Flexible Cotton Fabric-Based Ionizing Radiation Dosimeter for 2D Dose Distribution Measurements over a Wide Dose Range at High Dose Rates
Abstract
:1. Introduction
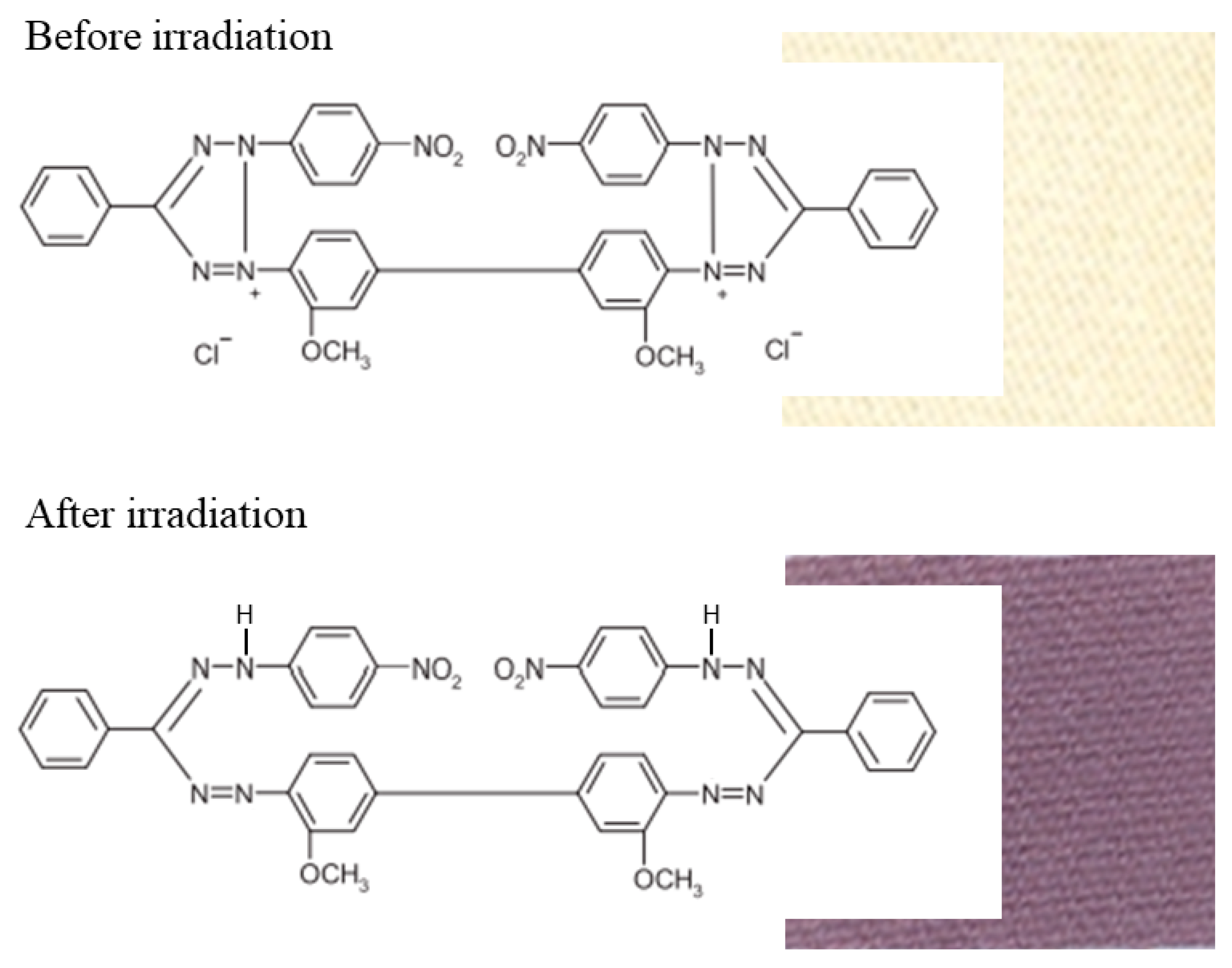
2. Results and Discussion
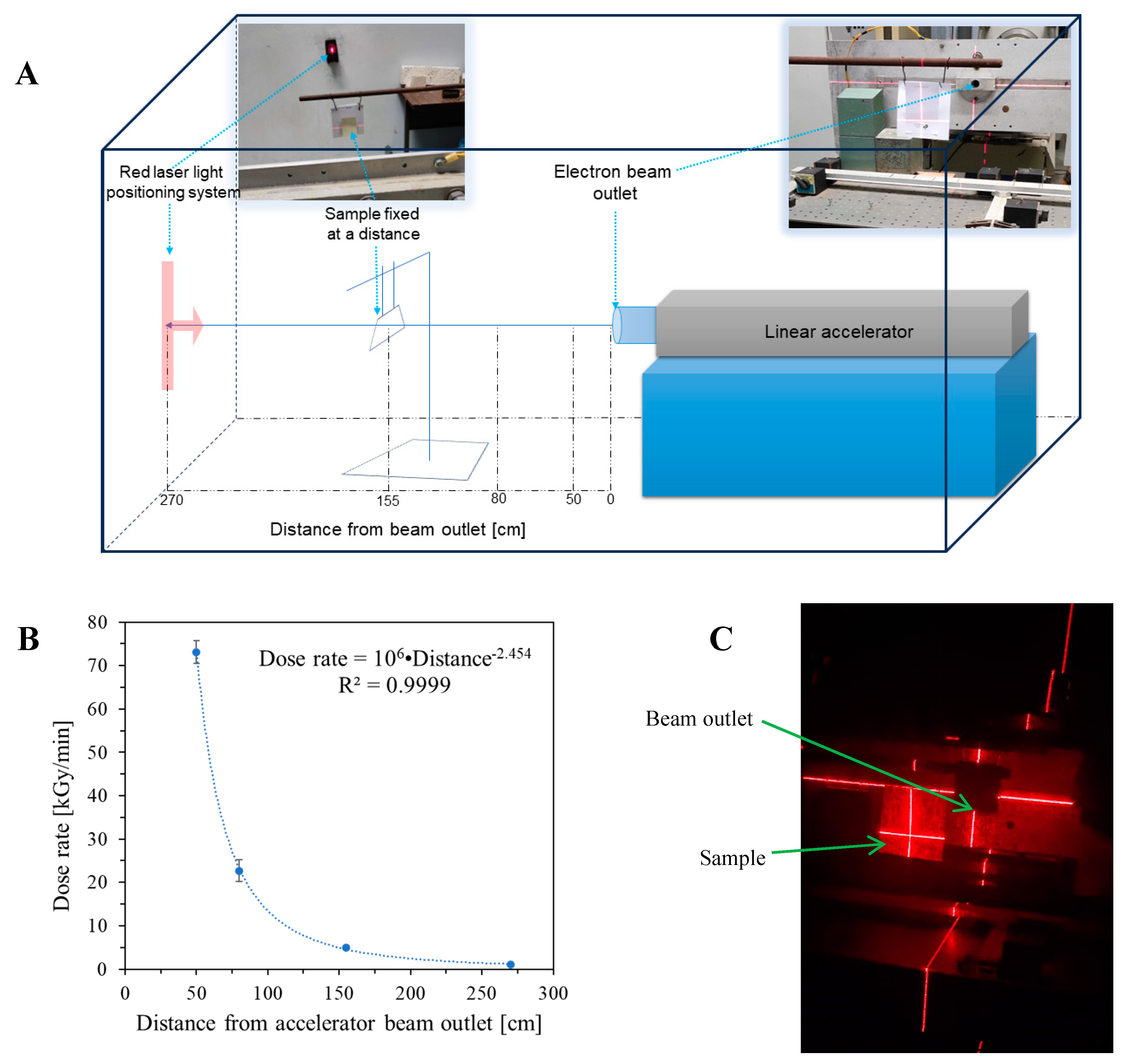
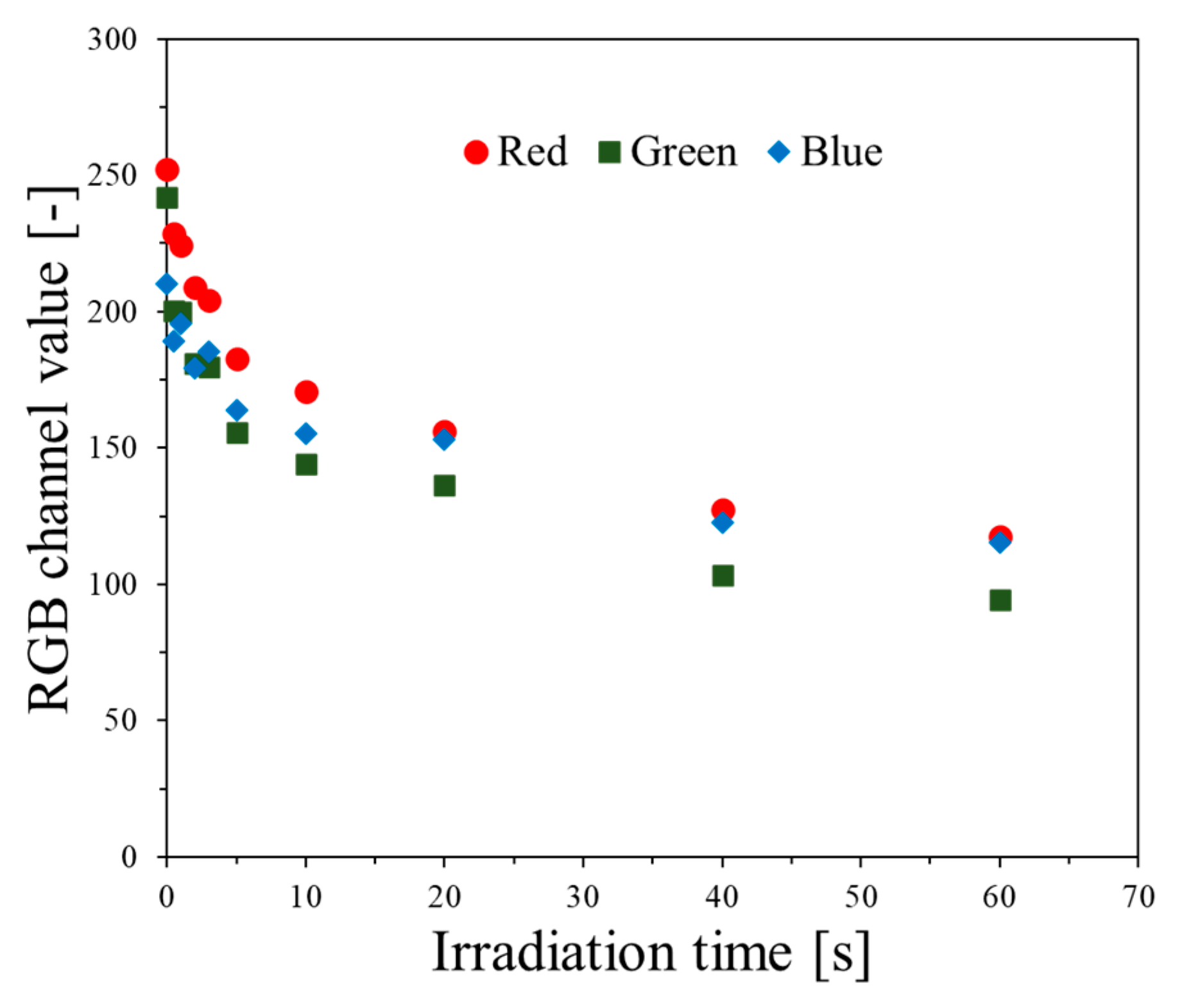
2.1. Impact of Dose and Dose Rate
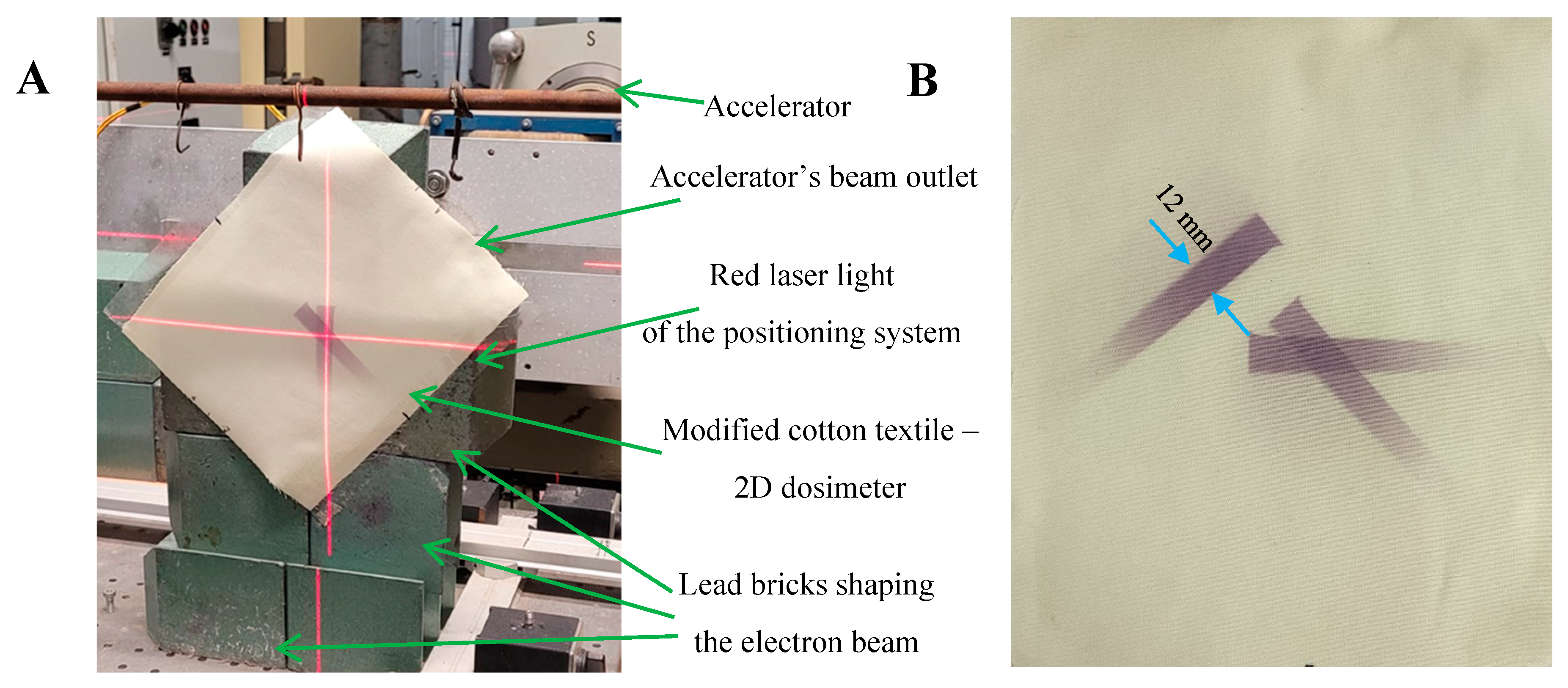
2.2. 2D Dose Distribution
3. Materials and Methods
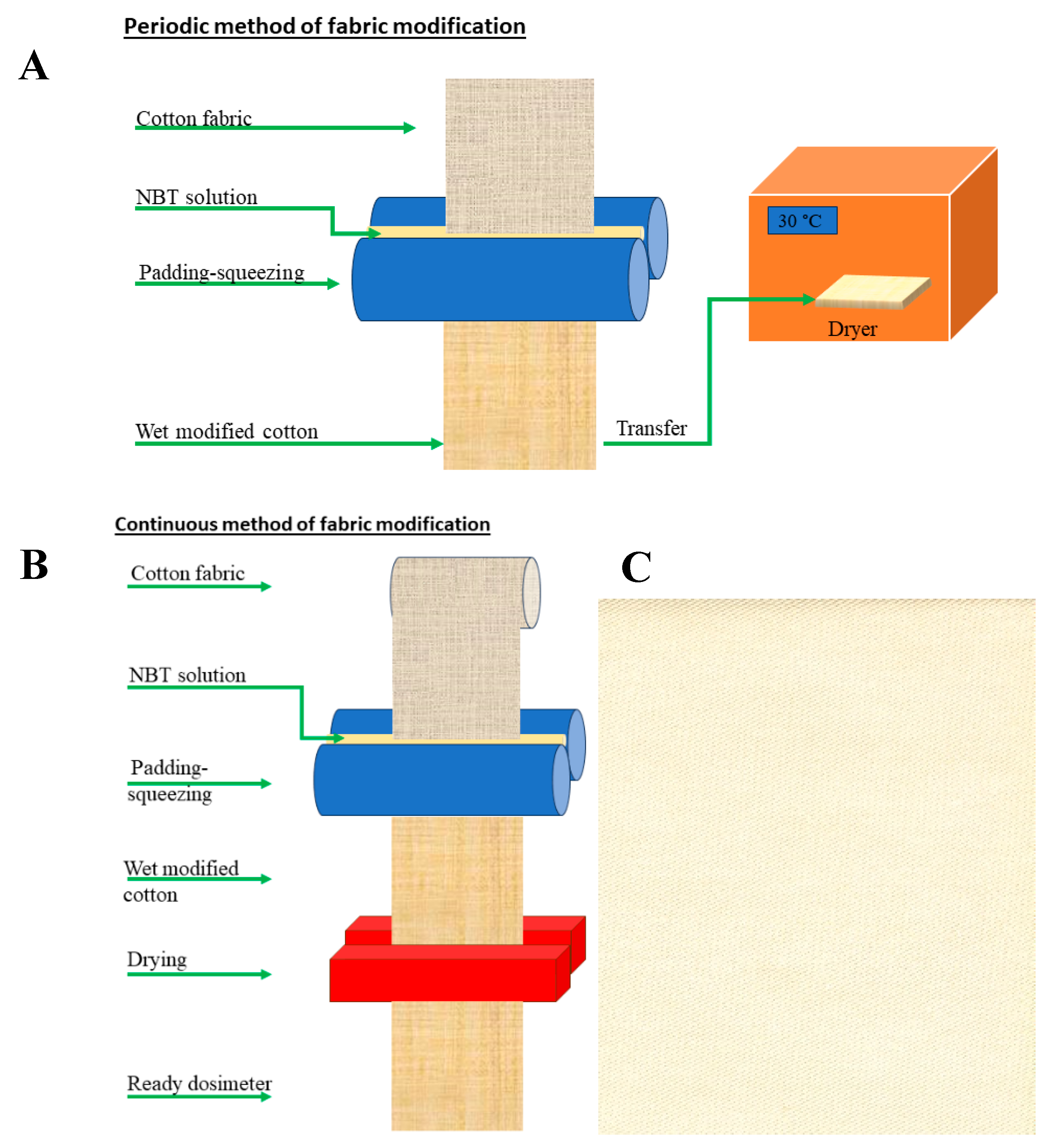
3.1. Preparation of Samples
3.2. Irradiation
3.3. 2D Measurements
3.4. Data Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | two dimensional |
| 3D | three dimensional |
| CT | computed tomography |
| dpi | dots per inch |
| HP | Hewlett-Packard |
| NBT | nitrotetrazolium blue chloride |
| RGB | Red-green-blue |
| TTC | 2,3,5-triphenyltetrazolium chloride |
| UV | ultraviolet radiation |
References
- Niroomand-Rad, A.; Blackwell, C.R.; Coursey, B.M.; Gall, K.P.; Galvin, J.M.; McLaughlin, W.L.; Meigooni, A.S.; Nath, R.; Rodgers, J.E.; Soares, C.G. Radiochromic film dosimetry: Recommendations of AAPM radiation therapy committee task group 55. Med. Phys. 1998, 25, 2093–2115. [Google Scholar] [CrossRef]
- McLaughlin, W.L. Radiation chemistry of anionic disazo dyes in cellophane films applications for high-dose dosimetry. Radiat. Phys. Chem. 2003, 67, 561–567. [Google Scholar] [CrossRef]
