High-Dose Fenofibrate Stimulates Multiple Cellular Stress Pathways in the Kidney of Old Rats
Abstract
1. Introduction
2. Results
2.1. Fenofibrate Treatment Increased the Collagenous Tissue Area in Old Rats and Mildly Affected Kidney Function Parameters in Both Age Groups
2.2. Low and High Doses of Fenofibrate Differently Affected the Expression of Stress-Associated Genes
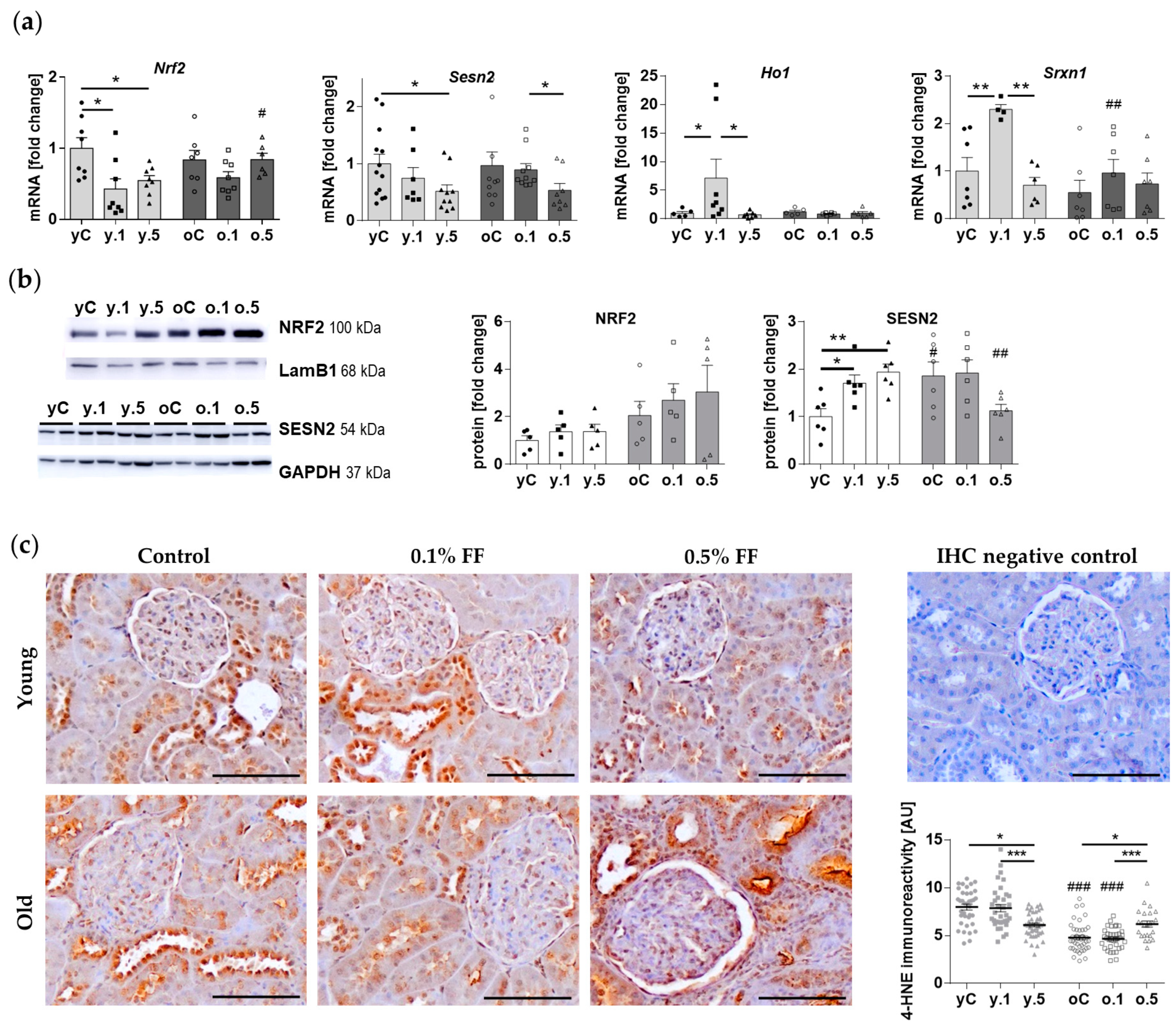
2.3. Fenofibrate Activated Components of Cellular Antioxidant Defences in the Kidney of Young but Not Old Animals
2.4. Fenofibrate Modulated the Expression of Factors Involved in Hypoxia and Osmotic Stress Signalling in a Similar Manner in Young and Old Rats
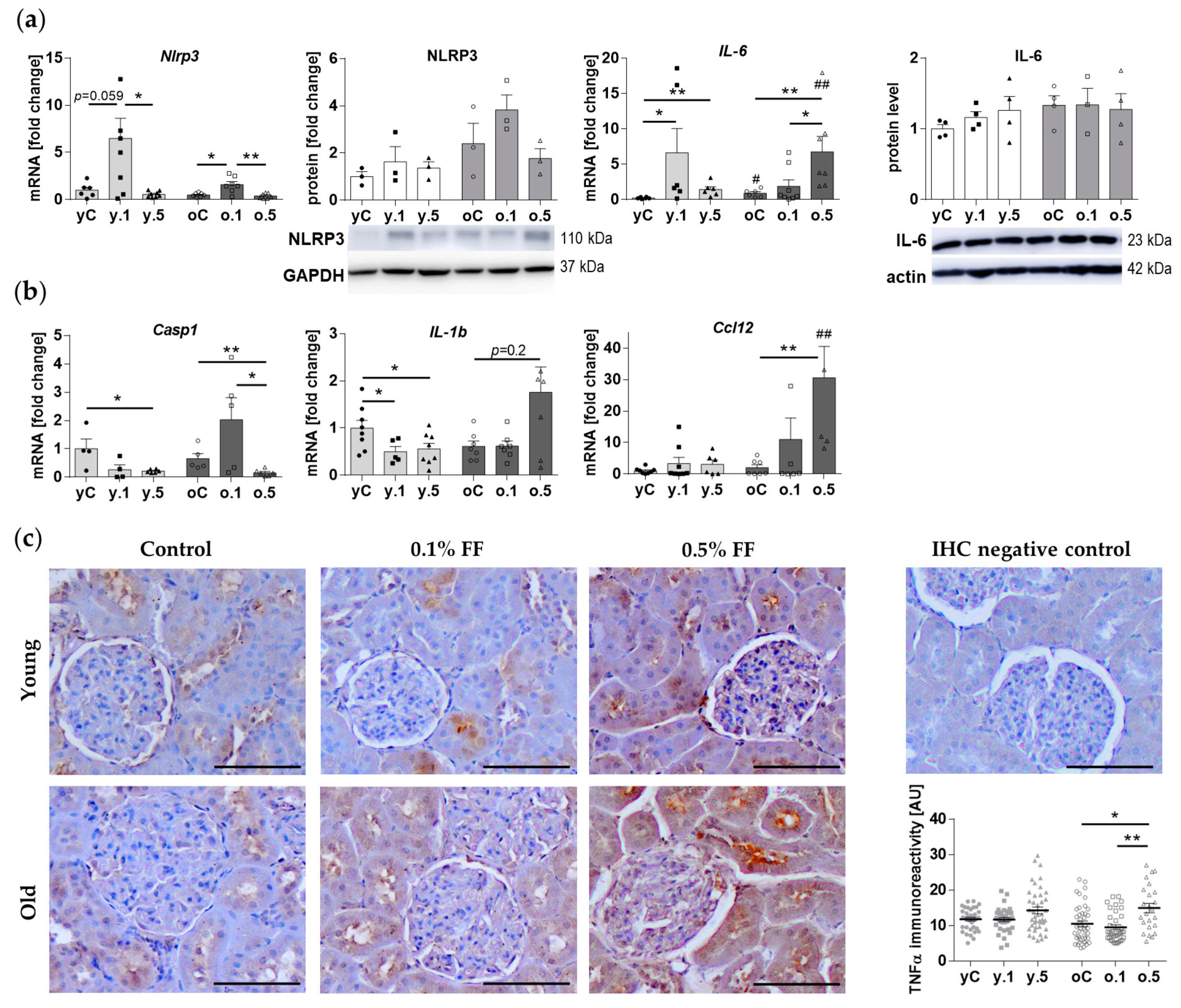
2.5. Inflammatory Response Genes Were Differently Affected by Fenofibrate in Young and Old Rats
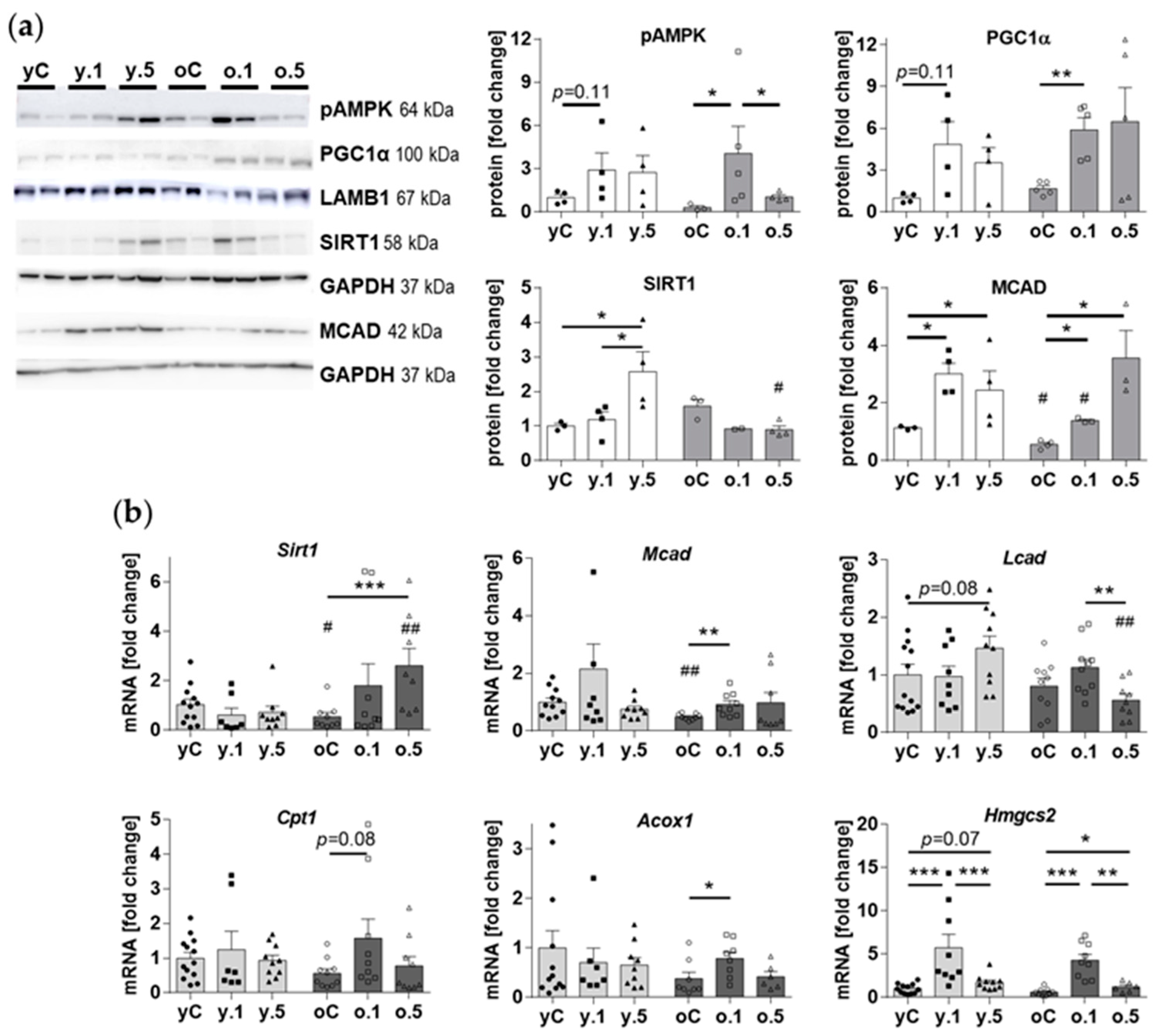
2.6. Effects of Fenofibrate on Kidney Nutrient-Sensing and Metabolic Effectors
3. Discussion
4. Materials and Methods
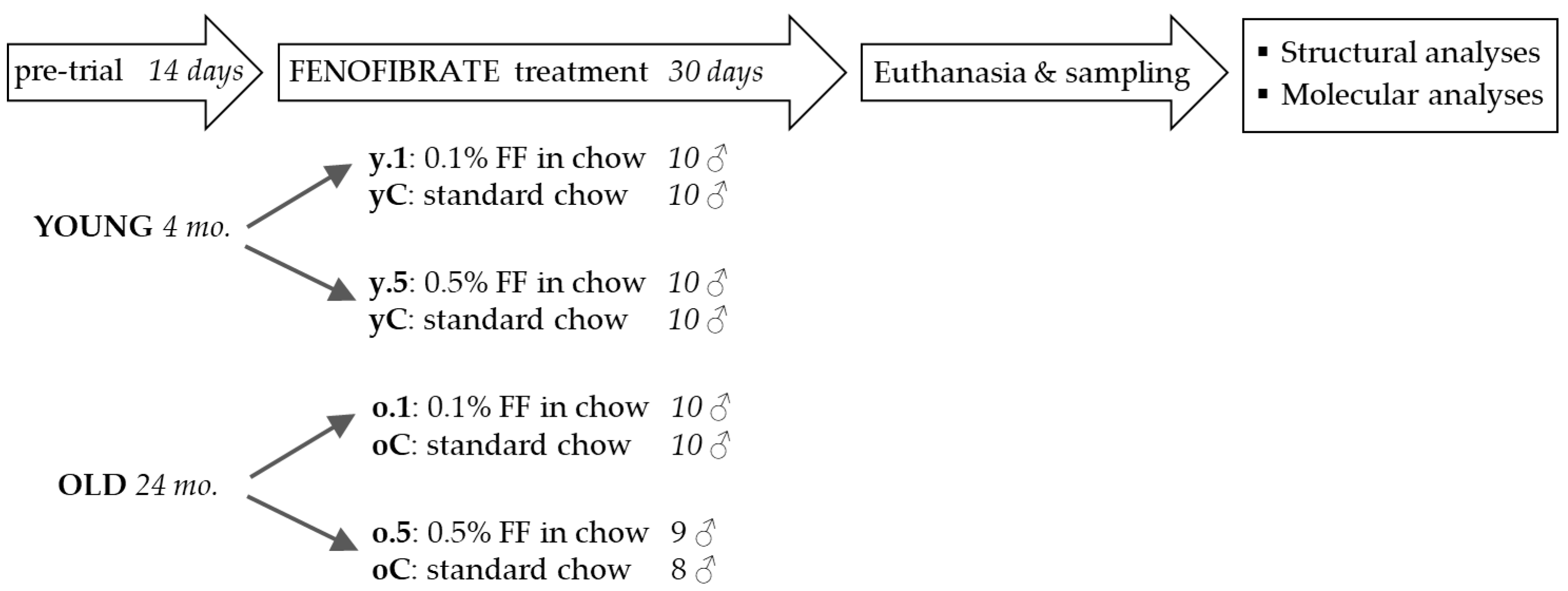
4.1. Animal Experiments
4.2. Serum Concentrations of Creatinine and Blood Urea Nitrogen
4.3. Histological Stains
4.4. Immunohistochemical Staining for Tumor Necrosis Factorα (TNFα) and 4-Hydroxynonenal (4-HNE)
4.5. qPCR Array
4.6. Quantification of Gene Expression Using Real-Time PCR
4.7. Assessment of Protein Content by Western Blotting
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, T.M.E.; Ting, R.; Best, J.D.; Donoghoe, M.W.; Drury, P.L.; Sullivan, D.R.; Jenkins, A.J.; O’Connell, R.L.; Whiting, M.J.; Glasziou, P.P.; et al. Effects of Fenofibrate on Renal Function in Patients with Type 2 Diabetes Mellitus: The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia 2011, 54, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-L.; Fan, P.-C.; Lin, M.-S.; Lee, C.-C.; Tu, K.-H.; Chen, C.-Y.; Hsiao, C.-C.; Hsu, H.-H.; Tian, Y.-C.; Chang, C.-H. Fenofibrate Delays the Need for Dialysis and Reduces Cardiovascular Risk Among Patients with Advanced CKD. J. Clin. Endocrinol. Metab. 2021, 106, 1594–1605. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Iseri, K.; Hida, N. Fibrates and the Risk of Cardiovascular Outcomes in Chronic Kidney Disease Patients. Nephrol. Dial. Transplant. 