Adult Neurogenesis of Teleost Fish Determines High Neuronal Plasticity and Regeneration
Abstract
:1. Introduction
2. Biological Features of NSCPs in Pacific Salmon
3. NSCs in the Adult Brain
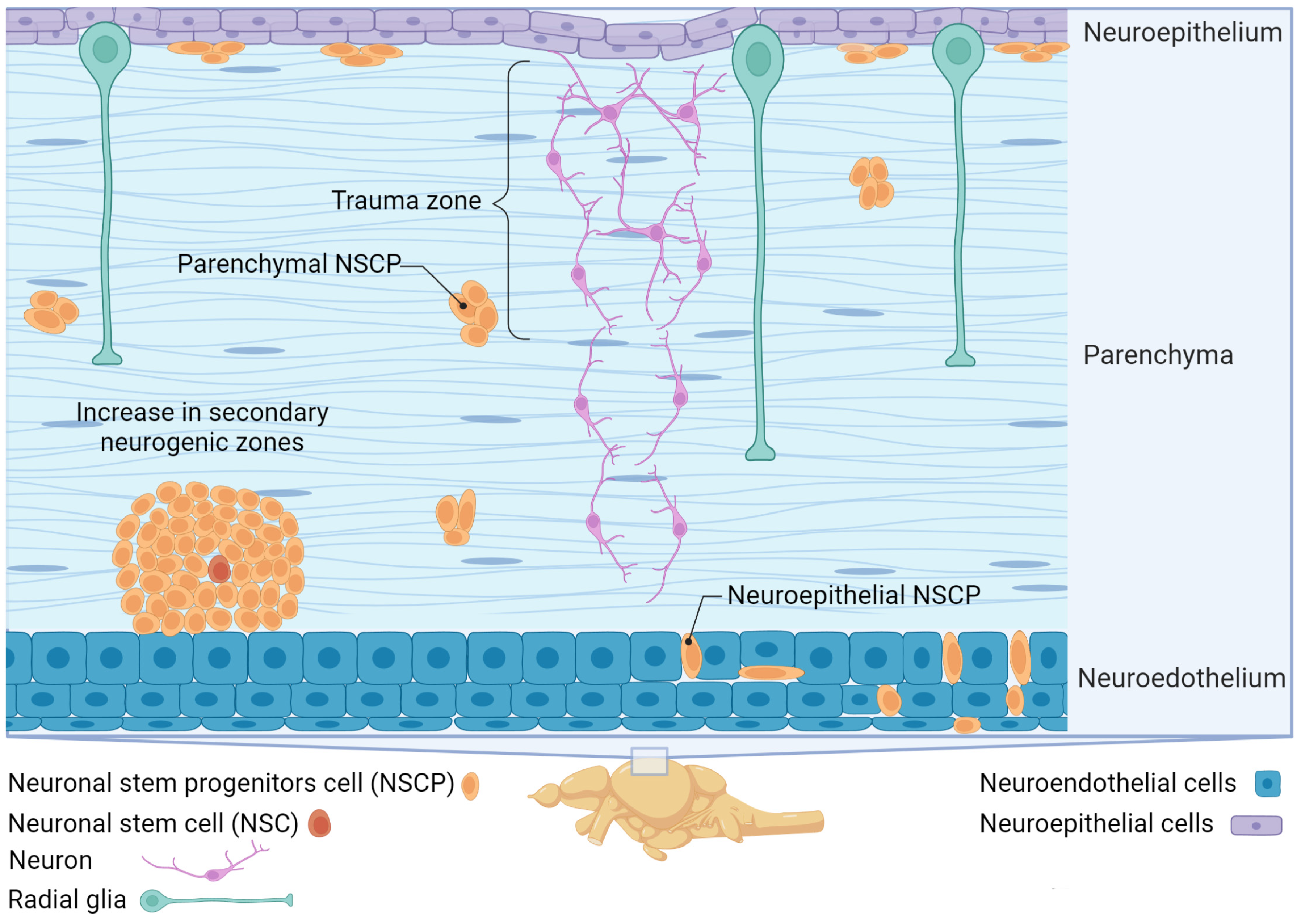
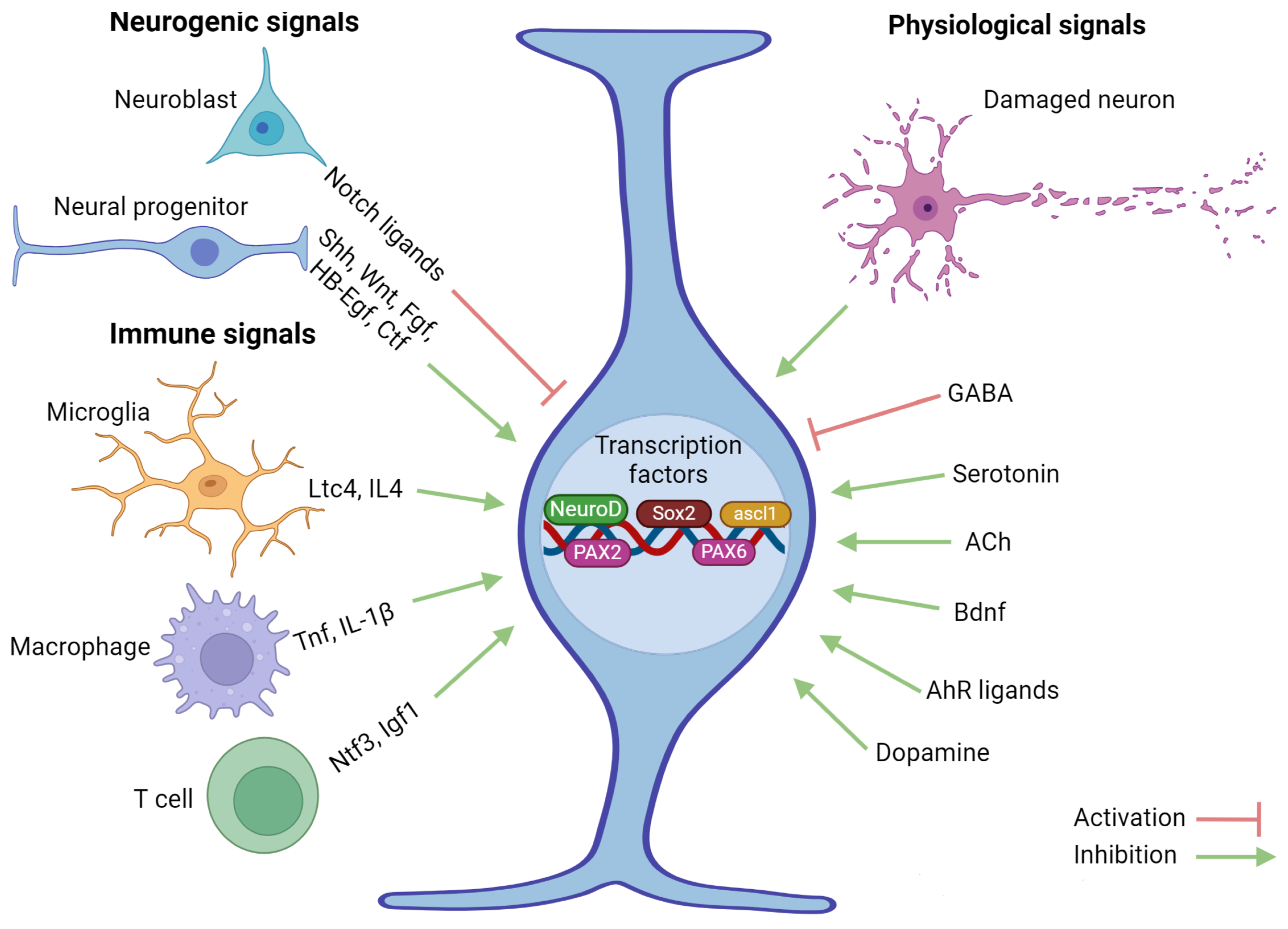
4. Creation of aNSCs in Intact and Damaged Brains
5. A Comparison of Functional, Structural and Physiological