- Kriminaskaya, Z.K.; Konarev, A.A.; Dyumaev, K.M.; Tolkachev, B.V.; Pikaev, A.K. Radiolysis of aqueous solutions of tetrazolium salts. Russ. Chem. Bull. 1988, 37, 1555–1559. [Google Scholar] [CrossRef]
- Kovács, A.; Baranyai, M.; Wojnárovits, L.; Moussa, A.; Othman, I.; McLaughlin, W.L. Aqueous-ethanol nitro blue tetrazolium solutions for high dose dosimetry. Radiat. Phys. Chem. 1999, 55, 799–803. [Google Scholar] [CrossRef]
- Kovács, A.; Wojnárovits, L.; Baranyai, M.; Moussa, A.; Othman, I.; McLaughlin, W.L. Radiolytic reactions of nitro blue tetrazolium under oxidative and reductive conditions: A pulse radiolysis study. Radiat. Phys. Chem. 1999, 55, 795–798. [Google Scholar] [CrossRef]
- Kovács, A.; Wojnárovits, L.; El-Assy, N.B.; Afeefy, H.Y.; Al-Sheikhly, M.; Walker, M.L.; McLaughlin, W.L. Alcohol solutions of triphenyl-tetrazolium chloride as high-dose radiochromic dosimeters. Radiat. Phys. Chem. 1995, 46, 1217–1225. [Google Scholar] [CrossRef]
- Kovács, A.; Wojnárovits, L.; Pálfi, T.; Emi-Reynolds, G.; Fletcher, J. Pulse radiolysis of tetrazolium violet in aqueous and aqueous-alcoholic solutions under oxidative and reductive conditions. Radiat. Phys. Chem. 2008, 77, 1088–1092. [Google Scholar] [CrossRef]