2023, gfad248. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.A.; Lim, J.H.; Kim, M.Y.; Kim, T.W.; Kim, Y.; Yang, K.S.; Park, H.S.; Choi, S.R.; Chung, S.; Kim, H.W.; et al. Fenofibrate Improves Renal Lipotoxicity through Activation of AMPK-PGC-1α in Db/Db Mice. PLoS ONE 2014, 9, e96147. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Attia, H.A.; Al-Amin, M.A.; Al-Ajmi, H.N.; Hasan, I.H.; Mohamad, R.A.; Sinjilawi, N.A. Renoprotective Effects of Fenofibrate via Modulation of LKB1/AMPK mRNA Expression and Endothelial Dysfunction in a Rat Model of Diabetic Nephropathy. Pharmacology 2015, 95, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Emmett, N.; Mann, D.; Zhao, X. Fenofibrate Attenuates Tubulointerstitial Fibrosis and Inflammation through Suppression of Nuclear Factor-κB and Transforming Growth Factor-Β1/Smad3 in Diabetic Nephropathy. Exp. Biol. Med. 2010, 235, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, A.; Hattori, Y.; Inoue, T.; Hattori, S.; Kasai, K. Fenofibrate Suppresses Microvascular Inflammation and Apoptosis through Adenosine Monophosphate-Activated Protein Kinase Activation. Metabolism 2011, 60, 513–522. [Google Scholar] [CrossRef]
- Hou, X.; Shen, Y.H.; Li, C.; Wang, F.; Zhang, C.; Bu, P.; Zhang, Y. PPARalpha Agonist Fenofibrate Protects the Kidney from Hypertensive Injury in Spontaneously Hypertensive Rats via Inhibition of Oxidative Stress and MAPK Activity. Biochem. Biophys. Res. Commun. 2010, 394, 653–659. [Google Scholar] [CrossRef]
- Sidhu, G.; Tripp, J. Fenofibrate. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mottl, A.K.; Buse, J.B.; Ismail-Beigi, F.; Sigal, R.J.; Pedley, C.F.; Papademetriou, V.; Simmons, D.L.; Katz, L.; Mychaleckyj, J.C.; Craven, T.E. Long-Term Effects of Intensive Glycemic and Blood Pressure Control and Fenofibrate Use on Kidney Outcomes. Clin. J. Am. Soc. Nephrol. 2018, 13, 1693–1702. [Google Scholar] [CrossRef]
- Bae, S.H.; Sung, S.H.; Oh, S.Y.; Lim, J.M.; Lee, S.K.; Park, Y.N.; Lee, H.E.; Kang, D.; Rhee, S.G. Sestrins Activate Nrf2 by Promoting P62-Dependent Autophagic Degradation of Keap1 and Prevent Oxidative Liver Damage. Cell Metab. 2013, 17, 73–84. [Google Scholar] [CrossRef]