Properties of RGCs in the Zebrafish Brain and Mammalian Glia
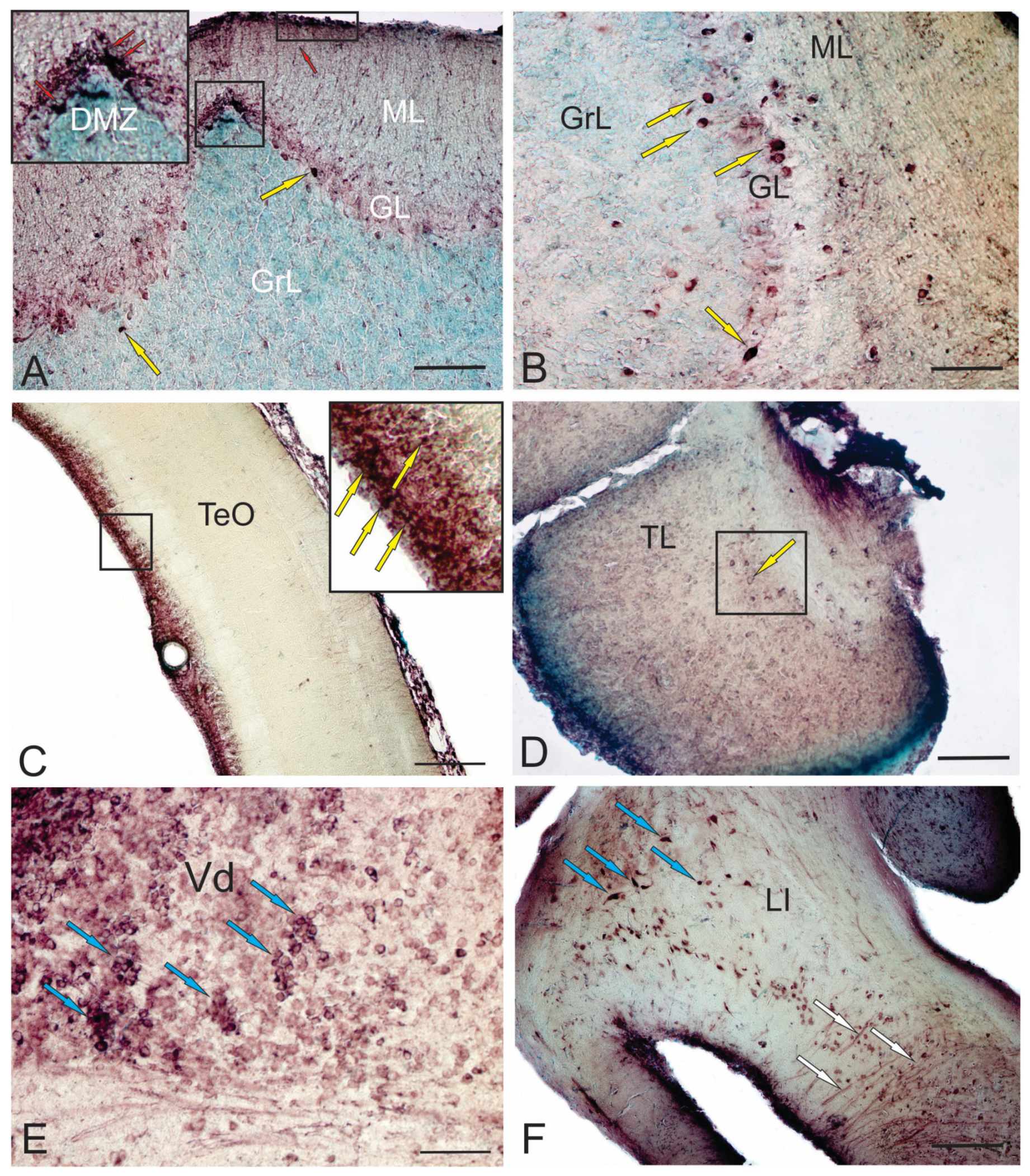
6. Constitutive and Reparative Neurogenesis in the Pacific Salmon Brain
7. Glial Plasticity in Response to Disease and Injury
8. Heterogeneity of Stem Cell Pools and Radial Glia
9. The Role of Astroglia in the Functioning of Neural Circuits and Animal Behavior
10. Conclusions
- (i).
