A Novel Bisquaternary Ammonium Compound as an Anion Sensor—ESI-MS and Fluorescence Study
Abstract
:1. Introduction
2. Results and Discussion
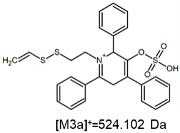
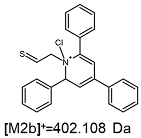
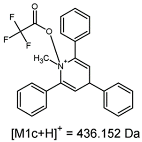
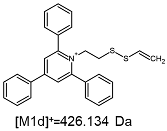
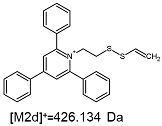
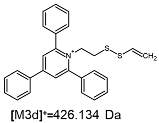
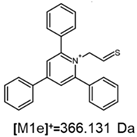
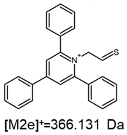
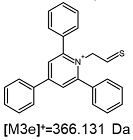
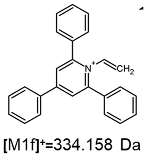
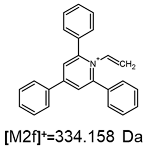
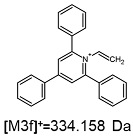
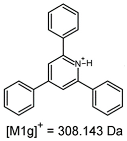
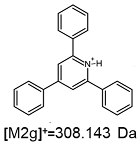
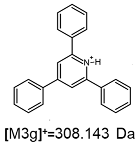
2.1. Mass Spectrometry Analysis
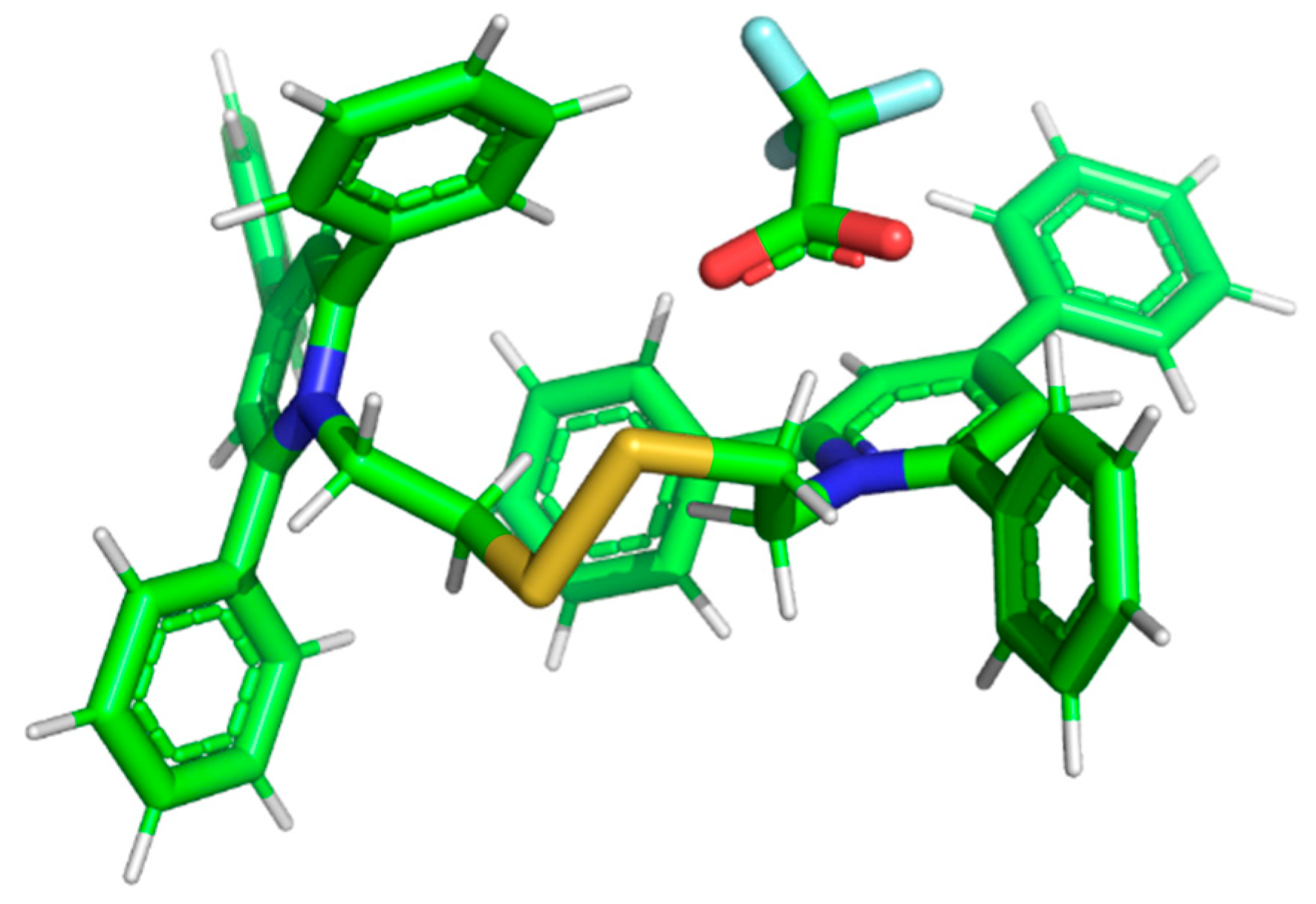
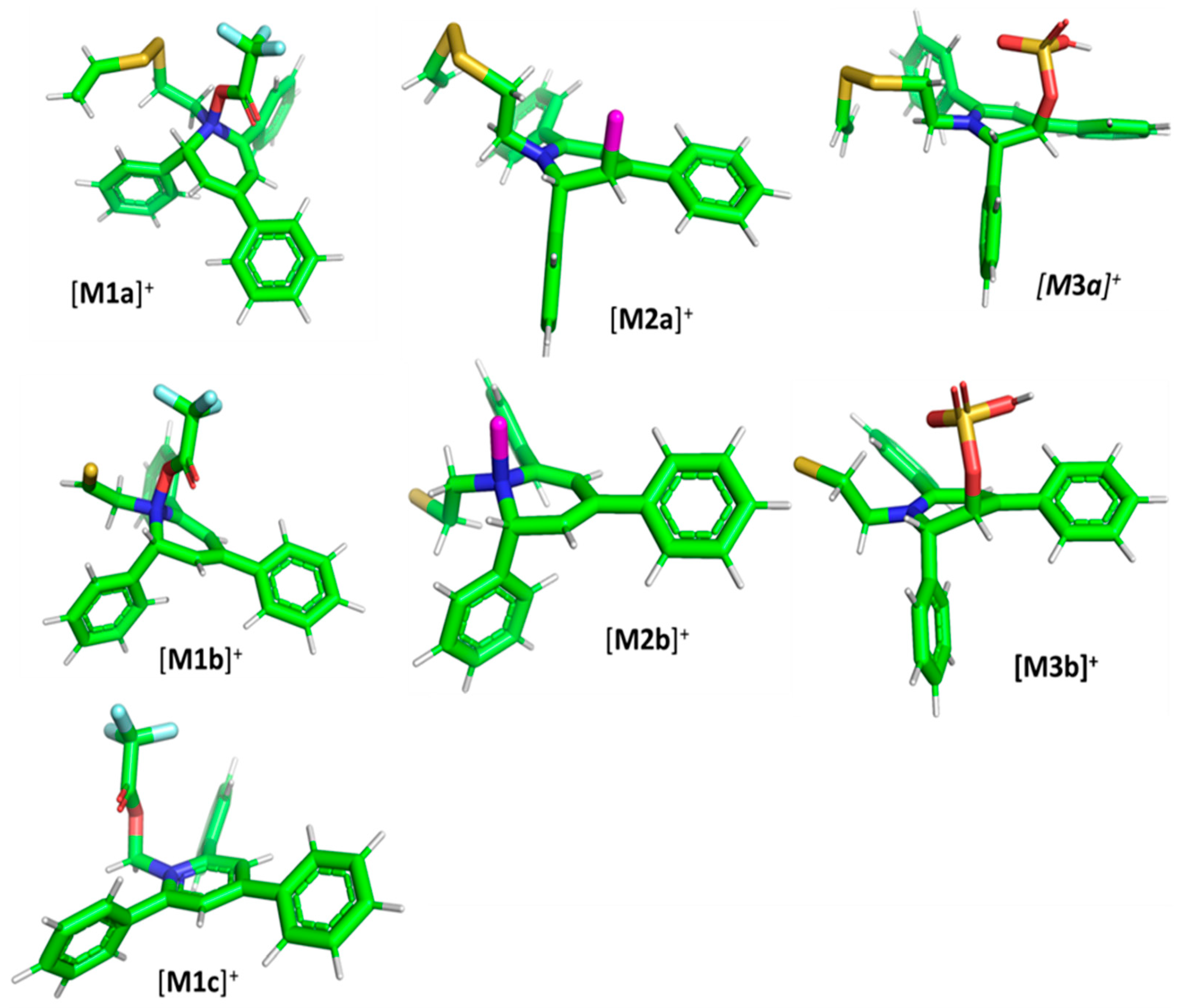
2.2. Computational Analysis
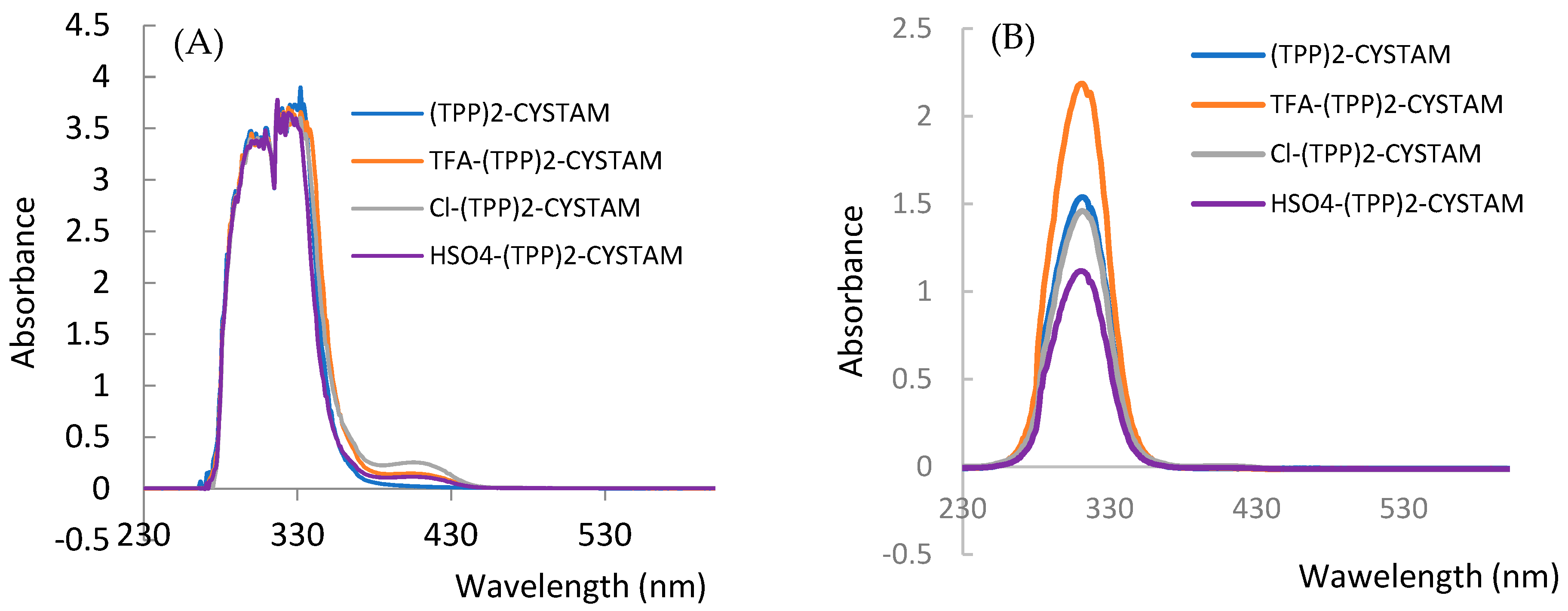
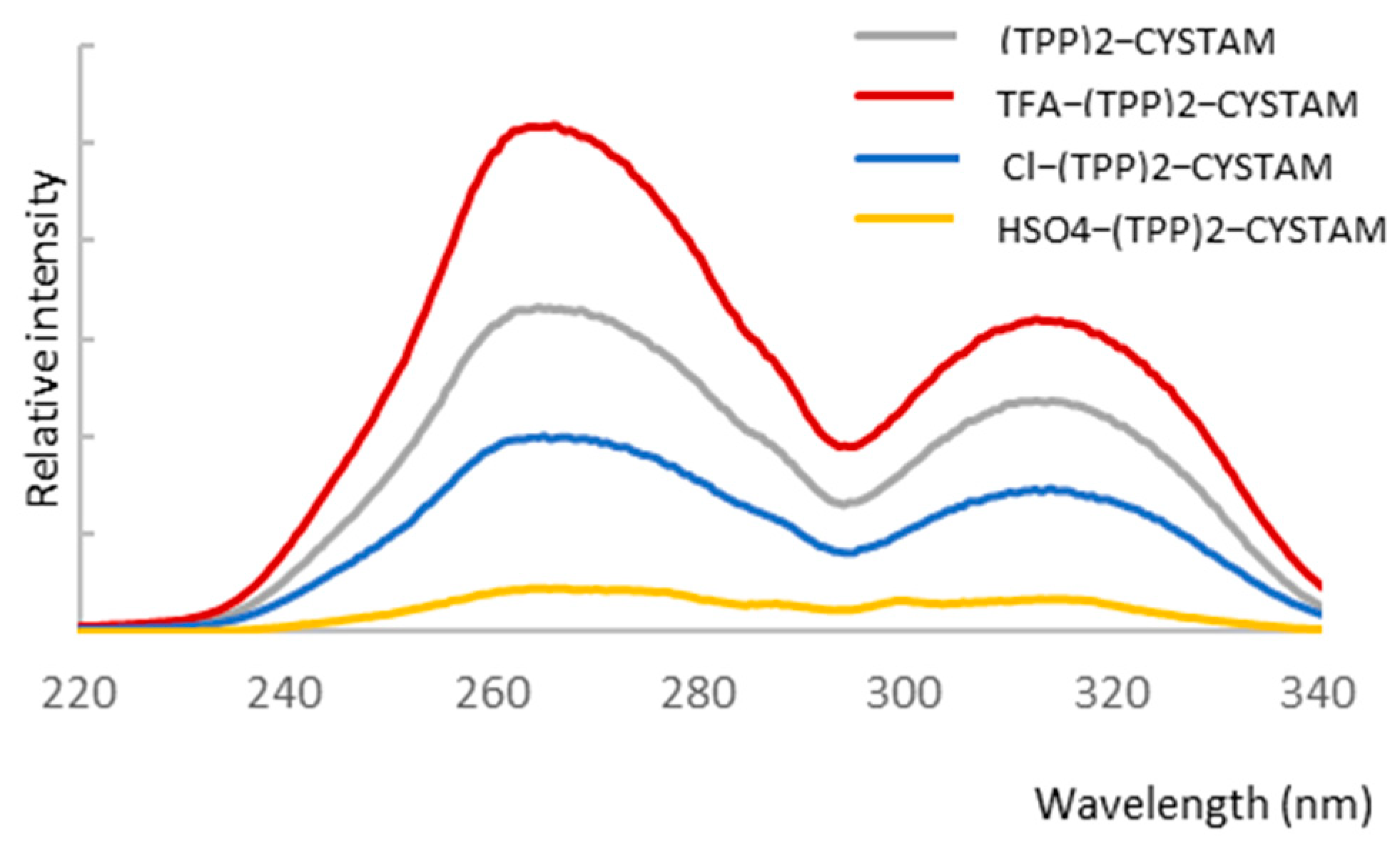
2.3. UV–Vis Absorption Analysis
2.4. Analysis of Anion Sensor Properties
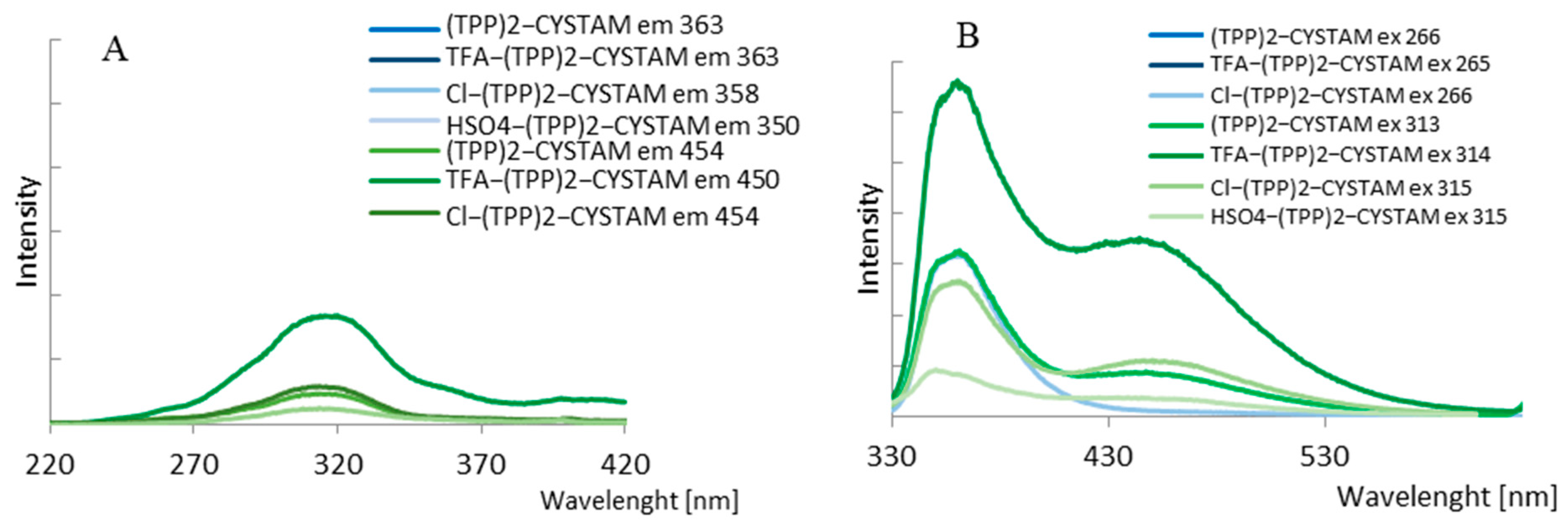
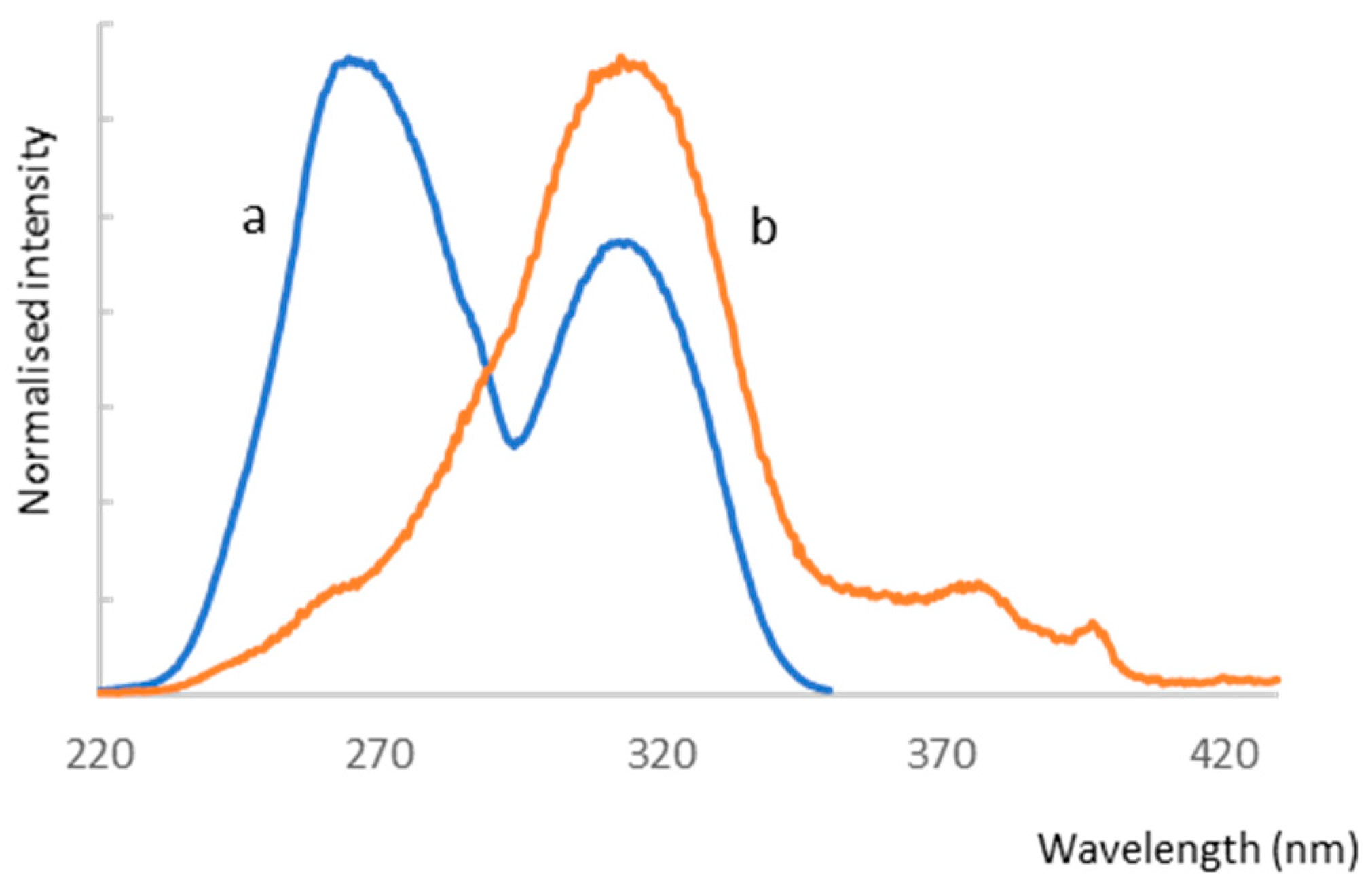
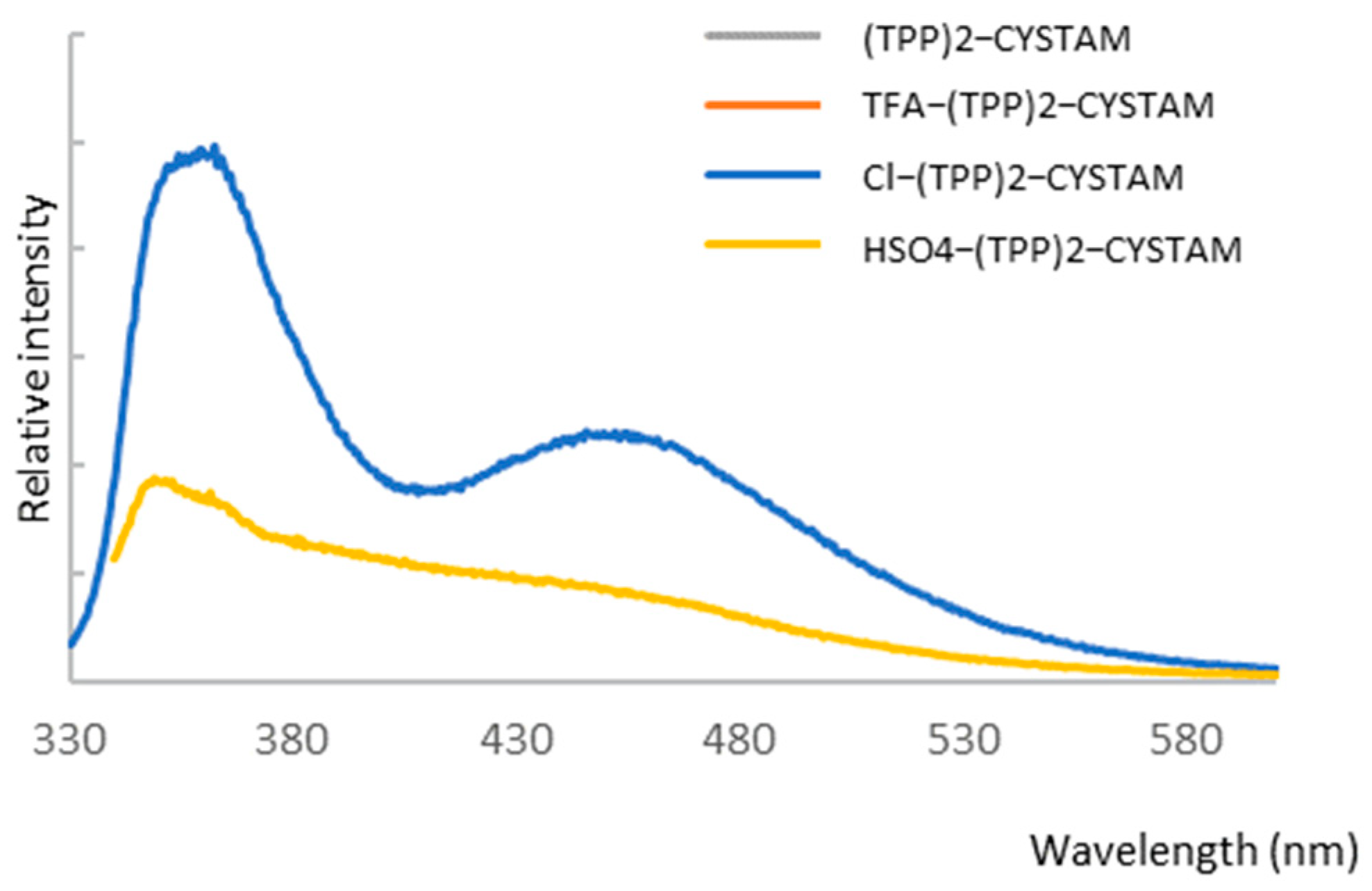
2.4.1. Analysis of Luminescent Properties
2.4.2. Sensitivity of Anion Sensing
3. Materials and Methods
3.1. Chemicals
3.2. (TPP)2-CYSTAM Synthesis
3.3. TFA, Cl and HSO4 Bisquaternary Ammonium Dication Adduct Preparation
3.4. Mass Spectrometry
3.5. High-Performance Liquid Chromatography
3.6. Computational Analysis
3.7. UV–Vis Measurement
3.8. Emission and Excitation Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kruve, A.; Kaupmees, K. Adduct Formation in ESI/MS by Mobile Phase Additives. J. Am. Soc. Mass Spectrom. 2017, 28, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Steckel, A.; Schlosser, G. An Organic Chemist’s Guide to Electrospray Mass Spectrometric Structure Elucidation. Molecules 2019, 24, 611. [Google Scholar] [CrossRef] [PubMed]
