Human Macrophages Activate Bystander Neutrophils’ Metabolism and Effector Functions When Challenged with Mycobacterium tuberculosis
Abstract
:1. Introduction
2. Results
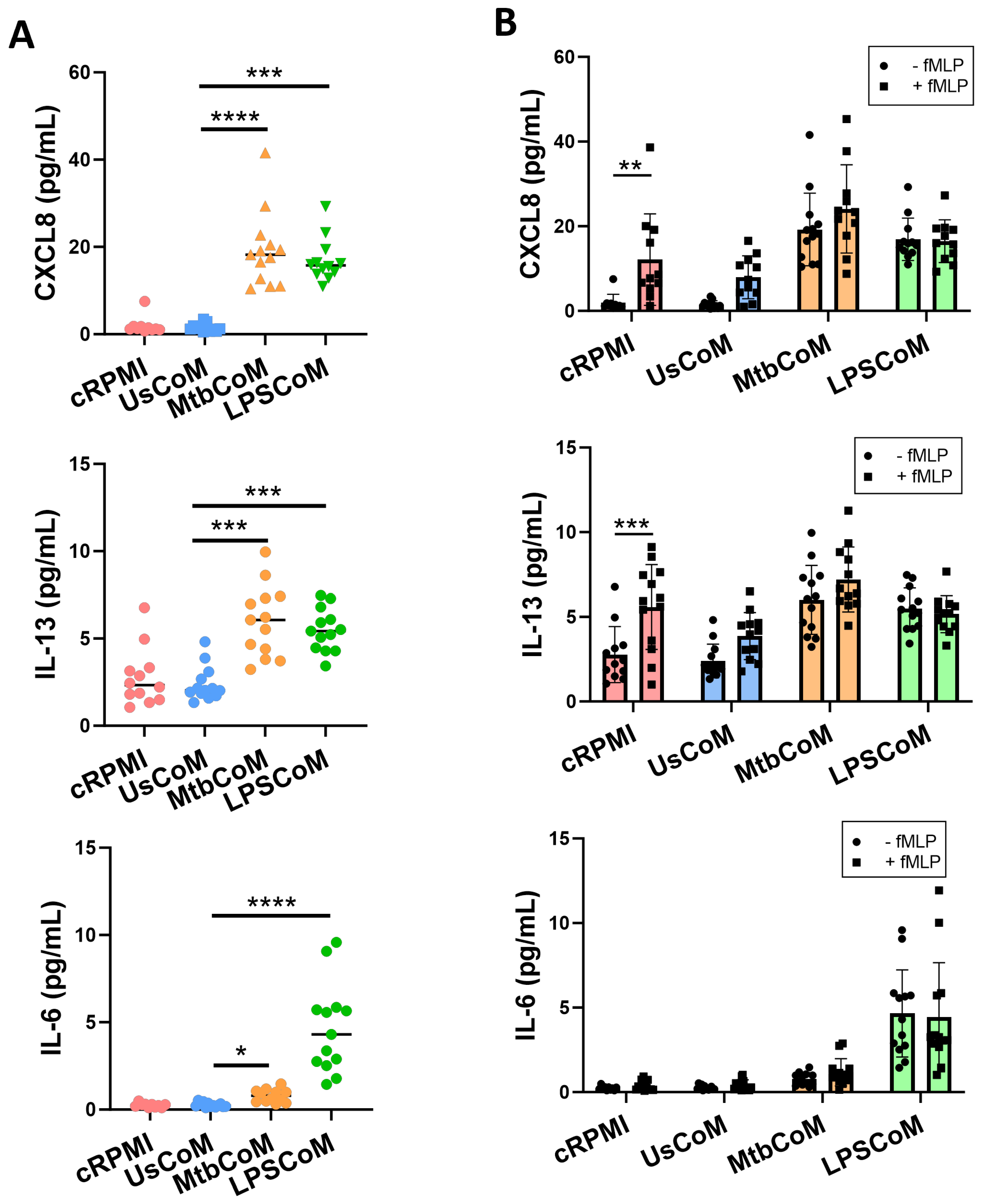
2.1. Mtb- and LPS-Stimulated hMDMs Secrete Cytokines and Chemokines Known to Modulate Neutrophil Biology
2.2. MΦ-CM from Mtb- and LPS-Stimulated hMDMs Activates Neutrophils and Alters Their Migratory Potential
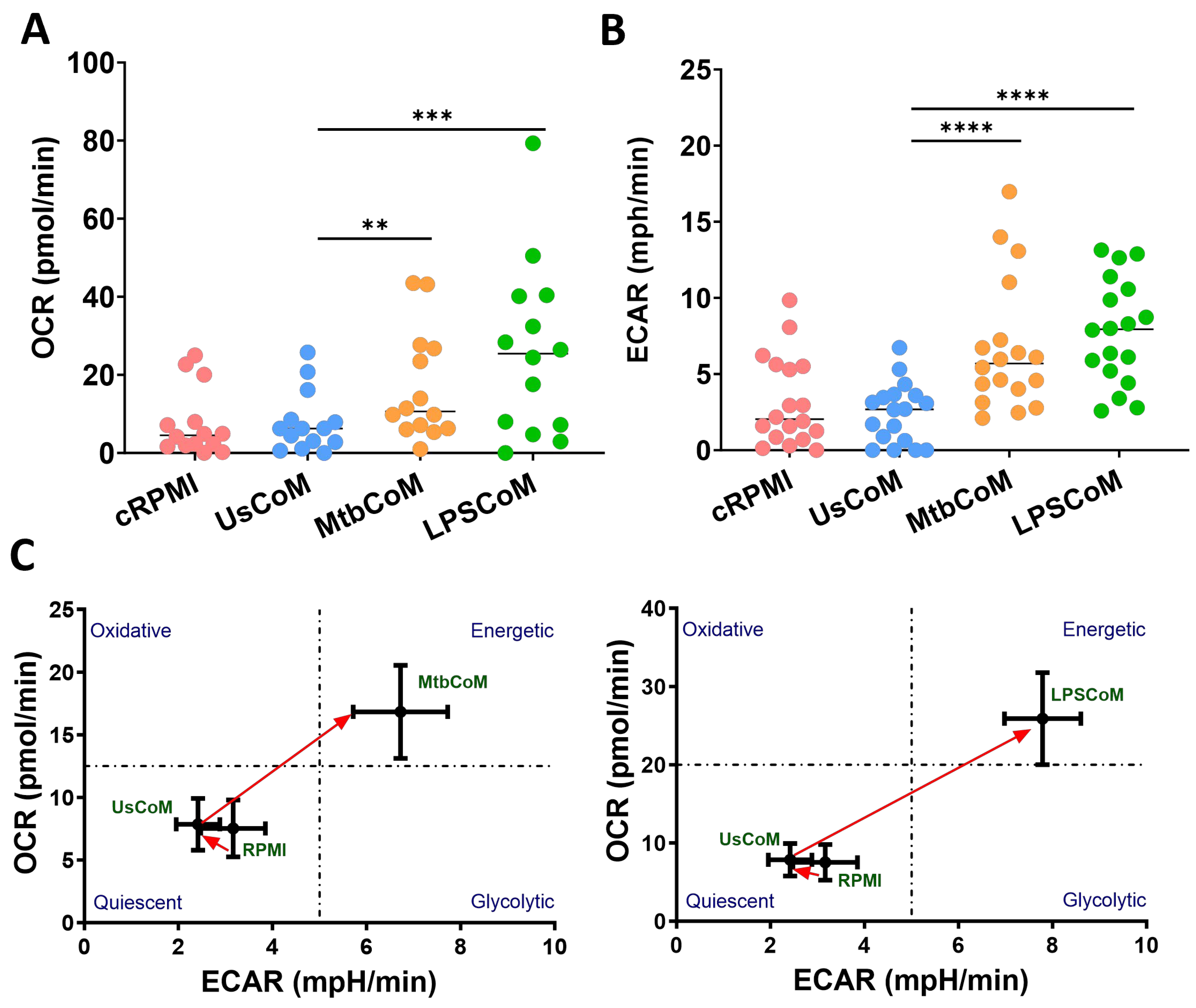
2.3. MΦ-CM from Mtb- and LPS-Stimulated hMDMs Augments Neutrophil Metabolism in Unstimulated and Stimulated Neutrophils
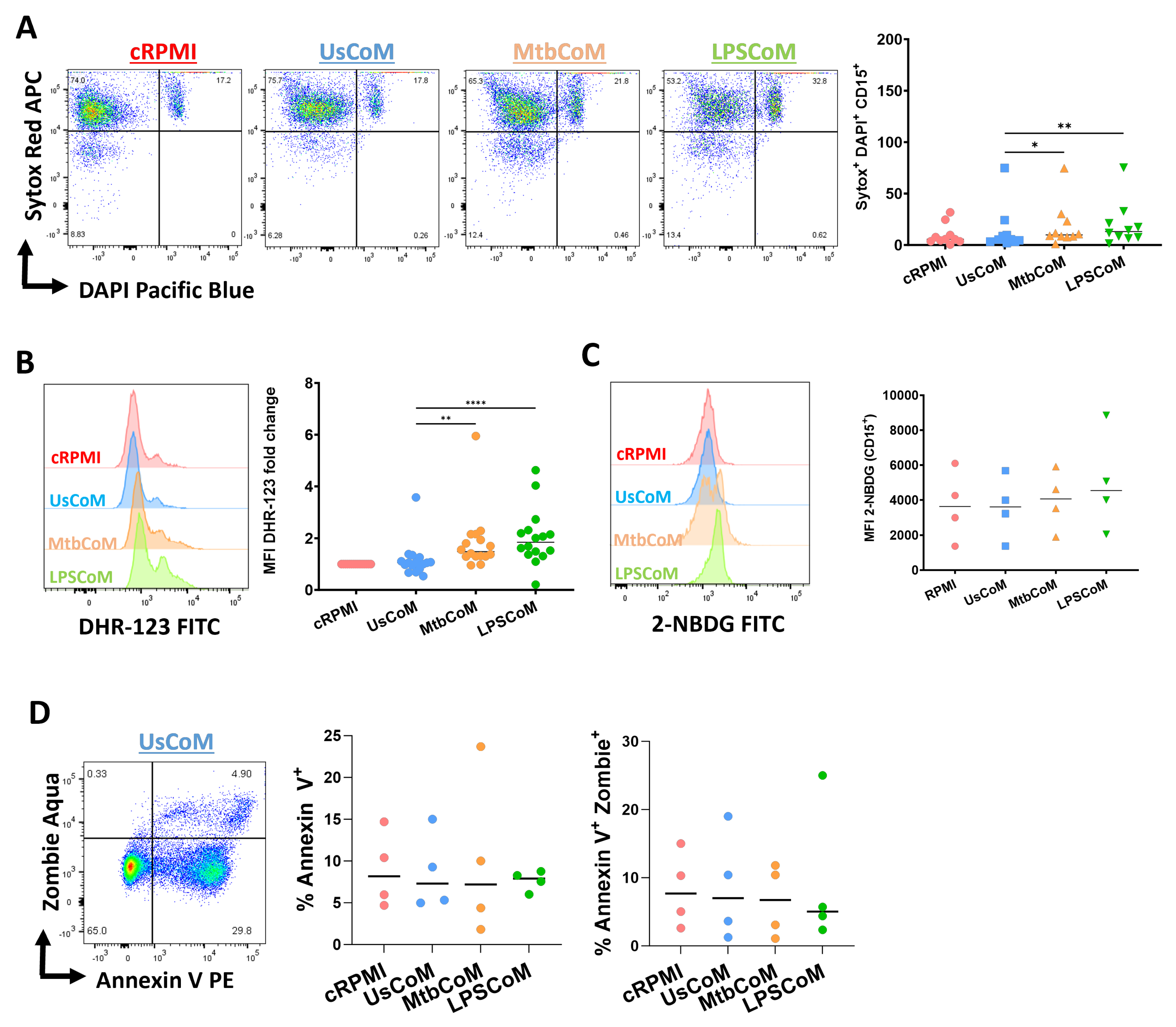
2.4. MtbCoM or LPSCoM Modulates Neutrophil Cytokine Secretion, NETosis, and ROS Production in Neutrophils
3. Discussion
Study Limitations
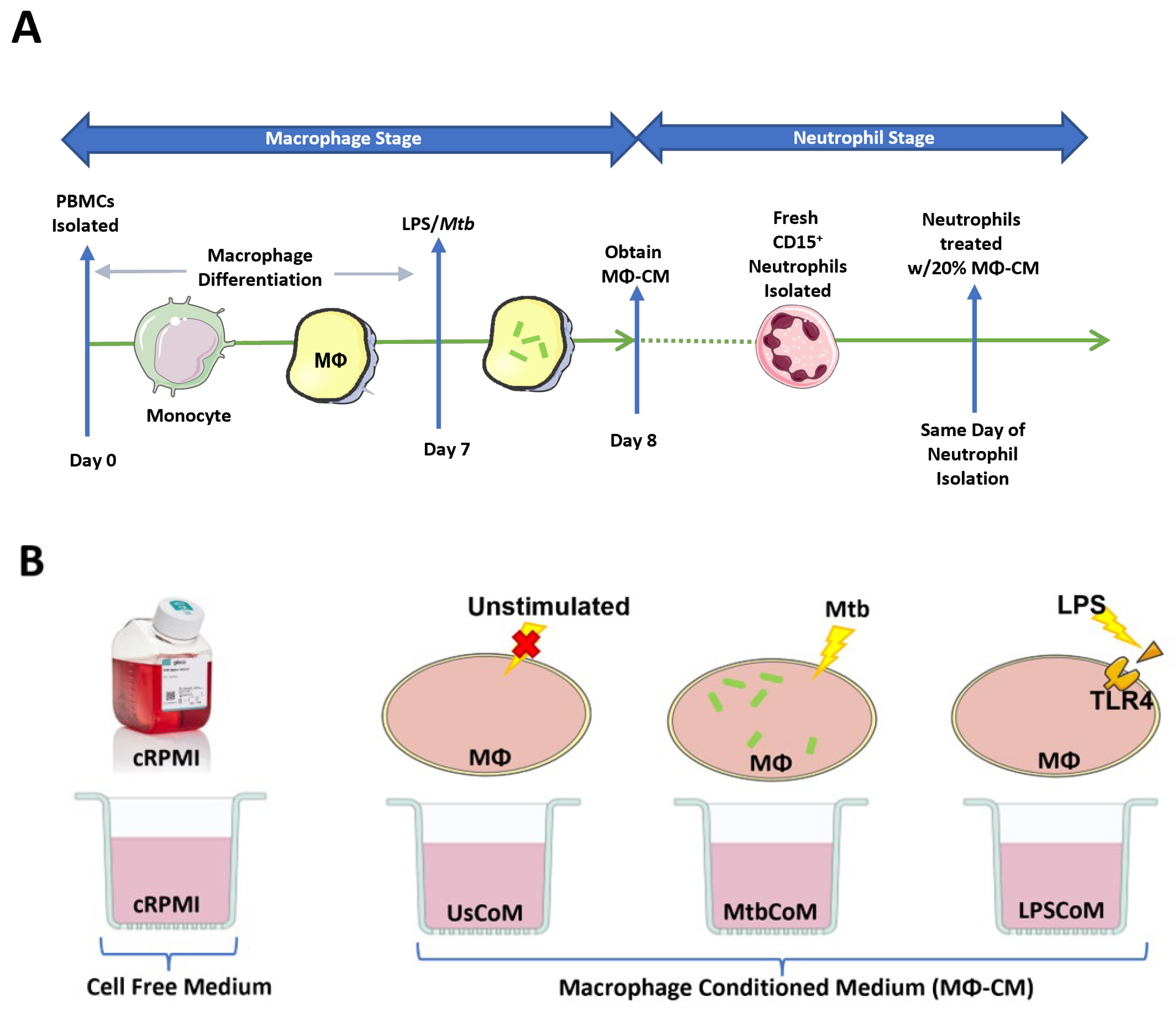
4. Materials and Methods
4.1. Obtaining MΦ-CM for Functional Neutrophil Analysis
4.2. Determination of Cytokine and Chemokine Levels in MΦ-CM in Response to Mtb and LPS Stimulation
4.3. Isolation of Neutrophils
4.4. Neutrophil Conditioning Using MΦ-CM
4.5. Stimulation of Neutrophils with Mtb
4.6. Characterizing the Effect of MΦ-CM on Neutrophil Metabolism Utilizing the Seahorse XFe24 Analyzer
4.7. Characterizing the Effect of MΦ-CM on Neutrophil Effector Functions Using Flow Cytometry
4.8. Neutrophil Transwell Assay and Quantification Using Counting Beads
4.9. Neutrophil Multiplex ELISAs
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Conditioned media abbreviations | |
| TB | Tuberculosis |
| LPS | Lipopolysaccharide |
| Mtb | Mycobacterium tuberculosis |
| hMDM | Human monocyte-derived macrophage |
| CoM | Conditioned media |
| MΦ-CM | Macrophage conditioned medium |
| UsCoM | Conditioned media from unstimulated hMDM |
| MtbCoM | Conditoned media from Mtb-stimulated hMDM |
| LPSCoM | Conditoned media from LPS-stimulated hMDM |
| Other abbreviations | |
| 2-NBDG | 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose |
| ANOVA | Analysis of variance |
| CD | Cluster of differentiation |
| cRPMI | Complete RPMI (RPMI + 10% humn serum) |
| CXCR2 | CXC motif chemokine receptor 2 |
| DAMP | Damage-associated molecular patterns |
| DHR123 | Dihydrorhodamine 123 |
| ECAR | Extracellular acidifcaction rate |
| FBS | Foetal bovine serum |
| fMLP | N-formylmethionine-leucyl-phenylalanine |
| IFN-γ | Interferon gamma |
| iH37Rv | Irradiated Mtb strain H37Rv |
| IL | Interluekin |