- Ala, M. Sestrin2 Signaling Pathway Regulates Podocyte Biology and Protects against Diabetic Nephropathy. J. Diabetes Res. 2023, 2023, 8776878. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, M.; Ansari, N.H.; Srivastava, S.K.; Ramana, K.V. 4-Hydroxynonenal in the Pathogenesis and Progression of Human Diseases. Curr. Med. Chem. 2014, 21, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Advani, A. VEGF and the Diabetic Kidney: More than Too Much of a Good Thing. J. Diabetes Complicat. 2017, 31, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kushida, N.; Nomura, S.; Mimura, I.; Fujita, T.; Yamamoto, S.; Nangaku, M.; Aburatani, H. Hypoxia-Inducible Factor-1α Activates the Transforming Growth Factor-β/SMAD3 Pathway in Kidney Tubular Epithelial Cells. Am. J. Nephrol. 2016, 44, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Duez, H.; Pourcet, B. Nuclear Receptors in the Control of the NLRP3 Inflammasome Pathway. Front. Endocrinol. 2021, 12, 630536. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Witkowski, J.M.; Olivieri, F.; Larbi, A. The Integration of Inflammaging in Age-Related Diseases. Semin. Immunol. 2018, 40, 17–35. [Google Scholar] [CrossRef]
- Mehaffey, E.; Majid, D.S.A. Tumor Necrosis Factor-α, Kidney Function, and Hypertension. Am. J. Physiol. Renal Physiol. 2017, 313, F1005–F1008. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Gai, Z.; Kullak-Ublick, G.A.; Liu, Z. TNF-α Deficiency Prevents Renal Inflammation and Oxidative Stress in Obese Mice. Kidney Blood Press. Res. 2017, 42, 416–427. [Google Scholar] [CrossRef]
- Stadler, K.; Goldberg, I.J.; Susztak, K. The Evolving Understanding of the Contribution of Lipid Metabolism to Diabetic Kidney Disease. Curr. Diab Rep. 2015, 15, 40. [Google Scholar] [CrossRef]
- Chung, H.W.; Lim, J.H.; Kim, M.Y.; Shin, S.J.; Chung, S.; Choi, B.S.; Kim, H.W.; Kim, Y.-S.; Park, C.W.; Chang, Y.S. High-Fat Diet-Induced Renal Cell Apoptosis and Oxidative Stress in Spontaneously Hypertensive Rat Are Ameliorated by Fenofibrate through the PPARα-FoxO3a-PGC-1α Pathway. Nephrol. Dial. Transplant. 2012, 27, 2213–2225. [Google Scholar] [CrossRef]