- Foreman, D.J.; McLuckey, S.A. Recent Developments in Gas-Phase Ion/Ion Reactions for Analytical Mass Spectrometry. Anal. Chem. 2020, 92, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Cech, N.B.; Enke, C.G. Practical implications of some recent studies in electrospray ionization fundamentals. Mass. Spectrom. Rev. 2001, 20, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Kuksis, A.; Marai, L.; Myher, J.J. Reversed-phase liquid chromatography-mass spectrometry of complex mixtures of natural triacylglycerols with chloride-attachment negative chemical ionization. J. Chromatogr. A 1991, 588, 73–87. [Google Scholar] [CrossRef]
- Kuksis, A.; Marai, L.; Myher, J.J. Plasma lipid profiling by liquid chromatography with chloride-attachment mass spectrometry. Lipids 1991, 26, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cole, R.B. Formation and decompositions of chloride adduct ions [M + Cl]−, in negative ion electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2000, 11, 932–941. [Google Scholar] [CrossRef]
- Winkler, G.; Wolschann, P.; Briza, P.; Heinz, F.X.; Kunz, C. Spectral properties of trifluoroacetic acid—Acetonitrile gradient systems for separation of picomole quantities of peptides by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1985, 347, 83. [Google Scholar] [CrossRef]
- Kuhlmann, F.E.; Apffel, A.; Fischer, S.M.; Goldberg, G.; Goodley, P.C. Signal enhancement for gradient reverse-phase high-performance liquid chromatography-electrospray ionization mass spectrometry analysis with trifluoroacetic and other strong acid modifiers by postcolumn addition of propionic acid and isopropanol. J. Am. Soc. Mass Spectrom. 1995, 6, 1221–1225. [Google Scholar] [CrossRef]
- Okusa, K.; Suita, Y.; Otsuka, Y.; Tahara, M.; Ikegami, T.; Tanaka, N.; Ohira, M.; Takahashi, M. Test compounds for detecting the silanol effect on the elution of ionized amines in reversed-phase LC. J. Sep. Sci. 2010, 33, 348–358. [Google Scholar] [CrossRef]