| IP-10 | Interferon gamma-induced protein 10 |
| MCP-1 | Human monocyte chemoattractant protein 1 |
| MCP-4 | Human monocyte chemoattractant protein 4 |
| MDC | Macrophage-derived chemokine |
| MFI | Mean fluorescent intensity |
| MIP-1β | Macrophage inflammatory protein 1 beta |
| MOI | Multiplicity of infection |
| NET | Neutrophil extracellular trap |
| OCR | Oxygen consumption rate |
| OXPHOS | Oxidative phosphorylation |
| PAMP | Pathogen-associated molecular patterns |
| PBMC | Peripheral blood mononuclear cell |
| PBS | Phosphate buffered saline |
| ROS | Reactive oxygen species |
| SEM | Standard error of the mean |
| TARC | Thymus- and activation-regulated chemokine |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumour necrosis factor alpha |
References
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Pechous, R.D. With Friends Like These: The Complex Role of Neutrophils in the Progression of Severe Pneumonia. Front. Cell. Infect. Microbiol. 2017, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.M.; Redford, P.S.; Wilkinson, R.J.; O’Garra, A.; Martineau, A.R. Neutrophils in tuberculosis: Friend or foe? Trends Immunol. 2012, 33, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Newton, S.M.; Wilkinson, K.A.; Kampmann, B.; Hall, B.M.; Nawroly, N.; Packe, G.E.; Davidson, R.N.; Griffiths, C.J.; Wilkinson, R.J. Neutrophil-mediated innate immune resistance to mycobacteria. J. Clin. Investig. 2007, 117, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.W.M.; Elkington, P.T.; Brilha, S.; Ugarte-Gil, C.; Tome-Esteban, M.T.; Tezera, L.B.; Pabisiak, P.J.; Moores, R.C.; Sathyamoorthy, T.; Patel, V.; et al. Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis. PLoS Pathog. 2015, 11, e1004917. [Google Scholar] [CrossRef] [PubMed]
- Sawant, K.V.; McMurray, D.N. Guinea Pig Neutrophils Infected with Mycobacterium tuberculosis Produce Cytokines Which Activate Alveolar Macrophages in Noncontact Cultures. Infect. Immun. 2007, 75, 1870–1877. [Google Scholar] [CrossRef]
- Appelberg, R. Mycobacterial Infection Primes T Cells and Macrophages for Enhanced Recruitment of Neutrophils. J. Leukoc. Biol. 1992, 51, 472–477. [Google Scholar] [CrossRef]
- Prame Kumar, K.; Nicholls, A.J.; Wong, C.H.Y. Partners in crime: Neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018, 371, 551–565. [Google Scholar] [CrossRef]