- How can astroglia contribute to the resistance or vulnerability of neural circuits to injury or disease?
- (ii).
- What are the potential therapeutic targets in astroglial signaling pathways?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Harris, L.; Zalucki, O.; Piper, M.; Heng, J.I.-T. Insights into the Biology and Therapeutic Applications of Neural Stem Cells. Stem Cells Int. 2016, 2016, 9745315. [Google Scholar] [CrossRef] [PubMed]
- Diotel, N.; Lübke, L.; Strähle, U.; Rastegar, S. Common and Distinct Features of Adult Neurogenesis and Regeneration in the Telencephalon of Zebrafish and Mammals. Front. Neurosci. 2020, 14, 568930. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, T. Vertebrate Neural Stem Cells: Development, Plasticity, and Regeneration. Keio J. Med. 2016, 65, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Clavreul, S.; Dumas, L.; Loulier, K. Astrocyte development in the cerebral cortex: Complexity of their origin, genesis, and maturation. Front. Neurosci. 2022, 16, 916055. [Google Scholar] [CrossRef] [PubMed]
- Butruille, L.; Sébillot, A.; Ávila, K.; Vancamp, P.; Demeneix, B.A.; Pifferi, F.; Remaud, S. Increased oligodendrogenesis and myelination in the subventricular zone of aged mice and gray mouse lemurs. Stem Cell Rep. 2023, 18, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.; Zhang, R.; Taylor, V. Notch and Neurogenesis. Adv. Exp. Med. Biol. 2018, 1066, 223–234. [Google Scholar]
- Ma, Y.; Xie, H.; Du, X.; Wang, L.; Jin, X.; Zhang, Q.; Han, Y.; Sun, S.; Wang, L.; Li, X.; et al. In vivo chemical reprogramming of astrocytes into neurons. Cell Discov. 2021, 7, 12. [Google Scholar] [CrossRef]
- Lampada, A.; Taylor, V. Notch signaling as a master regulator of adult neurogenesis. Front. Neurosci. 2023, 17, 1179011. [Google Scholar] [CrossRef]
- Tai, W.; Xu, X.-M.; Zhang, C.-L. Regeneration Through in vivo Cell Fate Reprogramming for Neural Repair. Front. Cell. Neurosci. 2020, 14, 107. [Google Scholar] [CrossRef]
- Stukaneva, M.E.; Pushchina, E.V. Constitutive Neurogenesis in the Brain of Different Vertebrate Groups. Neurophysiology 2020, 52, 456–470. [Google Scholar] [CrossRef]
- Mhalhel, K.; Sicari, M.; Pansera, L.; Chen, J.; Levanti, M.; Diotel, N.; Rastegar, S.; Germanà, A.; Montalbano, G. Zebrafish: A Model Deciphering the Impact of Flavonoids on Neurodegenerative Disorders. Cells 2023, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Alunni, A.; Bally-Cuif, L. A comparative view of regenerative neurogenesis in vertebrates. Development 2016, 143, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Bosak, V.; Murata, K.; Bludau, O.; Brand, M. Role of the immune response in initiating central nervous system regeneration in vertebrates: Learning from the fish. Int. J. Dev. Biol. 2018, 62, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Pushchina, E.V.; Zharikova, E.I.; Varaksin, A.A. Mechanical Brain Injury Increases Cells’ Production of Cystathionine β-Synthase and Glutamine Synthetase, but Reduces Pax2 Expression in the Telencephalon of Juvenile Chum Salmon, Oncorhynchus keta. Int. J. Mol. Sci. 2021, 22, 1279. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Zharikova, E.I.; Varaksin, A.A. Expression of Doublecortin, Glial Fibrillar Acidic Protein, and Vimentin in the Intact Subpallium and after Traumatic Injury to the Pallium in Juvenile Salmon, Oncorhynchus masou. Int. J. Mol. Sci. 2022, 23, 1334. [Google Scholar] [CrossRef]
- Too, L.K.; Gracie, G.; Hasic, E.; Iwakura, J.H.; Cherepanoff, S. Adult human retinal Müller glia display distinct peripheral and macular expression of CD117 and CD44 stem cell-associated proteins. Acta Histochem. 2017, 119, 142–149. [Google Scholar] [CrossRef]
- Krylov, A.; Yu, S.; Veen, K.; Newton, A.; Ye, A.; Qin, H.; He, J.; Jusuf, P.R. Heterogeneity in quiescent Müller glia in the uninjured zebrafish retina drive differential responses following photoreceptor ablation. Front. Mol. Neurosci. 2023, 16, 1087136. [Google Scholar] [CrossRef]
- Lourenço, R.; Brandão, A.S.; Borbinha, J.; Gorgulho, R.; Jacinto, A. Yap Regulates Müller Glia Reprogramming in Damaged Zebrafish Retinas. Front. Cell Dev. Biol. 2021, 9, 667796. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Kapustyanov, I.A.; Varaksin, A.A. Neural Stem Cells/Neuronal Precursor Cells and Postmitotic Neuroblasts in Constitutive Neurogenesis and After, Traumatic Injury to the Mesencephalic Tegmentum of Juvenile Chum Salmon, Oncorhynchus keta. Brain Sci. 2020, 10, 65. [Google Scholar] [CrossRef]
- Furlan, G.; Cuccioli, V.; Vuillemin, N.; Dirian, L.; Jan, L.Y.; Corti, P. The KCNQ channel opener retigabine inhibits zebrafish brain tumor initiation. PLoS ONE 2017, 12, e0188103. [Google Scholar]