- Fontecha-Barriuso, M.; Martin-Sanchez, D.; Martinez-Moreno, J.M.; Monsalve, M.; Ramos, A.M.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. The Role of PGC-1α and Mitochondrial Biogenesis in Kidney Diseases. Biomolecules 2020, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, C.; Copur, S.; Mutlu, A.; Peltek, I.B.; Galassi, A.; Ciceri, P.; Cozzolino, M.; Kanbay, M. Early Aging and Premature Vascular Aging in Chronic Kidney Disease. Clin. Kidney J. 2023, 16, 1751–1765. [Google Scholar] [CrossRef] [PubMed]
- Zubrzycki, A.; Wrońska, A.; Kotulak-Chrząszcz, A.; Wierzbicki, P.M.; Kmieć, Z. Fenofibrate Impairs Liver Function and Structure More Pronounced in Old than Young Rats. Arch. Gerontol. Geriatr. 2020, 91, 104244. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.; Kim, K.; Uddin, M.J.; Lee, G.; Hwang, I.; Kang, H.; Kim, H.; Lee, J.H.; Ha, H. Delayed Treatment with Fenofibrate Protects against High-Fat Diet-Induced Kidney Injury in Mice: The Possible Role of AMPK Autophagy. Am. J. Physiol. Renal Physiol. 2017, 312, F323–F334. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, L.; Pignieri, A.; Fiaschè, M.; Giudici, M.; Crestani, M.; Mitro, N.; Abbate, M.; Zoja, C.; Rottoli, D.; Foray, C.; et al. Fenofibrate Attenuates Cardiac and Renal Alterations in Young Salt-Loaded Spontaneously Hypertensive Stroke-Prone Rats through Mitochondrial Protection. J. Hypertens. 2018, 36, 1129–1146. [Google Scholar] [CrossRef]
- Chen, L.-L.; Zhang, J.-Y.; Wang, B.-P. Renoprotective Effects of Fenofibrate in Diabetic Rats Are Achieved by Suppressing Kidney Plasminogen Activator Inhibitor-1. Vascul Pharmacol. 2006, 44, 309–315. [Google Scholar] [CrossRef]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Kim, H.W.; Park, C.W.; Chang, Y.S.; Choi, B.S. PPARα Agonist, Fenofibrate, Ameliorates Age-Related Renal Injury. Exp. Gerontol. 2016, 81, 42–50. [Google Scholar] [CrossRef]
- Wiggins, J.E. Aging in the Glomerulus. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1358–1364. [Google Scholar] [CrossRef]