- Kovács, A.; Wojnárovits, L.; McLaughlin, W.L.; Ebrahim Eid, S.E.; Miller, A. Radiation-chemical reaction of 2,3,5-triphenyl-tetrazolium chloride in liquid and solid state. Radiat. Phys. Chem. 1996, 47, 483–486. [Google Scholar] [CrossRef]
- Kovács, A.; Baranyai, M.; Wojnárovits, L.; Slezsák, I.; McLaughlin, W.L.; Miller, A.; Moussa, A. Dose determination with nitro blue tetrazolium containing radiochromic dye films by measuring absorbed and reflected light. Radiat. Phys. Chem. 2000, 57, 711–716. [Google Scholar] [CrossRef]
- Ebraheem, S.; El-Kelany, M. Dosimeter film based on ethyl violet-bromophenol blue dyed poly(vinyl alcohol). J. Polym. Chem. 2013, 3, 1–5. [Google Scholar] [CrossRef]
- Pikaev, A.K.; Kriminskaya, Z.K. Use of tetrazolium salts in dosimetry of ionizing radiation. Radiat. Phys. Chem. 1998, 52, 555–561. [Google Scholar] [CrossRef]
- Basfar, A.A.; Rabaeh, K.A.; Moussa, A.A.; Msalam, R.I. Dosimetry characterization of nitro-blue tetrazolium polyvinylbutyral films for radiation processing. Radiat. Phys. Chem. 2011, 80, 763–766. [Google Scholar] [CrossRef]
- Al Zahrany, A.A.; Rabaeh, K.A.; Basfar, A.A. Radiation-induced color bleaching of methyl red in polyvinyl butyral film dosimeter. Radiat. Phys. Chem. 2011, 80, 1263–1267. [Google Scholar] [CrossRef]