- Zhang, G.; Lübke, L.; Chen, F.; Beil, T.; Takamiya, M.; Diotel, N.; Strähle, U.; Rastegar, S. Neuron-Radial Glial Cell Communication via BMP/Id1 Signaling Is Key to Long-Term Maintenance of the Regenerative Capacity of the Adult Zebrafish Telencephalon. Cells 2021, 10, 2794. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Bykova, M.E.; Varaksin, A.A. Posttraumatic expression of Aromatase B, Glutamine Synthetase, and Cysta-thionine-β-Synthase in the Cerebellum of Juvenile Chum Salmon, Oncorhynchus keta. Int. J. Mol. Sci. 2024, 25, 3299. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Stukaneva, M.E.; Varaksin, A.A. Hydrogen Sulfide Modulates Adult and Reparative Neurogenesis in the Cerebellum of Juvenile Masu Salmon, Oncorhynchus masou. Int. J. Mol. Sci. 2020, 21, 9638. [Google Scholar] [CrossRef] [PubMed]
- Pushchina, E.V.; Kapustyanov, I.A.; Varaksin, A.A. Proliferation and Neuro- and Gliogenesis in Normal and Mechanically Damaged Mesencephalic Tegmentum in Juvenile Chum Salmon, Oncorhynchus keta. Russ. J. Dev. Biol. 2019, 50, 59–76. [Google Scholar] [CrossRef]
- Ueda, H. Physiological mechanism of homing migration in Pacific salmon from behavioral to molecular biological approaches. Gen. Comp. Endocrinol. 2011, 170, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Strähle, U.; Scholpp, S. Neurogenesis in zebrafish—From embryo to adult. Neural Dev. 2013, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Pushchina, E.V.; Marinina, K.S.; Myasoyedov, S.D. Hydrogen Sulfide and Pathophysiology of the CNS. Neurophysiology 2020, 52, 308–321. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, D.X.; Wang, F.W.; Zhang, Q.; Du, Z.X.; Zhan, J.M.; Yuan, Q.H.; Ling, E.A.; Hao, A.J. L-Cysteine promotes the proliferation and differentiation of neural stem cells via the CBS/H2S pathway. Neuroscience 2013, 237, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Zupanc, G.K.; Sîrbulescu, R.F. Teleost fish as a model system to study successful regeneration of the central nervous system. Curr. Top. Microbiol. Immunol. 2013, 367, 193–233. [Google Scholar]
- Koch, I.J.; Narum, S.R. An evaluation of the potential factors affecting lifetime reproductive success in salmonids. Evol. Appl. 2021, 14, 1929–1957. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Varaksin, A.A.; Stukaneva, M.E.; Zharikova, E.I. Adult and Reparative Neurogenesis in Fish Brain. In Peripheral Nerve Regeneration—From Surgery to New Therapeutic Approaches Including Biomaterials and Cell-Based Therapies Development; IntechOpen: London, UK, 2017; pp. 175–195. [Google Scholar]
- Taniyama, N.; Kaneko, N.; Inatani, Y.; Miyakoshi, Y.; Shimizu, M. Effects of seawater transfer and fasting on the endocrine and biochemical growth indices in juvenile chum salmon (Oncorhynchus keta). Gen. Comp. Endocrinol. 2016, 236, 146–156. [Google Scholar] [CrossRef]
- Kitada, S.; Kishino, H. Population structure of chum salmon and selection on the markers collected for stock identification. Ecol. Evol. 2021, 11, 13972–13985. [Google Scholar] [CrossRef]
- Beacham, T.D.; Wallace, C.; Jonsen, K.; McIntosh, B.; Candy, J.R.; Rondeau, E.B.; Moore, J.S.; Bernatchez, L.; Withler, R.E. Accurate estimation of conservation unit contribution to coho salmon mixed-stock fisheries in British Columbia, Canada, using direct DNA sequencing for single nucleotide polymorphisms. Can. J. Fish. Aquat. Sci. 2020, 77, 1302–1315. [Google Scholar] [CrossRef]
- Dray, N.; Than-Trong, E.; Bally-Cuif, L. Neural stem cell pools in the vertebrate adult brain: Homeostasis from cell-autonomous decisions or community rules? Bioessays 2021, 43, e2000228. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.R.; Hong, C.J.; Kim, J.Y.; Kim, E.-K.; Sun, W.; Yu, S.-W. Control of adult neurogenesis by programmed cell death in the mammalian brain. Mol. Brain 2016, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef]
- Barbosa, J.S.; Ninkovic, J.; Pflanz, R.; Oberst, P.; Mizutani, K. N-cadherin and integrin α3β1 co-ordinately regulate actin dy-namics in migrating zebrafish neural crest. J. Cell Sci. 2015, 128, 3253–3266. [Google Scholar]
- Alunni, A.; Krecsmarik, M.; Bosco, A.; Galant, S.; Pan, L.; Moens, C.B.; Bally-Cuif, L. Notch3 signaling gates cell cycle entry and limits neural stem cell amplification in the adult pallium. Development 2013, 140, 3335–3347. [Google Scholar] [CrossRef] [PubMed]
- Labusch, M.; Mancini, L.; Morizet, D.; Bally-Cuif, L. Conserved and Divergent Features of Adult Neurogenesis in Zebrafish. Front. Cell Dev. Biol. 2020, 8, 525. [Google Scholar] [CrossRef]
- Ganz, J.; Brand, M. Adult Neurogenesis in Fish. Cold Spring Harb. Perspect. Biol. 2016, 8, a019018. [Google Scholar] [CrossRef] [PubMed]
- März, M.; Schmidt, R.; Rastegar, S.; Strähle, U. Expression of the transcription factor Olig2 in proliferating cells in the adult zebrafish telencephalon. Dev. Dyn. 2010, 239, 3336–3349. [Google Scholar] [CrossRef] [PubMed]