- Chan, C.C.; Bolgar, M.S.; Dalpathado, D.; Lloyd, D.K. Mitigation of signal suppression caused by the use of trifluoroacetic acid in liquid chromatography mobile phases during liquid chromatography/mass spectrometry analysis via post-column addition of ammonium hydroxide. Rapid Commun. Mass Spectrom. 2012, 26, 1507–1514. [Google Scholar] [CrossRef]
- Wouters, S.; Eeltink, S.; Haselberg, R.; Somsen, G.W.; Gargano, A.F.G. Microfluidic ion stripper for removal of trifluoroacetic acid from mobile phases used in HILIC-MS of intact proteins. Anal. Bioanal. Chem. 2021, 413, 4379–4386. [Google Scholar] [CrossRef]
- Shou, W.Z.; Naidong, W. Simple means to alleviate sensitivity loss by trifluoroacetic acid (TFA) mobile phases in the hydrophilic interaction chromatography-electrospray tandem mass spectrometric (HILIC-ESI/MS/MS) bioanalysis of basic compounds. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 825, 186–192. [Google Scholar] [CrossRef]
- Qiao, X.; Yang, Y.; Liu, S.; Chen, S.; Wang, X.; Li, G.; Yana, H.; Yang, X. Novel pyridinium-based tags: Synthesis and characterization for highly efficient analysis of thiol-containing peptides by mass spectrometry. Analyst 2015, 140, 407–413. [Google Scholar] [CrossRef]
- Zaia, J.; Biemann, K. Comparison of charged derivatives for high energy collision-induced dissociation tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 1995, 6, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.E.; Roberts, K.D.; Simpson, R.J.; O’Hair, R.A.J. Selective identification and quantitative analysis of methionine containing peptides by charge derivatization and tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2005, 16, 1131–1150. [Google Scholar] [CrossRef]
- Kowalska, M.; Bąchor, R. Catch, Modify and Analyze: Methods of Chemoselective Modification of Cysteine-Containing Peptides. Molecules 2022, 27, 1601. [Google Scholar] [CrossRef] [PubMed]