- Butson, M.J.; Yu, P.K.N.; Cheung, T.; Metcalfe, P. Radiochromic film for medical radiation dosimetry. Mater. Sci. Eng. 2003, 41, 61–120. [Google Scholar] [CrossRef]
- Kozicki, M.; Maras, P. Features of 2Day.QA® as a 2D radiation dosimeter. Phys. Medica 2022, 104, 23–31. [Google Scholar] [CrossRef]
- Fuss, M.; Sturtewagen, E.; De Wagte, C.; Georg, D. Dosimetric characterization of GafChromic EBT film and its implication on film dosimetry quality assurance. Phys. Med. Biol. 2007, 52, 4211–4225. [Google Scholar] [CrossRef]
- Devic, S. Radiochromic film dosimetry: Past, present, and future. Phys. Medica 2011, 27, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Chiu-Tsao, S.T.; Napoli, J.J.; Davis, S.D.; Hanley, J.; Rivard, M.J. Dosimetry for 131Cs and 125I seeds in solid water phantom using radiochromic EBT film. Appl. Radiat. Isotopes 2014, 92, 102–114. [Google Scholar] [CrossRef]
- Méndez, I.; Šljivic, Z.; Hudej, R.; Jenko, A.; Casar, V. Grid patterns, spatial inter-scan variations and scanning reading repeatability in radiochromic film dosimetry. Phys. Medica 2016, 32, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Devic, S.; Tomic, N.; Lewis, D. Reference radiochromic film dosimetry: Review of technical aspects. Phys. Medica 2016, 32, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Kłosowski, M.; Liszka, M.; Kopec, R.; Bilski, P.; Kedzierska, D. Dosimetric properties and stability of thermoluminescent foils made from LiF: Mg, Cu, P or CaSO4: Dy during long-term use. Radiat. Phys. Chem. 2014, 104, 212–215. [Google Scholar] [CrossRef]