- Adolf, B.; Chapouton, P.; Lam, C.S.; Topp, S.; Tannhauser, B.; Strahle, U. Conserved and acquired features of adult neuro-genesis in the zebrafish telencephalon. Dev. Biol. 2006, 295, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Grandel, H.; Kaslin, J.; Ganz, J.; Wenzel, I.; Brand, M. Neural stem cells and neurogenesis in the adult zebrafish brain: Origin, proliferation dynamics, migration and cell fate. Dev. Biol. 2006, 295, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Reimer, M.M.; Kuscha, V.; Wyatt, C.; Sörensen, I.; Frank, R.E.; Knüwer, M.; Becker, T.; Becker, C.G. Sonic Hedgehog Is a Polarized Signal for Motor Neuron Regeneration in Adult Zebrafish. J. Neurosci. 2009, 29, 15073–15082. [Google Scholar] [CrossRef]
- Meyer, T.J.; Rosenkrantz, J.L.; Carbone, L.; Chavez, S.L. Endogenous Retroviruses: With Us and against Us. Front. Chem. 2017, 5, 23. [Google Scholar] [CrossRef]
- Rothenaigner, I.; Krecsmarik, M.; Hayes, J.A.; Bahn, B.; Lepier, A.; Fortin, G.; Götz, M.; Jagasia, R.; Bally-Cuif, L. Clonal analysis by distinct viral vectors identifies bona fide neural stem cells in the adult zebrafish telencephalon and characterizes their division properties and fate. Development 2011, 138, 1459–1469. [Google Scholar] [CrossRef]
- Chapouton, P.; Skupien, P.; Hesl, B.; Coolen, M.; Moore, J.C.; Madelaine, R.; Kremmer, E.; Faus-Kessler, T.; Blader, P.; Lawson, N.D.; et al. Notch Activity Levels Control the Balance between Quiescence and Recruitment of Adult Neural Stem Cells. J. Neurosci. 2010, 30, 7961–7974. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Zharikova, E.I.; Varaksin, A.A.; Prudnikov, I.M.; Tsyvkin, V.N. Proliferation, Adult Neuronal Stem Cells and Cells Migration in Pallium during Constitutive Neurogenesis and after Traumatic Injury of Telencephalon of Juvenile Masu Salmon, Oncorhynchus masou. Brain Sci. 2020, 10, 222. [Google Scholar] [CrossRef]
- Hui, S.P.; Nag, T.C.; Ghosh, S. Neural cells and their progenitors in regenerating zebrafish spinal cord. Int. J. Dev. Biol. 2020, 64, 353–366. [Google Scholar] [CrossRef]
- Ernst, A.; Alkass, K.; Bernard, S.; Salehpour, M.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; Frisén, J. Neurogenesis in the Striatum of the Adult Human Brain. Cell 2014, 156, 1072–1083. [Google Scholar] [CrossRef]
- Skaggs, K.; Goldman, D.; Parent, J.M. Excitotoxic brain injury in adult zebrafish stimulates neurogenesis and long-distance neuronal integration. Glia 2014, 62, 2061–2079. [Google Scholar] [CrossRef]
- Ballout, N.; Frappé, I.; Péron, S.; Jaber, M.; Zibara, K.; Gaillard, A. Development and Maturation of Embryonic Cortical Neurons Grafted into the Damaged Adult Motor Cortex. Front. Neural Circuits 2016, 10, 55. [Google Scholar] [CrossRef]
- Tonchev, A.B. Brain ischemia, neurogenesis, and neurotrophic receptor expression in primates. Arch. Ital. Biol. 2011, 149, 225–231. [Google Scholar] [CrossRef]
- Jiang, M.; Jang, S.E.; Zeng, L. The Effects of Extrinsic and Intrinsic Factors on Neurogenesis. Cells 2023, 12, 1285. [Google Scholar] [CrossRef]
- Bartkowska, K.; Tepper, B.; Turlejski, K.; Djavadian, R. Postnatal and Adult Neurogenesis in Mammals, Including Marsupials. Cells 2022, 11, 2735. [Google Scholar] [CrossRef] [PubMed]
- Calzolari, F.; Michel, J.; Baumgart, E.V.; Theis, F.; Götz, M.; Ninkovic, J. Fast clonal expansion and limited neural stem cell self-renewal in the adult subependymal zone. Nat. Neurosci. 2015, 18, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Kroehne, V.; Freudenreich, D.; Hans, S.; Kaslin, J.; Brand, M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development 2011, 138, 4831–4841. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Ninomiya, M.; Acosta, P.H.; García-Verdugo, J.M.; Sunabori, T.; Sakaguchi, M.; Adachi, K.; Kojima, T.; Hirota, Y.; Kawase, T.; et al. Subventricular Zone-Derived Neuroblasts Migrate and Differentiate into Mature Neurons in the Post-Stroke Adult Striatum. J. Neurosci. 2006, 26, 6627–6636. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, G.; Momiyama, T.; Oya, S.; Takai, K.; Tanaka, J.I.; Higashiyama, S.; Saito, N.; Kirino, T.; Kawahara, N. Induction of striatal neurogenesis and generation of region-specific functional mature neurons after ischemia by growth factors: Laboratory investigation. J. Neurosurg. 2010, 113, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Kawasawa, Y.I.; Cheng, F.; Zhu, Y.; Xu, X.; Li, M.; Sousa, A.M.M.; Pletikos, M.; Meyer, K.A.; Sedmak, G.; et al. Spatio-temporal transcriptome of the human brain. Nature 2013, 478, 483–489. [Google Scholar] [CrossRef]
- Kolb, B.; Morshead, C.; Gonzalez, C.; Kim, M.; Gregg, C.; Shingo, T.; Weiss, S. Growth Factor-Stimulated Generation of New Cortical Tissue and Functional Recovery after Stroke Damage to the Motor Cortex of Rats. J. Cereb. Blood Flow Metab. 2013, 33, 340–348. [Google Scholar] [CrossRef]