- Cahill, C.; Cox, D.J.; O’connell, F.; Basdeo, S.A.; Gogan, K.M.; Ó’maoldomhnaigh, C.; O’sullivan, J.; Keane, J.; Phelan, J.J. The Effect of Tuberculosis Antimicrobials on the Immunometabolic Profiles of Primary Human Macrophages Stimulated with Mycobacterium tuberculosis. Int. J. Mol. Sci. 2021, 22, 12189. [Google Scholar] [CrossRef]
- Zhao, W.; Fogg, D.K.; Kaplan, M.J. A novel image-based quantitative method for the characterization of NETosis. J. Immunol. Methods 2015, 423, 104–110. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Gouwy, M.; Proost, P. Neutrophil chemoattractant receptors in health and disease: Double-edged swords. Cell. Mol. Immunol. 2020, 17, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, E.; Murphy, D.; Walsh, A.; Connolly, S.; Basdeo, S.A.; Keane, J.; Phelan, J.J. Defining the role of neutrophils in the lung during infection: Implications for tuberculosis disease. Front. Immunol. 2022, 13, 984293. [Google Scholar] [CrossRef] [PubMed]
- Sadiku, P.; Willson, J.A.; Ryan, E.M.; Sammut, D.; Coelho, P.; Watts, E.R.; Grecian, R.; Young, J.M.; Bewley, M.; Arienti, S.; et al. Neutrophils Fuel Effective Immune Responses through Gluconeogenesis and Glycogenesis. Cell Metab. 2021, 33, 1062–1064. [Google Scholar] [CrossRef] [PubMed]
- Burn, G.L.; Foti, A.; Marsman, G.; Patel, D.F.; Zychlinsky, A. The Neutrophil. Immunity 2021, 54, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, S.; Fröhlich, D.; Daniels, S. Characterization of the human fMLP receptor in neutrophils and in Xenopus oocytes. Br. J. Pharmacol. 2002, 135, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Lokuta, M.A.; Huttenlocher, A. TNF-alpha promotes a stop signal that inhibits neutrophil polarization and migration via a p38 MAPK pathway. J. Leukoc. Biol. 2005, 78, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Comen, E.; Wojnarowicz, P.; Seshan, V.E.; Shah, R.; Coker, C.; Norton, L.; Benezra, R. TNF is a key cytokine mediating neutrophil cytotoxic activity in breast cancer patients. NPJ Breast Cancer 2016, 2, 16009. [Google Scholar] [CrossRef]
- Keshari, R.S.; Jyoti, A.; Dubey, M.; Kothari, N.; Kohli, M.; Bogra, J.; Barthwal, M.K.; Dikshit, M. Cytokines induced neutrophil extracellular traps formation: Implication for the inflammatory disease condition. PLoS ONE 2012, 7, e48111. [Google Scholar] [CrossRef]
