholar] [CrossRef]
- D’Amelio, P.; Grimaldi, A.; Di Bella, S.; Tamone, C.; Brianza, S.Z.; Ravazzoli, M.G.; Bernabei, P.; Cristofaro, M.A.; Pescarmona, G.P.; Isaia, G. Risedronate reduces osteoclast precursors and cytokine production in postmenopausal osteoporotic women. J. Bone Miner. Res. 2008, 23, 373–379. [Google Scholar] [CrossRef]
- D’Amelio, P.; Grimaldi, A.; Cristofaro, M.A.; Ravazzoli, M.; Molinatti, P.A.; Pescarmona, G.P.; Isaia, G.C. Alendronate reduces osteoclast precursors in osteoporosis. Osteoporos. Int. 2010, 21, 1741–1750. [Google Scholar] [CrossRef]
- Shim, J.H.; Stavre, Z.; Gravallese, E.M. Bone Loss in Rheumatoid Arthritis: Basic Mechanisms and Clinical Implications. Calcif. Tissue Int. 2018, 102, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y. Managing Osteoporosis and Joint Damage in Patients with Rheumatoid Arthritis: An Overview. J. Clin. Med. 2021, 10, 1241. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.; Simon, N.; Steffen, U.; Andes, F.T.; Scholtysek, C.; Müller, D.I.H.; Weidner, D.; Andreev, D.; Kleyer, A.; Culemann, S.; et al. JAK inhibition increases bone mass in steady-state conditions and ameliorates pathological bone loss by stimulating osteoblast function. Sci. Transl. Med. 2020, 12, eaay4447. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Kobayashi, Y.; Uehara, S.; Suzuki, T.; Koide, M.; Yamashita, T.; Nakamura, M.; Takahashi, N.; Kato, H.; Udagawa, N.; et al. A Jak1/2 inhibitor, baricitinib, inhibits osteoclastogenesis by suppressing RANKL expression in osteoblasts in vitro. PLoS ONE 2017, 12, e0181126. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Yamaoka, K.; Kondo, M.; Yamagata, K.; Zhao, J.; Iwata, S.; Tanaka, Y. The JAK inhibitor, tofacitinib, reduces the T cell stimulatory capacity of human monocyte-derived dendritic cells. Ann. Rheum. Dis. 2014, 73, 2192–2198. [Google Scholar] [CrossRef]
- Gaber, T.; Brinkman, A.C.K.; Pienczikowski, J.; Diesing, K.; Damerau, A.; Pfeiffenberger, M.; Lang, A.; Ohrndorf, S.; Burmester, G.R.; Buttgereit, F.; et al. Impact of Janus Kinase Inhibition with Tofacitinib on Fundamental Processes of Bone Healing. Int. J. Mol. Sci. 2020, 21, 865. [Google Scholar] [CrossRef]
- Yari, S.; Kikuta, J.; Shigyo, H.; Miyamoto, Y.; Okuzaki, D.; Furusawa, Y.; Minoshima, M.; Kikuchi, K.; Ishii, M. JAK inhibition ameliorates bone destruction by simultaneously targeting mature osteoclasts and their precursors. Inflamm. Regen. 2023, 43, 18. [Google Scholar] [CrossRef] [PubMed]
- Wollenhaupt, J.; Silverfield, J.; Lee, E.B.; Curtis, J.R.; Wood, S.P.; Soma, K.; Nduaka, C.I.; Benda, B.; Gruben, D.; Nakamura, H.; et al. Safety and efficacy of tofacitinib, an oral janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J. Rheumatol. 2014, 41, 837–852. [Google Scholar] [CrossRef]
- Fleischmann, R.; Kremer, J.; Cush, J.; Schulze-Koops, H.; Connell, C.A.; Bradley, J.D.; Gruben, D.; Wallenstein, G.V.; Zwillich, S.H.; Kanik, K.S.; et al. ORAL Solo Investigators. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N. Engl. J. Med. 2012, 367, 495–507. [Google Scholar] [CrossRef]
- Tracey, D.; Klareskog, L.; Sasso, E.H.; Salfeld, J.G.; Tak, P.P. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol. Ther. 2008, 117, 244–279. [Google Scholar] [CrossRef]