- Waliczek, M.; Bąchor, R.; Kijewska, M.; Gąszczyk, D.; Panek-Laszczyńska, K.; Konieczny, A.; Dąbrowska, K.; Witkiewicz, W.; Marek-Bukowiec, K.; Tracz, J.; et al. Isobaric duplex based on a combination of 16O/18O enzymatic exchange and labeling with pyrylium salts. Anal. Chim. Acta 2019, 1048, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Grocholska, P.; Konieczny, A.; Kaźmierczak, Z.; Dąbrowska, K.; Panek-Laszczyńska, K.; Kłak, M.; Witkiewicz, W.; Szewczuk, Z.; Bąchor, R. Peptide Charge Derivatization as a Tool for Early Detection of Preeclampsia by Mass Spectrometry—A Comparison with the ELISA Test. Molecules 2021, 26, 7102. [Google Scholar] [CrossRef]
- Grocholska, P.; Kowalska, M.; Wieczorek, R.; Bąchor, R. A Non-Covalent Dimer Formation of Quaternary Ammonium Cation with Unusual Charge Neutralization in Electrospray-Ionization Mass Spectrometry. Molecules 2021, 26, 5868. [Google Scholar] [CrossRef]
- Gale, P.A.; Howe, E.N.W.; Wu, X. Anion Receptor Chemistry. Chem 2016, 1, 351–422. [Google Scholar] [CrossRef]
- He, X.; Thompson, R.R.; Clawson, S.A.; Fronczek, F.R.; Lee, S. Anion receptors with nitrone C-H hydrogen bond donors. Chem. Commun. 2023, 59, 4624–4627. [Google Scholar] [CrossRef]
- Gale, P.A.; Sessler, J.L.; Král, V.; Lynch, V. Calix[4]pyrroles: Old yet new anion-binding agents. J. Am. Chem. Soc. 1996, 118, 5140–5141. [Google Scholar] [CrossRef]
- Chang, K.J.; Moon, D.; Lah, M.S.; Jeong, K.S. Indole-based macrocycles as a class of receptors for anions. Angew. Chem. Int. Ed. Engl. 2005, 44, 7926–7929. [Google Scholar] [CrossRef] [PubMed]
- Eytel, L.M.; Brueckner, A.C.; Lohrman, J.A.; Haley, M.M.; Cheong, P.H.Y.; Johnson, D.W. Conformationally flexible arylethynyl bis-urea receptors bind disparate oxoanions with similar, high affinities. Chem. Commun. 2018, 54, 13208–13211. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Kang, S.O.; Powell, D.; Bowman-James, K. Anion receptors: A new class of amide/quaternized amine macrocycles and the chelate effect. Inorg. Chem. 2003, 42, 1397–1399. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Hartley, A.; Turner, P.; Elmes, R.B.P.; Jolliffe, K.A. Macrocyclic squaramides: Anion receptors with high sulfate binding affinity and selectivity in aqueous media. Chem. Sci. 2016, 7, 4563–4572. [Google Scholar] [CrossRef]