- Palmer, A.L.; Bradley, D.; Nisbet, A. Evaluation and implementation of triple-channel radiochromic film dosimetry in brachytherapy. J. Appl. Clin. Med. Phys. 2014, 15, 280–296. [Google Scholar] [CrossRef]
- Lagendijk, J.J.W.; Raaymakers, B.W.; Raaijmakers, A.J.E.; Overweg, J.; Brown, K.J.; Kerkhof, E.M.; van der Put, R.W.; Hårdemark, B.; van Vulpen, M.; van der Heide, U.A. MRI/linac integration. Radiother. Oncol. 2008, 86, 25–29. [Google Scholar] [CrossRef]
- Fallone, B.G.; Murray, B.; Rathee, S.; Stanescu, T.; Steciw, S.; Vidakovic, S.; Blosser, E.; Tymofichuk, D. First MR images obtained during megavoltage photon irradiation from a prototype integrated linac-MR system. Med. Phys. 2009, 36, 2084–2088. [Google Scholar] [CrossRef] [PubMed]
- Keall, P.J.; Barton, M.; Crozier, S. The Australian magnetic resonance imaging–linac program. Semin. Radiat. Oncol. 2014, 24, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Klavsen, M.F.; Ankjærgaard, C.; Behrens, C.P.; Vogelius, I.R.; Boye, K.; Hvass Hansen, R.; Andersen, C.E. Time-resolved plastic scintillator dosimetry in MR linear accelerators without image distortion. Radiat. Meas. 2022, 154, 106759. [Google Scholar] [CrossRef]