- Meda, L.; Gasperini, S.; Ceska, M.; Cassatella, M.A. Modulation of proinflammatory cytokine release from human polymorphonuclear leukocytes by gamma interferon. Cell. Immunol. 1994, 157, 448–461. [Google Scholar] [CrossRef]
- Florentin, J.; Zhao, J.; Tai, Y.-Y.; Vasamsetti, S.B.; O’neil, S.P.; Kumar, R.; Arunkumar, A.; Watson, A.; Sembrat, J.; Bullock, G.C.; et al. Interleukin-6 mediates neutrophil mobilization from bone marrow in pulmonary hypertension. Cell. Mol. Immunol. 2021, 18, 374–384. [Google Scholar] [CrossRef]
- Fielding, C.A.; McLoughlin, R.M.; McLeod, L.; Colmont, C.S.; Najdovska, M.; Grail, D.; Ernst, M.; Jones, S.A.; Topley, N.; Jenkins, B.J. IL-6 Regulates Neutrophil Trafficking during Acute Inflammation via STAT3. J. Immunol. 2008, 181, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Mateer, S.W.; Mathe, A.; Bruce, J.; Liu, G.; Maltby, S.; Fricker, M.; Goggins, B.J.; Tay, H.L.; Marks, E.; Burns, G.; et al. IL-6 Drives Neutrophil-Mediated Pulmonary Inflammation Associated with Bacteremia in Murine Models of Colitis. Am. J. Pathol. 2018, 188, 1625–1639. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.L.; Cross, A.L.; Edwards, S.W.; Moots, R.J. Effects of IL-6 and IL-6 blockade on neutrophil function in vitro and in vivo. Rheumatology 2014, 53, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.L.; Selby, P. Clinical applications of tumour necrosis factor. Prog. Growth Factor Res. 1989, 1, 107–122. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.P.; Bald, A.; Cassatella, M.A. Activation of the NF-κB Pathway by Inflammatory Stimuli in Human Neutrophils. Blood 1997, 89, 3421–3433. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Sironi, M.; Vecchi, A.; Colotta, F.; Mantovani, A.; Locati, M. IL-8 induces a specific transcriptional profile in human neutrophils: Synergism with LPS for IL-1 production. Eur. J. Immunol. 2004, 34, 2286–2292. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.N.; Beaman, B.L. Interferon-gamma activation of polymorphonuclear neutrophil function. Immunology 2004, 112, 2–12. [Google Scholar] [CrossRef]
- Borish, L.; Rosenbaum, R.; Albury, L.; Clark, S. Activation of neutrophils by recombinant interleukin 6. Cell. Immunol. 1989, 121, 280–289. [Google Scholar] [CrossRef]
- Netea, M.G.; Simon, A.; van de Veerdonk, F.; Kullberg, B.J.; Van der Meer, J.W.; Joosten, L.A. IL-1beta processing in host defense: Beyond the inflammasomes. PLoS Pathog. 2010, 6, e1000661. [Google Scholar] [CrossRef]