- Kilah, N.L.; Wise, M.D.; Serpell, C.J.; Thompson, A.L.; White, N.G.; Christensen, K.E.; Beer, P.D. Enhancement of Anion Recognition Exhibited by a Halogen-Bonding Rotaxane Host System. J. Am. Chem. Soc. 2010, 132, 11893–11895. [Google Scholar] [CrossRef]
- Lim, J.Y.; Marques, I.; Thompson, A.L.; Christensen, K.E.; Félix, V.; Beer, P.D. Chalcogen bonding macrocycles and [2] rotaxanes for anion recognition. J. Am. Chem. Soc. 2017, 139, 3122–3133. [Google Scholar] [CrossRef]
- Benz, S.; Macchione, M.; Verolet, Q.; Mareda, J.; Sakai, N.; Matile, S. Anion Transport with Chalcogen Bonds. J. Am. Chem. Soc. 2016, 138, 9093–9096. [Google Scholar] [CrossRef]
- Kilah, N.L.; Beer, P.D. Pyridine and Pyridinium-Based Anion Receptors. Top. Heterocycl. Chem. 2010, 24, 301–340. [Google Scholar] [CrossRef]
- Picci, G.; Montis, R.; Gilchrist, A.M.; Gale, P.A.; Caltagirone, C. Fluorescent and colorimetric sensors for anions: Highlights from 2020 to 2022. Coord. Chem. Rev. 2024, 501, 215561. [Google Scholar] [CrossRef]
- González-Ruiz, V.; Cores, Á.; Caja, M.M.; Sridharan, V.; Villacampa, M.; Martín, M.A.; Olives, A.I.; Menéndez, J.C. Fluorescence Sensors Based on Hydroxycarbazole for the Determination of Neurodegeneration-Related Halide Anions. Biosensors 2022, 12, 175. [Google Scholar] [CrossRef]
- Velpandian, T.; Nirmal, J.; Arora, B.; Ravi, A.K.; Kotnala, A. Understanding the Charge Issues in Mono and di-Quaternary Ammonium Compounds for Their Determination by LC/ESI-MS/MS. Anal. Lett. 2012, 45, 2367–2376. [Google Scholar] [CrossRef]
- Breitbach, Z.S.; Wanigasekara, E.; Dodbiba, E.; Schug, K.A.; Armstrong, D.W. Mechanisms of ESI-MS Selectivity and Sensitivity Enhancements When Detecting Anions in the Positive Mode Using Cationic Pairing Agents. Anal. Chem. 2010, 82, 9066–9073. [Google Scholar] [CrossRef]