- Sweetwyne, M.T.; Pippin, J.W.; Eng, D.G.; Hudkins, K.L.; Chiao, Y.A.; Campbell, M.D.; Marcinek, D.J.; Alpers, C.E.; Szeto, H.H.; Rabinovitch, P.S.; et al. The Mitochondrial-Targeted Peptide, SS-31, Improves Glomerular Architecture in Mice of Advanced Age. Kidney Int. 2017, 91, 1126–1145. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, J.; Guo, W.; Li, F.; Sun, W.; Chen, J.; Zhang, C.; Lu, X.; Tan, Y.; Feng, W.; et al. Up-Regulation of Nrf2 Is Involved in FGF21-Mediated Fenofibrate Protection against Type 1 Diabetic Nephropathy. Free Radic. Biol. Med. 2016, 93, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Jao, T.-M.; Nangaku, M.; Wu, C.-H.; Sugahara, M.; Saito, H.; Maekawa, H.; Ishimoto, Y.; Aoe, M.; Inoue, T.; Tanaka, T.; et al. ATF6α Downregulation of PPARα Promotes Lipotoxicity-Induced Tubulointerstitial Fibrosis. Kidney Int. 2019, 95, 577–589. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, L.; Zhang, Y.; Lin, J.; Zhou, H. Fenofibrate Improved Interstitial Fibrosis of Renal Allograft through Inhibited Epithelial-Mesenchymal Transition Induced by Oxidative Stress. Oxid. Med. Cell Longev. 2019, 2019, 8936856. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Liu, B.; Meng, Q.; Zhang, D.; Yang, H.; Li, G.; Wang, Y.; Zhou, H.; Xu, Z.-X.; Wang, Y. Targeted Changes in Blood Lipids Improves Fibrosis in Renal Allografts. Lipids Health Dis. 2023, 22, 215. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Miao, X.; Cui, W.; Miao, L.; Cai, L. A Minireview: Role of AMP-Activated Protein Kinase (AMPK) Signaling in Obesity-Related Renal Injury. Life Sci. 2021, 265, 118828. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, X.; Ma, F.; Sun, W.; Wang, W.; Yu, J.; Shi, Y.; Cai, L.; Xu, Z. The Role of Akt2 in the Protective Effect of Fenofibrate against Diabetic Nephropathy. Int. J. Biol. Sci. 2020, 16, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Mohammadi, M.T.; Rezaee, R.; Sahebkar, A. Fenofibrate Improves Renal Function by Amelioration of NOX-4, IL-18, and P53 Expression in an Experimental Model of Diabetic Nephropathy. J. Cell Biochem. 2018, 119, 7458–7469. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Saad, E.B.; El-Rhman, R.H.A.; El-Raouf, O.M.A.; Gad, A.M. Impact of Peroxisome Proliferator Activated Receptor Agonist Drugs in a Model of Nephrotoxicity in Rats. J. Biochem. Mol. Toxicol. 