- Prince, L.R.; Allen, L.; Jones, E.C.; Hellewell, P.G.; Dower, S.K.; Whyte, M.K.; Sabroe, I. The role of interleukin-1beta in direct and toll-like receptor 4-mediated neutrophil activation and survival. Am. J. Pathol. 2004, 165, 1819–1826. [Google Scholar] [CrossRef]
- Li, J.; Gyorffy, S.; Lee, S.; Kwok, C.S. Effect of recombinant human interleukin 2 on neutrophil adherence to endothelial cells in vitro. Inflammation 1996, 20, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.X.; Wang, Y.; Liu, Q.; Schramm, R.; Thorlacius, H. CC chemokines induce P-selectin-dependent neutrophil rolling and recruitment in vivo: Intermediary role of mast cells. Br. J. Pharmacol. 2003, 138, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Smalley, D.M.; Ley, K. L-selectin: Mechanisms and physiological significance of ectodomain cleavage. J. Cell. Mol. Med. 2005, 9, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Kazemier, K.M.; Grutters, J.C.; Koenderman, L.; Van den Bosch, V.J. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin. Exp. Immunol. 2009, 115, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Woodfin, A.; Voisin, M.B.; Beyrau, M.; Colom, B.; Caille, D.; Diapouli, F.M.; Nash, G.B.; Chavakis, T.; Albelda, S.M.; Rainger, G.E. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat. Immunol. 2011, 12, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Herbold, W.; Maus, R.; Hahn, I.; Ding, N.; Srivastava, M.; Christman, J.W.; Mack, M.; Reutershan, J.; Briles, D.E.; Paton, J.C. Importance of CXC chemokine receptor 2 in alveolar neutrophil and exudate macrophage recruitment in response to pneumococcal lung infection. Infect. Immun. 2010, 78, 2620–2630. [Google Scholar] [CrossRef] [PubMed]
- Reutershan, J.; Morris, M.A.; Burcin, T.L.; Smith, D.F.; Chang, D.; Saprito, M.S.; Ley, K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J. Clin. Investig. 2006, 116, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Sawant, K.V.; Poluri, K.M.; Dutta, A.K.; Sepuru, K.M.; Troshkina, A.; Garofalo, R.P.; Rajarathnam, K. Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci. Rep. 2016, 6, 33123. [Google Scholar] [CrossRef]
- Li, L.; Xu, L.; Yan, J.; Zhen, Z.-J.; Ji, Y.; Liu, C.-Q.; Lau, W.Y.; Zheng, L.; Xu, J. CXCR2–CXCL1 axis is correlated with neutrophil infiltration and predicts a poor prognosis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 129. [Google Scholar] [CrossRef]