- Borisov, R.S.; Zakirov, M.I.; Ovcharov, M.V.; Zaikin, V.G. Investigation of 1,1′-disubstituted 4,4′-bipyridinium salts by various mass spectrometry techniques. J. Anal. Chem. 2013, 68, 1183–1187. [Google Scholar] [CrossRef]
- Soukup-Hein, R.J.; Remsburg, J.W.; Dasgupta, P.K.; Armstrong, D.W. A General, Positive Ion Mode ESI-MS Approach for the Analysis of Singly Charged Inorganic and Organic Anions Using a Dicationic Reagent. Anal. Chem. 2007, 79, 7346–7352. [Google Scholar] [CrossRef] [PubMed]
- Mielke, Z.; Latajka, Z.; Olbert-Majkut, A.; Wieczorek, R. Matrix Infrared Spectra and ab Initio Calculations of the Nitrous Acid Complexes with Nitrogen Monoxide. J. Phys. Chem. A 2000, 104, 3764–3769. [Google Scholar] [CrossRef]
- Wieczorek, R.; Latajka, Z.; Lundell, J. Quantum Chemical Study of the Bimolecular Complex of HONO. J. Phys. Chem. A 1999, 103, 6234–6239. [Google Scholar] [CrossRef]
- Olszewski, T.K.; Wojaczyńska, E.; Wieczorek, R.; Bąkowicz, J. α-Hydroxyphosphonic acid derivatives of 2-azanorbornane: Synthesis, DFT calculations, and crystal structure analysis. Tetrahedron Asymmetry 2015, 26, 601–607. [Google Scholar] [CrossRef]
- Gumienna-Kontecka, E.; Berthon, G.; Fritsky, I.O.; Wieczorek, R.; Latajka, Z.; Kozłowski, H. 2-(Hydroxyimino)propanohydroxamic acid, a new effective ligand for aluminium. J. Chem. Soc. Dalton Trans. 2000, 22, 4201–4208. [Google Scholar] [CrossRef]
- Laine, M.; Barbosa, N.A.; Wieczorek, R.; Melnikov, M.Y.; Filarowski, A. Calculations of BODIPY dyes in the ground and excited states using the M06-2X and PBE0 functionals. J. Mol. Model. 2016, 22, 260. [Google Scholar] [CrossRef]
- NIST Computational Chemistry Comparison and Benchmark Database, NIST Standard Reference Database Number 101 Release 21, August 2020; Johnson, R.D., III, Ed. Available online: http://cccbdb.nist.gov (accessed on 21 January 2024).