2023, 37, e23350. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, L.; Jiang, H. Integrated Bioinformatics and Clinical Correlation Analysis of Key Genes, Pathways, and Potential Therapeutic Agents Related to Diabetic Nephropathy. Dis. Markers 2022, 2022, 9204201. [Google Scholar] [CrossRef] [PubMed]
- Mondragón-García, A.; Luna-Luna, M.; Flores-Castillo, C.; Aranda-Fraustro, A.; Carreón-Torres, E.; López-Olmos, V.; Fragoso, J.M.; Vargas-Alarcón, G.; Pérez-Méndez, Ó. Atorvastatin and Fenofibrate Exert Opposite Effects on the Vascularization and Characteristics of Visceral Adipose Tissue in New Zealand White Rabbits. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Hu, Y.; Wang, H.; Sun, C.; Wang, Y.; He, W.; Zhang, X. Inhibitory Effects of Fenofibrate on Plasminogen Activator Inhibitor-1 Expression in Human Endothelial Cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2006, 26, 192–193, 198. [Google Scholar] [CrossRef]
- Li, M.; Wang, D.; Liu, Z.; Huang, Y.; Zhang, Q.; Pan, C.; Lin, Y.; Sun, L.; Zheng, Y. Assessing the Effects of Aging on the Renal Endothelial Cell Landscape Using Single-Cell RNA Sequencing. Front. Genet. 2023, 14, 1175716. [Google Scholar] [CrossRef]
- Takiar, V.; Nishio, S.; Seo-Mayer, P.; King, J.D.J.; Li, H.; Zhang, L.; Karihaloo, A.; Hallows, K.R.; Somlo, S.; Caplan, M.J. Activating AMP-Activated Protein Kinase (AMPK) Slows Renal Cystogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 2462–2467. [Google Scholar] [CrossRef]
- Losacco, M.C.; de Almeida, C.F.T.; Hijo, A.H.T.; Bargi-Souza, P.; Gama, P.; Nunes, M.T.; Goulart-Silva, F. High-Fat Diet Affects Gut Nutrients Transporters in Hypo and Hyperthyroid Mice by PPAR-a Independent Mechanism. Life Sci. 2018, 202, 35–43. [Google Scholar] [CrossRef]
- Glineur, C.; Gross, B.; Neve, B.; Rommens, C.; Chew, G.T.; Martin-Nizard, F.; Rodríguez-Pascual, F.; Lamas, S.; Watts, G.F.; Staels, B. Fenofibrate Inhibits Endothelin-1 Expression by Peroxisome Proliferator-Activated Receptor α-Dependent and Independent Mechanisms in Human Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 621–628. [Google Scholar] [CrossRef]