- Cheng, W.-L.; Wang, C.-S.; Huang, Y.-H.; Tsai, M.-M.; Liang, Y.; Lin, K.-H. Overexpression of CXCL1 and its receptor CXCR2 promote tumor invasion in gastric cancer. Ann. Oncol. 2011, 22, 2267–2276. [Google Scholar] [CrossRef]
- Phelan, J.J.; Sheedy, F.J. Phagocyte metabolism: Neutrophils have their cake but don’t eat it. Trends Immunol. 2021, 42, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dikshit, M. Metabolic Insight of Neutrophils in Health and Disease. Front. Immunol. 2019, 10, 2099. [Google Scholar] [CrossRef]
- Heitmann, L.; Abad Dar, M.; Schreiber, T.; Erdmann, H.; Behrends, J.; Mckenzie, A.N.; Brombacher, F.; Ehlers, S.; Hölscher, C. The IL-13/IL-4Rα axis is involved in tuberculosis-associated pathology. J. Pathol. 2014, 234, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Kurdowska, A.N.N.A.; Noble, J.M.; Steinberg, K.P.; Ruzinski, J.T.; Hudson, L.D.; Martin, T.R. Anti-interleukin 8 autoantibody: Interleukin 8 complexes in the acute respiratory distress syndrome. Relationship between the complexes and clinical disease activity. Am. J. Respir. Crit. Care Med. 2001, 163, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Swaroopa, D.; Bhaskar, K.; Mahathi, T.; Katkam, S.; Raju, Y.S.; Chandra, N.; Kutala, V.K. Association of serum interleukin-6, interleukin-8, and Acute Physiology and Chronic Health Evaluation II score with clinical outcome in patients with acute respiratory distress syndrome. Indian J. Crit. Care Med. 2016, 20, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y. Pneumonia, Acute Respiratory Distress Syndrome, and Early Immune-Modulator Therapy. Int. J. Mol. Sci. 2017, 18, 388. [Google Scholar] [CrossRef]
- Zimmermann, M.; Arruda-Silva, F.; Bianchetto-Aguilera, F.; Finotti, G.; Calzetti, F.; Scapini, P.; Lunardi, C.; Cassatella, M.A.; Tamassia, N. IFNα enhances the production of IL-6 by human neutrophils activated via TLR8. Sci. Rep. 2016, 6, 19674. [Google Scholar] [CrossRef] [PubMed]
- Cicco, N.A.; Lindemann, A.; Content, J.; Vandenbussche, P.; Lubbert, M.; Gauss, J.; Mertelsmann, R.; Herrmann, F. Inducible production of interleukin-6 by human polymorphonuclear neutrophils: Role of granulocyte-macrophage colony-stimulating factor and tumor necrosis factor-alpha. Blood 1990, 75, 2049–2052. [Google Scholar] [CrossRef]