- McClelland, B.W.; Hedberg, L.; Hedberg, K.; Hagen, K. Molecular structure of dinitrogen pentoxide in the gas phase. Large amplitude motion in a system of coupled rotors. J. Am. Chem. Soc. 1983, 105, 3789–3793. [Google Scholar] [CrossRef]
- Davis, F.A.; Lamendola, J.; Nadir, J.U.; Kluger, E.W.; Sedergran, T.C.; Panunto, T.W.; Billmers, R.; Jenkins, R.; Turchi, J.I.J.; Watson, W.H.; et al. Chemistry of oxaziridines. 1. Synthesis and structure of 2-arenesulfonyl-3-aryloxaziridines. A new class of oxaziridines. J. Am. Chem. Soc. 1980, 102, 2000–2005. [Google Scholar] [CrossRef]
- Knyazhansky, M.I.; Kharlanov, V.A.; Tymiansky, Y.R. Adiabatic structural relaxation in heterocyclic nitrogen-containing cations: The structure, absorption and fluorescence of the 2,4,6-triarylsubstituted pyridinium cations. J. Photochem. Photobiol. 1998, 118, 151–156. [Google Scholar] [CrossRef]
- Kharlanov, V.A.; Knyazhansky, M.I. The dependence of photoinduced adiabatic transformations and fluorescence in 2,4,6-triarylsubstituted pyridinium cations on environment. J. Photochem. Photobiol. 1999, 125, 21–27. [Google Scholar] [CrossRef]
- Kharlanov, V.; Papper, V. Geometry of Structurally Non-Rigid Pyridinium Cations in an Excited State. J. Fluoresc. 2020, 30, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Mooney, J.; Kambhampati, P. Correction to “Get the Basics Right: Jacobian Conversion of Wavelength and Energy Scales for Quantitative Analysis of Emission Spectra”. J. Phys. Chem. Lett. 2013, 4, 3316–3318. [Google Scholar] [CrossRef]
- Niu, X.; Zhang, H.; Wu, X.; Zhu, S.; Feng, H.; Liu, W. A novel “turn-on” fluorescent sensor based on Tetraphenylethylene-planarized bis-Schiff base for dual-state TFA detection. J. Mol. Struct. 2022, 1259, 132754. [Google Scholar] [CrossRef]