- Henriksen, K.; Bollerslev, J.; Everts, V.; Karsdal, M.A. Osteoclast activity and subtypes as a function of physiology and pathology--implications for future treatments of osteoporosis. Endocr. Rev. 2011, 32, 31–63. [Google Scholar] [CrossRef]
- Shevde, N.K.; Bendixen, A.C.; Dienger, K.M.; Pike, J.W. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc. Natl. Acad. Sci. USA 2000, 97, 7829–7834. [Google Scholar] [CrossRef]
- Oursler, M.J.; Osdoby, P.; Pyfferoen, J.; Riggs, B.L.; Spelsberg, T.C. Avian osteoclasts as estrogen target cells. Proc. Natl. Acad. Sci. USA 1991, 88, 6613–6617. [Google Scholar] [CrossRef] [PubMed]
- Oursler, M.J.; Pederson, L.; Fitzpatrick, L.; Riggs, B.L.; Spelsberg, T. Human giant cell tumors of the bone (osteoclastomas) are estrogen target cells. Proc. Natl. Acad. Sci. USA 1994, 91, 5227–5231. [Google Scholar] [CrossRef] [PubMed]
- Michael, H.; Härkönen, P.L.; Kangas, L.; Väänänen, H.K.; Hentunen, T.A. Differential effects of selective oestrogen receptor modulators (SERMs) tamoxifen, ospemifene and raloxifene on human osteoclasts in vitro. Br. J. Pharmacol. 2007, 151, 384–395. [Google Scholar] [CrossRef]
- Taranta, A.; Brama, M.; Teti, A.; De Luca, V.; Scandurra, R.; Spera, G.; Agnusdei, D.; Termine, J.D.; Migliaccio, S. The selective estrogen receptor modulator raloxifene regulates osteoclast and osteoblast activity in vitro. Bone 2002, 30, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Oursler, M.J.; Pederson, L.; Pyfferoen, J.; Osdoby, P.; Fitzpatrick, L.; Spelsberg, T.C. Estrogen modulation of avian osteoclast lysosomal gene expression. Endocrinology 1993, 132, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Kremer, M.; Judd, J.; Rifkin, B.; Auszmann, J.; Oursler, M.J. Estrogen modulation of osteoclast lysosomal enzyme secretion. J. Cell Biochem. 1995, 57, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Chen, W.; McConnell, M.; Zhu, Z.; Li, S.; Reddy, M.; Eleazer, P.D.; Wang, M.; Li, Y.P. A small molecule, odanacatib, inhibits inflammation and bone loss caused by endodontic disease. Infect. Immun. 2015, 83, 1235–1245. [Google Scholar] [CrossRef]
- Matsumoto, F.; Saitoh, S.; Fukui, R.; Kobayashi, T.; Tanimura, N.; Konno, K.; Kusumoto, Y.; Akashi-Takamura, S.; Miyake, K. Cathepsins are required for Toll-like receptor 9 responses. Biochem. Biophys. Res. Commun. 2008, 367, 693–699. [Google Scholar] [CrossRef]
- Hao, L.; Chen, J.; Zhu, Z.; Reddy, M.S.; Mountz, J.D.; Chen, W.; Li, Y.P. Odanacatib, A Cathepsin K-Specific Inhibitor, Inhibits Inflammation and Bone Loss Caused by Periodontal Diseases. J. Periodontol. 2015, 86, 972–983. [Google Scholar] [CrossRef]
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef]
- Recker, R.; Dempster, D.; Langdahl, B.; Giezek, H.; Clark, S.; Ellis, G.; de Villiers, T.; Valter, I.; Zerbini, C.A.; Cohn, D.; et al. Effects of Odanacatib on Bone Structure and Quality in Postmenopausal Women with Osteoporosis: 5-Year Data from the Phase 3 Long-Term Odanacatib Fracture Trial (LOFT) and its Extension. J. Bone Miner. Res. 2020, 35, 1289–1299. [Google Scholar] [CrossRef]
- Zhao, Z.; Hua, Z.; Luo, X.; Li, Y.; Yu, L.; Li, M.; Lu, C.; Zhao, T.; Liu, Y. Application and pharmacological mechanism of methotrexate in rheumatoid arthritis. Biomed. Pharmacother. 2022, 150, 113074. [Google Scholar] [CrossRef]