- Cassatella, M.A. Neutrophil-Derived Proteins: Selling Cytokines by the Pound. In Advances in Immunology; Dixon, F.J., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 73, pp. 369–509. [Google Scholar]
- Rodríguez-Espinosa, O.; Rojas-Espinosa, O.; Moreno-Altamirano, M.M.B.; López-Villegas, E.O.; Sánchez-García, F.J. Metabolic requirements for neutrophil extracellular traps formation. Immunology 2015, 145, 213–224. [Google Scholar] [CrossRef]
- Kirchner, T.; Möller, S.; Klinger, M.; Solbach, W.; Laskay, T.; Behnen, M. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediat. Inflamm. 2012, 2012, 849136. [Google Scholar] [CrossRef]
- Fossati, G.; Moulding, D.A.; Spiller, D.G.; Moots, R.J.; White, M.R.; Edwards, S.W. The mitochondrial network of human neutrophils: Role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J. Immunol. 2003, 170, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Adrover, J.M.; Carrau, L.; Daßler-Plenker, J.; Bram, Y.; Chandar, V.; Houghton, S.; Redmond, D.; Merrill, J.R.; Shevik, M.; Tenoever, B.R.; et al. Disulfiram inhibits neutrophil extracellular trap formation and protects rodents from acute lung injury and SARS-CoV-2 infection. J. Clin. Investig. 2022, 7, e157342. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, S.; Hug, S.; Stratmann, A.E.P.; Erber, M.; Vidoni, L.; Knapp, C.L.; Thomaß, B.D.; Fauler, M.; Nilsson, B.; Ekdahl, K.N.; et al. Interleukin 8 Elicits Rapid Physiological Changes in Neutrophils That Are Altered by Inflammatory Conditions. J. Innate Immun. 2021, 13, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Lahoz-Beneytez, J.; Elemans, M.; Zhang, Y.; Ahmed, R.; Salam, A.; Block, M.; Niederalt, C.; Asquith, B.; Macallan, D. Human neutrophil kinetics: Modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood 2016, 127, 3431–3438. [Google Scholar] [CrossRef] [PubMed]
- Pillay, J.; Braber, I.D.; Vrisekoop, N.; Kwast, L.M.; de Boer, R.J.; Borghans, J.A.M.; Tesselaar, K.; Koenderman, L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 2010, 116, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, M.C.; Fingerhut, L.; Alfonso-Castro, A.; Mergani, A.; Schwennen, C.; von Köckritz-Blickwede, M.; de Buhr, N. How Long Does a Neutrophil Live?—The Effect of 24 h Whole Blood Storage on Neutrophil Functions in Pigs. Biomedicines 2020, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.T.; Barker, J.H.; Kaufman, J.; Fayram, D.C.; McCracken, J.M.; Allen, L.-A.H. Francisella tularensis Inhibits the Intrinsic and Extrinsic Pathways To Delay Constitutive Apoptosis and Prolong Human Neutrophil Lifespan. J. Immunol. 2012, 188, 3351–3363. [Google Scholar] [CrossRef]