- Jumaah, M.; Khairuddean, M.; Owaid, S.J. Benzothiazole Pyrazoline: Acid-Switchable Absorption and Fluorescence of Photoinduced Electron Transfer (PET). J. Fluoresc. 2022, 32, 937–948. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- The PyMOL Molecular Graphics System; Version 2.0; Schrödinger, LLC: New York, NY, USA, 2015.


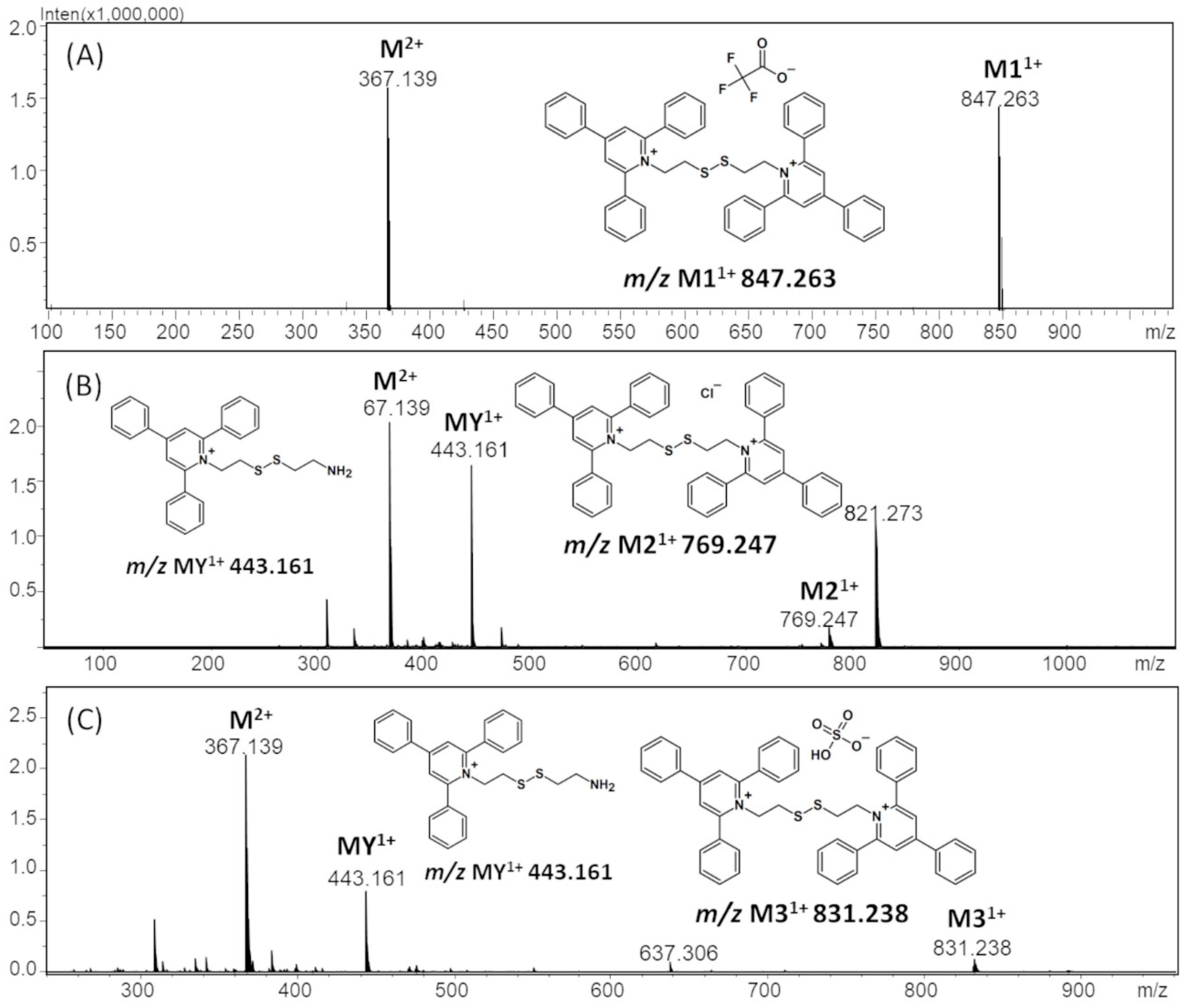
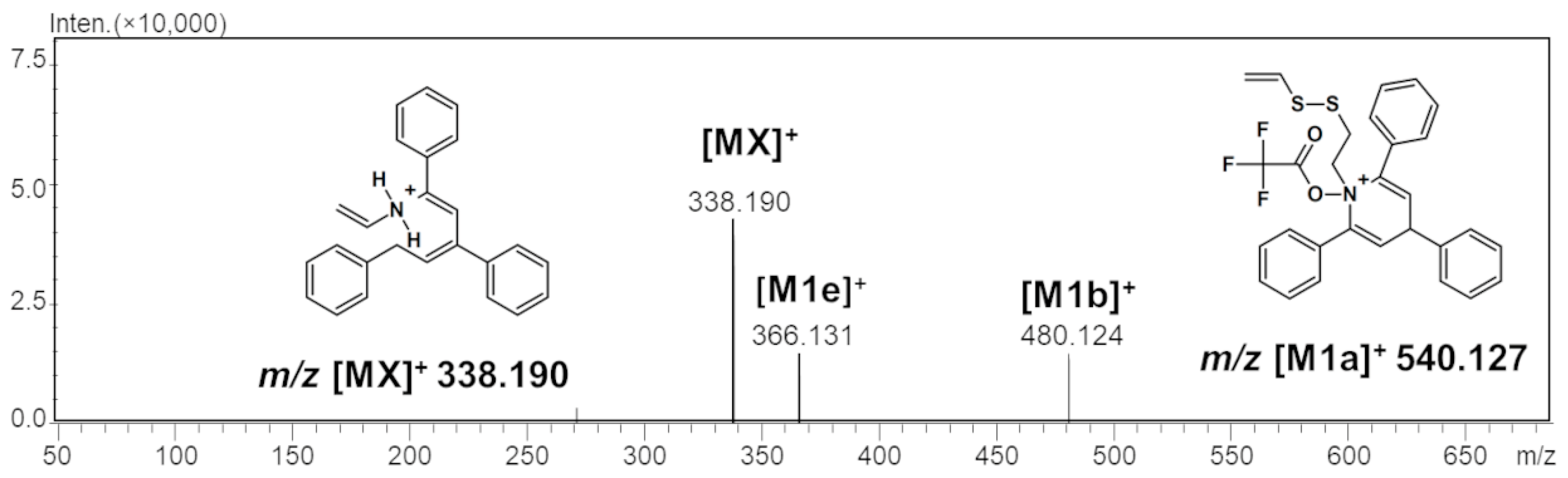




| TFA-(TPP)2-CYSTAM Parent Ion: m/z 847.270 (M1+) | Cl-(TPP)2-CYSTAM Parent Ion: m/z 769.245 (M2+) | HSO4-(TPP)2-CYSTAM Parent Ion: m/z 831.238 (M3+) |
|---|---|---|
 |  |  |
 |  |  |
 | - | - |
 |  |  |
 |  |  |
 |  |  |
 |  |  |
| - | 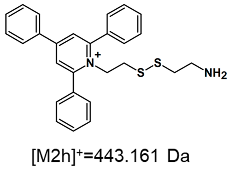 | 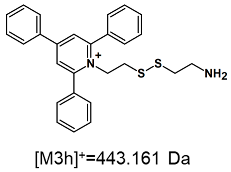 |
| Amount of TFA in the Analyzed Sample | ||
|---|---|---|
| Sample | Percent Concentrations [%] | Amount of Mole |
| 1 | 1 | 1.31 × 10−4 |
| 2 | 0.1 | 1.31 × 10−5 |
| 3 | 0.01 | 1.31 × 10−6 |
| 4 | 0.001 | 1.31 × 10−7 |
| 5 | 0.0005 | 0.655 × 10−7 |
| 6 | 0.0001 | 1.31 × 10−8 |
| 7 | 0.00001 | 1.31 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, M.; Wieczorek, R.; Gawryszewska, P.; Bąchor, R. A Novel Bisquaternary Ammonium Compound as an Anion Sensor—ESI-MS and Fluorescence Study. Int. J. Mol. Sci. 2024, 25, 3467. https://doi.org/10.3390/ijms25063467
Kowalska M, Wieczorek R, Gawryszewska P, Bąchor R. A Novel Bisquaternary Ammonium Compound as an Anion Sensor—ESI-MS and Fluorescence Study. International Journal of Molecular Sciences. 2024; 25(6):3467. https://doi.org/10.3390/ijms25063467
Chicago/Turabian StyleKowalska, Marta, Robert Wieczorek, Paula Gawryszewska, and Remigiusz Bąchor. 2024. "A Novel Bisquaternary Ammonium Compound as an Anion Sensor—ESI-MS and Fluorescence Study" International Journal of Molecular Sciences 25, no. 6: 3467. https://doi.org/10.3390/ijms25063467