- Szalay, B.; Vásárhelyi, B.; Cseh, A.; Tulassay, T.; Deák, M.; Kovács, L.; Balog, A. The impact of conventional DMARD and biological therapies on CD4+ cell subsets in rheumatoid arthritis: A follow-up study. Clin. Rheumatol. 2014, 33, 175–185. [Google Scholar] [CrossRef]
- Xinqiang, S.; Fei, L.; Nan, L.; Yuan, L.; Fang, Y.; Hong, X.; Lixin, T.; Juan, L.; Xiao, Z.; Yuying, S.; et al. Therapeutic efficacy of experimental rheumatoid arthritis with low-dose methotrexate by increasing partially CD4+CD25+ Treg cells and inducing Th1 to Th2 shift in both cells and cytokines. Biomed. Pharmacother. 2010, 64, 463–471. [Google Scholar] [CrossRef]
- Herman, S.; Zurgil, N.; Langevitz, P.; Ehrenfeld, M.; Deutsch, M. Methotrexate selectively modulates TH1/TH2 balance in active rheumatoid arthritis patients. Clin. Exp. Rheumatol. 2008, 26, 317–323. [Google Scholar]
- Cribbs, A.P.; Kennedy, A.; Penn, H.; Amjadi, P.; Green, P.; Read, J.E.; Brennan, F.; Gregory, B.; Williams, R.O. Methotrexate Restores Regulatory T Cell Function Through Demethylation of the FoxP3 Upstream Enhancer in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2015, 67, 1182–1192. [Google Scholar] [CrossRef]
- Zerbini, C.A.F.; Clark, P.; Mendez-Sanchez, L.; Pereira, R.M.R.; Messina, O.D.; Uña, C.R.; Adachi, J.D.; Lems, W.F.; Cooper, C.; Lane, N.E.; et al. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos Int. 2017, 28, 429–446. [Google Scholar] [CrossRef]
- Haugeberg, G.; Conaghan, P.G.; Quinn, M.; Emery, P. Bone loss in patients with active early rheumatoid arthritis: Infliximab and methotrexate compared with methotrexate treatment alone. Explorative analysis from a 12-month randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2009, 68, 1898–1901. [Google Scholar] [CrossRef]
- Kume, K.; Amano, K.; Yamada, S.; Kanazawa, T.; Ohta, H.; Hatta, K.; Amano, K.; Kuwaba, N. The effect of tocilizumab on bone mineral density in patients with methotrexate-resistant active rheumatoid arthritis. Rheumatology 2014, 53, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, J.; Wu, L.; Xu, Y.; Xiao, C.; Yang, X.; Sun, R.; Zhao, J.; Xu, J.; Liu, Q.; et al. CYT387, a JAK-Specific Inhibitor Impedes Osteoclast Activity and Oophorectomy-Induced Osteoporosis via Modulating RANKL and ROS Signaling Pathways. Front. Pharmacol. 2022, 13, 829862. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, F.; Del Giglio, M.; Gisondi, P.; Girolomoni, G. Targeting tumor necrosis factor α in psoriasis and psoriatic arthritis. Expert Opin. Ther. Targets 2008, 12, 1085–1096. [Google Scholar] [CrossRef] [PubMed]


| Cytokines | Sources | Effect on Osteoclast Differentiation | Function in Bone Homeostasis | Ref. |
|---|---|---|---|---|
| IL-4 | Th2 cells | Suppression | Reduce the expression of TRAP and RANKL to inhibit bone resorption. | [126] |
| IL-6 | Th2 cells | Promotion | Boost the expression of M-CSF and RANKL, promoting bone resorption. | [115] |
| IL-7 | Thymic stromal cells | Promotion | Promote RANKL-mediated osteoclast bone resorption. | [101,127] |
| IL-10 | Treg cells Breg cells | Suppression | Downregulate NFATc1, TNF-α, and IL-6 production; induce OPG expression. | [128,129] |