- Mhaonaigh, A.U.; Coughlan, A.M.; Dwivedi, A.; Hartnett, J.; Cabral, J.; Moran, B.; Brennan, K.; Doyle, S.L.; Hughes, K.; Lucey, R.; et al. Low Density Granulocytes in ANCA Vasculitis Are Heterogenous and Hypo-Responsive to Anti-Myeloperoxidase Antibodies. Front. Immunol. 2019, 10, 2603. [Google Scholar] [CrossRef]
- Sabroe, I.; Jones, E.C.; Usher, L.R.; Whyte, M.K.B.; Dower, S.K. Toll-Like Receptor (TLR)2 and TLR4 in Human Peripheral Blood Granulocytes: A Critical Role for Monocytes in Leukocyte Lipopolysaccharide Responses. J. Immunol. 2002, 168, 4701–4710. [Google Scholar] [CrossRef]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, D.M.; Walsh, A.; Stein, L.; Petrasca, A.; Cox, D.J.; Brown, K.; Duffin, E.; Jameson, G.; Connolly, S.A.; O’Connell, F.; et al. Human Macrophages Activate Bystander Neutrophils’ Metabolism and Effector Functions When Challenged with Mycobacterium tuberculosis. Int. J. Mol. Sci. 2024, 25, 2898. https://doi.org/10.3390/ijms25052898
Murphy DM, Walsh A, Stein L, Petrasca A, Cox DJ, Brown K, Duffin E, Jameson G, Connolly SA, O’Connell F, et al. Human Macrophages Activate Bystander Neutrophils’ Metabolism and Effector Functions When Challenged with Mycobacterium tuberculosis. International Journal of Molecular Sciences. 2024; 25(5):2898. https://doi.org/10.3390/ijms25052898
Chicago/Turabian StyleMurphy, Dearbhla M., Anastasija Walsh, Laura Stein, Andreea Petrasca, Donal J. Cox, Kevin Brown, Emily Duffin, Gráinne Jameson, Sarah A. Connolly, Fiona O’Connell, and et al. 2024. "Human Macrophages Activate Bystander Neutrophils’ Metabolism and Effector Functions When Challenged with Mycobacterium tuberculosis" International Journal of Molecular Sciences 25, no. 5: 2898. https://doi.org/10.3390/ijms25052898
APA StyleMurphy, D. M., Walsh, A., Stein, L., Petrasca, A., Cox, D. J., Brown, K., Duffin, E., Jameson, G., Connolly, S. A., O’Connell, F., O’Sullivan, J., Basdeo, S. A., Keane, J., & Phelan, J. J. (2024). Human Macrophages Activate Bystander Neutrophils’ Metabolism and Effector Functions When Challenged with Mycobacterium tuberculosis. International Journal of Molecular Sciences, 25(5), 2898. https://doi.org/10.3390/ijms25052898