- Li, C.; Mpollo, M.-S.E.M.; Gonsalves, C.S.; Tahara, S.M.; Malik, P.; Kalra, V.K. Peroxisome Proliferator-Activated Receptor-α-Mediated Transcription of miR-199a2 Attenuates Endothelin-1 Expression via Hypoxia-Inducible Factor-1α. J. Biol. Chem. 2014, 289, 36031–36047. [Google Scholar] [CrossRef] [PubMed]
- Jen, H.-L.; Liu, P.-L.; Chen, Y.-H.; Yin, W.-H.; Chen, J.-W.; Lin, S.-J. Peroxisome Proliferator-Activated Receptor α Reduces Endothelin-1-Caused Cardiomyocyte Hypertrophy by Inhibiting Nuclear Factor-κB and Adiponectin. Mediat. Inflamm. 2016, 2016, 5609121. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, Y.; Song, Y.; Gao, X.; Deng, H. AP-1 Is a Regulatory Transcription Factor of Inflammaging in the Murine Kidney and Liver. Aging Cell 2023, 22, e13858. [Google Scholar] [CrossRef] [PubMed]
- Sinning, J.; Funk, N.D.; Soerensen-Zender, I.; Wulfmeyer, V.C.; Liao, C.M.; Haller, H.; Hinze, C.; Schmidt-Ott, K.M.; Melk, A.; Schmitt, R. The Aging Kidney Is Characterized by Tubuloinflammaging, a Phenotype Associated with MHC-II Gene Expression. Front. Immunol. 2023, 14, 1222339. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-J.; He, Y.-H.; Zhou, J.-H. Fenofibrate Pre-Treatment Suppressed Inflammation by Activating Phosphoinositide 3 Kinase/Protein Kinase B (PI3K/Akt) Signaling in Renal Ischemia-Reperfusion Injury. J. Huazhong Univ. Sci. Technol. Med. Sci. 2015, 35, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Helmy, M.M.; Helmy, M.W.; El-Mas, M.M. Additive Renoprotection by Pioglitazone and Fenofibrate against Inflammatory, Oxidative and Apoptotic Manifestations of Cisplatin Nephrotoxicity: Modulation by PPARs. PLoS ONE 2015, 10, e0142303. [Google Scholar] [CrossRef]
- Noureldein, M.H.; Abd El-Razek, R.S.; El-Hefnawy, M.H.; El-Mesallamy, H.O. Fenofibrate Reduces Inflammation in Obese Patients with or without Type 2 Diabetes Mellitus via Sirtuin 1/Fetuin A Axis. Diabetes Res. Clin. Pract. 2015, 109, 513–520. [Google Scholar] [CrossRef]
- Jin, L.; Hua, H.; Ji, Y.; Jia, Z.; Peng, M.; Huang, S. Anti-Inflammatory Role of Fenofibrate in Treating Diseases. Biomol. Biomed. 2023, 23, 376–391. [Google Scholar] [CrossRef]