| IL-17 | Th17 cells | Promotion | Promote RANKL expression levels, trigger a cascade of pro-inflammatory responses, and amplify the effects on osteoclasts. | [59,130] |
| IL-35 | Treg cells, B cell | Suppression | Reduce IL-17 levels and indirectly inhibit RANKL expression. | [131,132] |
| TNF-α | Th17 cells, Macrophages | Promotion | Indirectly stimulate osteoclast production through RANKL production by B cells; enhance RANK/RANKL expression and reduce OPG production to induce osteoclast differentiation. | [90,133] |
| IFN-γ | Th1 cells | Suppression | Inhibit RANKL- and TNF-α-induced osteoclast differentiation; stimulate osteoclast apoptosis. | [54,134] |
| RANKL | Th cells | Promotion | Directly activate osteoclast function-related genes through binding to RANKL. | [133] |
| Classification | Drug(s) | Modes of Action and Characteristics | Ref. |
|---|---|---|---|
| RANKL monoclonal antibody | Denosumab | Blocks RANKL activation on the surface of osteoclasts and their precursors and inhibits osteoclast activation and maturation. | [156,158] |
| Romosozumab | Binds with and inhibits sclerostin through Wnt signaling, which has a dual effect on increasing bone formation and decreasing bone resorption. | [164] | |
| Bisphosphonate | Zoledronate | Induces osteoclast apoptosis and reduces systemic RANKL levels. | [167,168] |
| JAK inhibitor | Baricitinib | Reduces RANKL and IL-6 levels; does not affect osteoclast function and activity; decreases RANKL levels produced by T lymphocytes. | [174] |
| JAK inhibitor | Tofacitinib | Reduces the ratio of RANKL/OPG in serum. | |
| JAK inhibitor | CYT387 | Attenuates the formation of osteoclasts; suppresses the bone reabsorption function and the expression and activation of osteoclasts; inhibits the intracellular Ca2+ influx. | [202] |
| TNF-α antagonist | Infliximab | Reduces RANKL expression and osteoclast precursors. | [203] |
| Selective estrogen receptor modulator | Tamoxifen | Inhibits osteoclast occurrence and bone resorption; promotes osteoclast apoptosis. | [166,187] |
| Ctsk inhibitor | Odanacatib | Reduces the bone resorption capacity of osteoclasts but maintains the activities and functions of osteoclasts with less impact on osteoblasts; does not disrupt bone remodeling at the baseline level. | [30] |
| Traditional synthetic DMARD | MTX | Decreases the production of Th1 cells, improves the levels of Th2 cytokines, and reduces the Th17/Treg ratio; osteoclasts are indirectly inhibited. | [197,198] |
| Biologic response modifiers | Infliximab, tocilizumab | Target specific inflammatory mediators to reduce inflammation and indirectly inhibit osteoclasts. | [199] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Zhu, L. Osteoimmunology: The Crosstalk between T Cells, B Cells, and Osteoclasts in Rheumatoid Arthritis. Int. J. Mol. Sci. 2024, 25, 2688. https://doi.org/10.3390/ijms25052688
Yang M, Zhu L. Osteoimmunology: The Crosstalk between T Cells, B Cells, and Osteoclasts in Rheumatoid Arthritis. International Journal of Molecular Sciences. 2024; 25(5):2688. https://doi.org/10.3390/ijms25052688
Chicago/Turabian StyleYang, Mei, and Lei Zhu. 2024. "Osteoimmunology: The Crosstalk between T Cells, B Cells, and Osteoclasts in Rheumatoid Arthritis" International Journal of Molecular Sciences 25, no. 5: 2688. https://doi.org/10.3390/ijms25052688