- Jurkowski, M.P.; Bettio, L.; Woo, E.K.; Patten, A.; Yau, S.-Y.; Gil-Mohapel, J. Beyond the Hippocampus and the SVZ: Adult Neurogenesis Throughout the Brain. Front. Cell. Neurosci. 2020, 14, 576444. [Google Scholar] [CrossRef]
- Christie, K.J.; Turnley, A.M. Regulation of endogenous neural stem/progenitor cells for neural repair—Factors that promote neurogenesis and gliogenesis in the normal and damaged brain. Front. Cell. Neurosci. 2013, 6, 70. [Google Scholar] [CrossRef]
- Gil, J.M.; Mohapel, P.; Araújo, I.M.; Popovic, N.; Li, J.-Y.; Brundin, P.; Petersén, Ǻ. Reduced hippocampal neurogenesis in R6/2 transgenic Huntington’s disease mice. Neurobiol. Dis. 2005, 20, 744–751. [Google Scholar] [CrossRef]
- Simpson, J.M.; Gil-Mohapel, J.; Pouladi, M.A.; Ghilan, M.; Xie, Y.; Hayden, M.R.; Christie, B.R. Altered adult hippocampal neurogenesis in the YAC128 transgenic mouse model of Huntington disease. Neurobiol. Dis. 2011, 41, 249–260. [Google Scholar] [CrossRef]
- Batista, C.M.C.; Kippin, T.E.; Willaime-Morawek, S.; Shimabukuro, M.K.; Akamatsu, W.; van der Kooy, D. A Progressive and Cell Non-Autonomous Increase in Striatal Neural Stem Cells in the Huntington’s Disease R6/2 Mouse. J. Neurosci. 2006, 26, 10452–10460. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Gage, F.H. Adult neurogenesis: Bridging the gap between mice and humans. Trends Cell Biol. 2014, 24, 558–563. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Varaksin, A.A.; Obukhov, D.K. Molecular Markers of Adult Neurogenesis in the Telencephalon and Tectum of Rainbow Trout, Oncorhynchus mykiss. Int. J. Mol. Sci. 2022, 23, 1188. [Google Scholar] [CrossRef] [PubMed]
- Robins, S.C.; Trudel, E.; Rotondi, O.; Liu, X.; Djogo, T.; Kryzskaya, D.; Bourque, C.W.; Kokoeva, M.V. Evidence for NG2-glia Derived, Adult-Born Functional Neurons in the Hypothalamus. PLoS ONE 2013, 8, e78236. [Google Scholar] [CrossRef] [PubMed]
- Ganz, J.; Kroehne, V.; Freudenreich, D.; Machate, A.; Geffarth, M.; Braasch, I.; Kaslin, J.; Brand, M. Subdivisions of the adult zebrafish pallium based on molecular marker analysis. F1000Research 2014, 3, 308. [Google Scholar] [CrossRef]
- Kizil, C.; Kaslin, J.; Kroehne, V.; Brand, M. Adult neurogenesis and brain regeneration in zebrafish. Dev. Neurobiol. 2012, 72, 429–461. [Google Scholar] [CrossRef]
- Ganz, J.; Kaslin, J.; Freudenreich, D.; Machate, A.; Geffarth, M.; Brand, M. Subdivisions of the adult zebrafish subpallium by molecular marker analysis. J. Comp. Neurol. 2012, 520, 633–655. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Ziman, M. Pax genes during neural development and their potential role in neuroregeneration. Prog. Neurobiol. 2011, 95, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, E.V.; Barbosa, J.S.; Bally-Cuif, L.; Götz, M.; Ninkovic, J. Stab wound injury of the zebrafish telencephalon: A model for comparative analysis of reactive gliosis. Glia 2012, 60, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Encinas, J.M.; Deudero, J.J.; Chancey, J.H.; Enikolopov, G.; Overstreet-Wadiche, L.S.; Tsirka, S.E.; Maletic-Savatic, M. Microglia Shape Adult Hippocampal Neurogenesis through Apoptosis-Coupled Phagocytosis. Cell Stem Cell 2015, 7, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Dirian, L.; Galant, S.; Coolen, M.; Chen, W.; Bedu, S.; Houart, C.; Bally-Cuif, L. Spatial regionalization and heterochrony in the formation of adult pallial neural stem cells in zebrafish. Development 2014, 141, 4831–4840. [Google Scholar]
- Li, P.; Quan, W.; Wang, Z.; Chen, Y.; Zhang, H.; Zhou, Y. AD7c-NTP Impairs Adult Striatal Neurogenesis by Affecting the Biological Function of MeCP2 in APP/PSl Transgenic Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2021, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Takahashi, K.; Takata, K.; Eguchi, A.; Yamato, M.; Kume, S.; Nakano, M.; Watanabe, Y.; Kataoka, Y. Noninvasive Evaluation of Cellular Proliferative Activity in Brain Neurogenic Regions in Rats under Depression and Treatment by Enhanced [18F]FLT-PET Imaging. J. Neurosci. 2016, 36, 8123–8131. [Google Scholar] [CrossRef]
- Leal-Galicia, P.; Chávez-Hernández, M.E.; Mata, F.; Mata-Luévanos, J.; Rodríguez-Serrano, L.M.; Tapia-De-Jesús, A.; Buenrostro-Jáuregui, M.H. Adult Neurogenesis: A Story Ranging from Controversial New Neurogenic Areas and Human Adult Neurogenesis to Molecular Regulation. Int. J. Mol. Sci. 2021, 22, 11489. [Google Scholar] [CrossRef]
- Chang, Z.; Wang, Q.-Y.; Li, L.-H.; Jiang, B.; Zhou, X.-M.; Zhu, H.; Sun, Y.-P.; Pan, X.; Tu, X.-X.; Wang, W.; et al. Potential Plausible Role of Stem Cell for Treating Depressive Disorder: A Retrospective Review. Mol. Neurobiol. 2023, 1–19. [Google Scholar] [CrossRef]
- Pacary, E.; Martynoga, B.; Guillemot, F. Crucial first steps: The evolution of the neocortex. Neuron 2012, 76, 209–221. [Google Scholar] [CrossRef]
- Götz, M. Radial glia: Progenitor, pathway, and partner. Neuron 2012, 74, 415–432. [Google Scholar]