- Chalkiadaki, A.; Guarente, L. High-Fat Diet Triggers Inflammation-Induced Cleavage of SIRT1 in Adipose Tissue to Promote Metabolic Dysfunction. Cell Metab. 2012, 16, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Grabacka, M.; Pierzchalska, M.; Dean, M.; Reiss, K. Regulation of Ketone Body Metabolism and the Role of PPARα. Int. J. Mol. Sci. 2016, 17, 2093. [Google Scholar] [CrossRef] [PubMed]
- Lakhia, R.; Yheskel, M.; Flaten, A.; Quittner-Strom, E.B.; Holland, W.L.; Patel, V. PPARα Agonist Fenofibrate Enhances Fatty Acid β-Oxidation and Attenuates Polycystic Kidney and Liver Disease in Mice. Am. J. Physiol. Renal Physiol. 2018, 314, F122–F131. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, J.A. Histological and Histochemical Methods: Theory and Practice, 5th ed.; Scion Publishing: Wickford, UK, 2015. [Google Scholar]
- Kiezun, J.; Godlewski, J.; Krazinski, B.E.; Kozielec, Z.; Kmiec, Z. Galanin Receptors (GalR1, GalR2, and GalR3) Expression in Colorectal Cancer Tissue and Correlations to the Overall Survival and Poor Prognosis of CRC Patients. Int. J. Mol. Sci. 2022, 23, 3735. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrońska, A.; Kieżun, J.; Kmieć, Z. High-Dose Fenofibrate Stimulates Multiple Cellular Stress Pathways in the Kidney of Old Rats. Int. J. Mol. Sci. 2024, 25, 3038. https://doi.org/10.3390/ijms25053038
Wrońska A, Kieżun J, Kmieć Z. High-Dose Fenofibrate Stimulates Multiple Cellular Stress Pathways in the Kidney of Old Rats. International Journal of Molecular Sciences. 2024; 25(5):3038. https://doi.org/10.3390/ijms25053038
Chicago/Turabian StyleWrońska, Agata, Jacek Kieżun, and Zbigniew Kmieć. 2024. "High-Dose Fenofibrate Stimulates Multiple Cellular Stress Pathways in the Kidney of Old Rats" International Journal of Molecular Sciences 25, no. 5: 3038. https://doi.org/10.3390/ijms25053038
APA StyleWrońska, A., Kieżun, J., & Kmieć, Z. (2024). High-Dose Fenofibrate Stimulates Multiple Cellular Stress Pathways in the Kidney of Old Rats. International Journal of Molecular Sciences, 25(5), 3038. https://doi.org/10.3390/ijms25053038