- Bertuccio, G.; Puglisi, D.; Macera, D.; Di Liberto, R.; Lamborizio, M.; Mantovani, L. Silicon Carbide Detectors for in vivo Dosimetry. IEEE Trans. Nucl. Sci. 2014, 61, 961–966. [Google Scholar] [CrossRef]
- Pettinato, S.; Girolami, M.; Olivieri, R.; Stravato, A.; Caruso, C.; Salvatori, S. A Diamond-Based Dose-per-Pulse X-ray Detector for Radiation Therapy. Materials 2021, 14, 5203. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Sasiadek, E. Textile, UV detector with 2,3,5-triphenyltetrazolium chloride as an active compound. Radiat. Meas. 2011, 46, 510–526. [Google Scholar] [CrossRef]
- Kozicki, M.; Sasiadek, E. UV dosimeter based on polyamide woven fabric and nitro blue tetrazolium chloride as an active compound. Radiat. Meas. 2011, 46, 1123–1137. [Google Scholar] [CrossRef]
- Kozicki, M.; Sąsiadek, E. Polyamide woven fabrics with 2,3,5-triphenyltetrazoluim chloride or nitro blue tetrazolium chloride as 2D ionizing radiation dosimeters. Radiat. Meas. 2012, 47, 614–621. [Google Scholar] [CrossRef]
- Sąsiadek, E.; Andrzejczak, R.; Kozicki, M. The importance of fabric structure in the construction of 2D textile radiation dosimeters. Radiat. Meas. 2012, 47, 622–627. [Google Scholar] [CrossRef]