- Hansen, D.V.; Lui, J.H.; Parker, P.R.; Kriegstein, A.R. Neurogenic radial glia in the outer subventricular zone of human neo-cortex. Nature 2010, 464, 554–561. [Google Scholar] [CrossRef]
- Lui, J.H.; Hansen, D.V.; Kriegstein, A.R. Development and Evolution of the Human Neocortex. Cell 2011, 146, 18–36. [Google Scholar] [CrossRef]
- Raponi, E.; Agenes, F.; Delphin, C.; Assard, N.; Baudier, J.; Legraverend, C.; Deloulme, J. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia 2006, 55, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Buffo, A.; Götz, M. The novel roles of glial cells revisited: The contribution of radial glia and astrocytes to neurogenesis. Curr. Top. Dev. Biol. 2005, 69, 67–99. [Google Scholar] [PubMed]
- Götz, M.; Sirko, S.; Beckers, J.; Irmler, M. Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, In vitro potential, and Genome-wide expression analysis. Glia 2015, 63, 1452–1468. [Google Scholar] [CrossRef]
- Steiner, B.; Tata, M.; Frisén, J. A restricted population of cortical interneurons from primate: A comparison to neurogenesis in rodents. Cereb. Cortex 2019, 29, 2668–2675. [Google Scholar]
- Van Den Broeck, W.; Kempermann, G. The neurogenic niche in the postnatal and adult mouse brain. In Neurogenesis in the Adult Brain; Springer: Berlin/Heidelberg, Germany, 2012; Volume 1, pp. 107–127. [Google Scholar]
- Urbã¡N, N.; Guillemot, F. Neurogenesis in the embryonic and adult brain: Same regulators, different roles. Front. Cell. Neurosci. 2014, 8, 396. [Google Scholar] [CrossRef]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Rodriguez, J.J.; Verkhratsky, A. Neurogenesis in the subventricular zone of the adult brain. Eur. J. Neurosci. 2011, 33, 1155–1163. [Google Scholar]
- Tincer, G.; Mashkaryan, V.; Bhattarai, P.; Kizil, C. Neural stem/progenitor cells in Alzheimer’s disease. Yale J. Biol. Med. 2016, 89, 23–35. [Google Scholar]
- Nato, G.; Caramello, A.; Trova, S.; Avataneo, V.; Rolando, C.; Taylor, V.; Buffo, A.; Peretto, P.; Luzzati, F. Striatal astrocytes produce neuroblasts in an excitotoxic model of Huntington’s disease. Development 2015, 142, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Tanzi, R.E. Is Alzheimer’s Disease a Neurogenesis Disorder? Cell Stem Cell Previews 2019, 25, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Ahlenius, H.; Devaraju, K.; Monni, E.; Oki, K.; Wattananit, S.; Darsalia, V.; Iosif, R.E.; Torper, O.; Wood, J.C.; Braun, S.; et al. Adaptor Protein LNK Is a Negative Regulator of Brain Neural Stem Cell Proliferation after Stroke. J. Neurosci. 2012, 32, 5151–5164. [Google Scholar] [CrossRef]
- Prins, M.; Greco, T.; Alexander, D.; Giza, C.C. The pathophysiology of traumatic brain injury at a glance. Dis. Model. Mech. 2013, 6, 1307–1315. [Google Scholar] [CrossRef]
- Llorens-Bobadilla, E.; Zhao, S.; Baser, A.; Saiz-Castro, G.; Zwadlo, K.; Martin-Villalba, A. Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells that Become Activated upon Brain Injury. Cell Stem Cell 2015, 17, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; März, M.; Strähle, U. gfap and nestin reporter lines reveal characteristics of neural progenitors in the adult zebrafish brain. Dev. Dyn. 2009, 238, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Wullimann, M.F. BrdU-, neuroD (nrd)- and Hu-studies reveal unusual non-ventricular neurogenesis in the postembryonic zebrafish forebrain. Mech. Dev. 2009, 126, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Than-Trong, E.; Bally-Cuif, L. Radial glia and neural progenitors in the adult zebrafish central nervous system. Glia 2015, 63, 1406–1428. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.L.; Marz, M.; Strahle, U. Notch and Neurogenesis. Neuroscientist 2016, 22, 277–287. [Google Scholar]
- Cosacak, M.I.; Bhattarai, P.; Reinhardt, S.; Petzold, A.; Dahl, A.; Zhang, Y.; Kizil, C. Single-cell transcriptomics of the adult zebrafish brain. J. Exp. Biol. 2019, 222, jeb201008. [Google Scholar]
- Than-Trong, E.; Labusch, M.; Mannioui, A. Endothelial cell-derived non-glioblastoma cancer stem cells contribute to tumor vascularization in zebrafish xenotransplants. Dis. Mod. Mech. 2020, 13, dmm045971. [Google Scholar]
- Dorsemans, A.C.; Soule, J.; Deloulme, J.C.; Macari, F.; Steinbusch, H.W.; Boudin, H. Age-related neuronal degeneration: Com-plementary roles of nucleolin and PARP1. Neurobiol. Aging 2017, 50, 169–178. [Google Scholar]
- Rastegar, S.; Parvin, S.; Bakhtiarizadeh, M.R.; Ghaderian, S.M.; Rahimi-Mianji, G. The apoptotic genes. Bcl-2 and Bax, and their ratio in the development of hypoxic-ischemic encephalopathy. J. Cell. Biochem. 2019, 120, 1976–1984. [Google Scholar]
- Kalamakis, G.; Brüne, D.; Ravichandran, S.; Bolz, J.; Fan, W.; Ziebell, F.; Stiehl, T.; Catalá-Martinez, F.; Kupke, J.; Zhao, S.; et al. Quiescence Modulates Stem Cell Maintenance and Regenerative Capacity in the Aging Brain. Cell 2019, 176, 1407–1419.e14. [Google Scholar] [CrossRef]