- Kozicki, M.; Sąsiadek, E. Scanning of flat textile-based radiation dosimeters: Influence of parameters on the quality of results. Radiat. Measur. 2013, 58, 87–93. [Google Scholar] [CrossRef]
- Kozicki, M.; Sasiadek, E.; Karbownik, I.; Maniukiewicz, W. Doped polyacrylonitrile fibres as UV radiation sensors. Sensor. Actuators B-Chem. 2015, 213, 234–243. [Google Scholar] [CrossRef]
- Kozicki, M.; Sąsiadek, E.; Kadlubowski, S.; Dudek, M.; Karbownik, I. Radiation sensitive polyacrylonitrile microfibres doped with PDA nanoparticles. Radiat. Phys. Chem. 2020, 169, 107751. [Google Scholar] [CrossRef]
- Vujcic, I.; Masic, S.; Medic, M.; Milicevic, B.; Dramicanin, M. The influence of gamma irradiation on the color change of wool, linen, silk, and cotton fabrics used in cultural heritage artifacts. Radiat. Phys. Chem. 2019, 156, 307–313. [Google Scholar] [CrossRef]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer gel dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef] [PubMed]
- Trapp, J.V.; Michael, G.; Evans, P.M.; Baldock, C.; Leach, C.; Webb, S. Dose resolution in gel dosimetry: Effect of uncertainty in the calibration function. Phys. Med. Biol. 2004, 49, N139–N146. [Google Scholar] [CrossRef]
- Maras, P.; Jaszczak, M.; Kozicki, M. Basic features of VIC-T dosimeter with spiral CT readout. CT scanning conditions and data processing with a new polyGeVero-CT software package. Radiat. Phys. Chem. 2021, 189, 109730. [Google Scholar] [CrossRef]
- Szucs, P.; Civan, F.; Virag, M. Applicability of the most frequent value method in groundwater modelling. Hydrogeol. J. 2006, 14, 31–43. [Google Scholar] [CrossRef]
- Kozicki, M.; Maras, P.; Karwowski, A.C. Software for 3D radiotherapy dosimetry. Validation. Phys. Med. Biol. 2014, 59, 4111–4136. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozicki, M.; Sąsiadek-Andrzejczak, E.; Wach, R.; Maras, P. Flexible Cotton Fabric-Based Ionizing Radiation Dosimeter for 2D Dose Distribution Measurements over a Wide Dose Range at High Dose Rates. Int. J. Mol. Sci. 2024, 25, 2916. https://doi.org/10.3390/ijms25052916
Kozicki M, Sąsiadek-Andrzejczak E, Wach R, Maras P. Flexible Cotton Fabric-Based Ionizing Radiation Dosimeter for 2D Dose Distribution Measurements over a Wide Dose Range at High Dose Rates. International Journal of Molecular Sciences. 2024; 25(5):2916. https://doi.org/10.3390/ijms25052916
Chicago/Turabian StyleKozicki, Marek, Elżbieta Sąsiadek-Andrzejczak, Radosław Wach, and Piotr Maras. 2024. "Flexible Cotton Fabric-Based Ionizing Radiation Dosimeter for 2D Dose Distribution Measurements over a Wide Dose Range at High Dose Rates" International Journal of Molecular Sciences 25, no. 5: 2916. https://doi.org/10.3390/ijms25052916