- Mizrak, D.; Bayin, N.S.; Yuan, J.; Liu, Z.; Suciu, R.M.; Niphakis, M.J.; Ngo, N.; Lum, K.M.; Cravatt, B.F.; Joyner, A.L.; et al. Single-Cell Profiling and SCOPE-Seq Reveal Lineage Dynamics of Adult Ventricular-Subventricular Zone Neurogenesis and NOTUM as a Key Regulator. Cell Rep. 2019, 31, 107805. [Google Scholar] [CrossRef]
- Petrik, D.; Myoga, M.H.; Grade, S.; Gerkau, N.J.; Pusch, M.; Rose, C.R.; Grothe, B.; Götz, M. Epithelial Sodium Channel Regulates Adult Neural Stem Cell Proliferation in a Flow-Dependent Manner. Cell Stem Cell 2018, 22, 865–878.e8. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Wang, G.; Wang, Y.; Han, D.; Bi, J.; Yuan, Y. Neurogenesis in the adult brain and its implications for Alzheimer’s disease. J. Cell. Mol. Med. 2018, 22, 3103–3110. [Google Scholar]
- Ming, G.-L.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.J.; Kim, J.Y. Stereotaxic Infusion of 17β-Estradiol Enhances Recovery After Traumatic Brain Injury in Female Rats. Endocrinology 2015, 156, 4351–4360. [Google Scholar]
- Hu, L.; Lu, M.; Tiong, C.X.; Dawe, G.S.; Hu, G.; Bian, J. Neuroprotective effects of hydrogen sulfide on Parkinson’s disease rat models. Aging Cell 2010, 9, 135–146. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen Sulfide as a Neuromodulator. Mol. Neurobiol. 2002, 26, 013–020. [Google Scholar] [CrossRef]
- Alexandrou, M.A.; Swartz, B.A.; Matzke, N.J.; Oakley, T.H. Genome duplication and multiple evolutionary origins of complex migratory behavior in Salmonidae. Mol. Phylogenet. Evol. 2013, 69, 514–523. [Google Scholar] [CrossRef]
- Berthelot, C.; Brunet, F.; Chalopin, D.; Juanchich, A.; Bernard, M.; Noël, B.; Bento, P.; Da Silva, C.; Labadie, K.; Alberti, A.; et al. The rainbow trout genome provides novel insights into evolution after whole-genome duplication in vertebrates. Nat. Commun. 2014, 5, 3657. [Google Scholar] [CrossRef]
- Ohno, S. Evolution by Gene Duplication; Springer: New York, NY, USA, 1970; 160p, ISBN 978-3-642-86661-6. [Google Scholar]
- Sémon, M.; Wolfe, K.H. Consequences of genome duplication. Curr. Opin. Genet. Dev. 2007, 17, 505–512. [Google Scholar] [CrossRef]
- Waples, R.S.; Pess, G.R.; Beechie, T. Evolutionary history of Pacific salmon in dynamic environments. Evol. Appl. 2008, 1, 189–206. [Google Scholar] [CrossRef]
- Zhivotovsky, L.A.; Yurchenko, A.A.; Nikitin, V.D.; Safronov, S.N.; Shitova, M.V.; Zolotukhin, S.F.; Makeev, S.S.; Weiss, S.; Rand, P.S.; Semenchenko, A.Y. Eco-geographic units, population hierarchy, and a two-level conservation strategy with reference to a critically endangered salmonid, Sakhalin taimen Parahucho perryi. Conserv. Genet. 2014, 16, 431–441. [Google Scholar] [CrossRef]
- Allendorf, F.W.; Thorgaard, G.H. Tetraploidy and Evolution of Salmonid Fishes. In Evolutionary Genetics of Fish; Turner, B.J., Ed.; Springer: Boston, MA, USA, 1984; pp. 1–53. [Google Scholar]
- Afanas’ev, K.I.; Rubtsova, G.A.; Shitova, M.V.; Malinina, T.V.; Rakitskaya, T.A.; Prokhorovskaya, V.D.; Shevlyakov, E.A.; Zavarina, L.O.; Bachevskaya, L.T.; Chereshnev, I.A.; et al. Population structure of chum salmon Oncorhynchus keta in the Russian Far East, as revealed by microsatellite markers. Russ. J. Mar. Biol. 2011, 37, 42–51. [Google Scholar] [CrossRef]
- Lu, J.; Peatman, E.; Tang, H.; Lewis, J.; Liu, Z. Profiling of gene duplication patterns of sequenced teleost genomes: Evidence for rapid lineage-specific genome expansion mediated by recent tandem duplications. BMC Genom. 2012, 13, 246. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Varaksin, A.A.; Obukhov, D.K. The Pax2 and Pax6 Transcription Factors in the Optic Nerve and Brain of Trout Oncorhynchus mykiss after a Mechanical Eye Injury. Russ. J. Dev. Biol. 2018, 49, 264–290. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Varaksin, A.A. Neurolin expression in the optic nerve and immunoreactivity of Pax6-positive niches in the brain of rainbow trout Oncorhynchus mykiss after unilateral eye injury. Neural Regen. Res. 2019, 14, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Pushchina, E.V.; Kapustyanov, I.A.; Shamshurina, E.V.; Varaksin, A.A. A Confocal Microscopic Study of Gene Transfer into the Mesencephalic Tegmentum of Juvenile Chum Salmon, Oncorhynchus keta, Using Mouse Adeno-Associated Viral Vectors. Int. J. Mol. Sci. 2021, 22, 5661. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ueda, Y.; Ohshima, T. Wnt signaling regulates proliferation and differentiation of radial glia in regenerating zebrafish spinal cord. Dev. Neurobiol. 2018, 78, 955–964. [Google Scholar]
- Ghaddar, B.; Lübke, L.; Couret, D.; Rastegar, S.; Diotel, N. Cellular Mechanisms Participating in Brain Repair of Adult Zebrafish and Mammals after Injury. Cells 2021, 10, 391. [Google Scholar] [CrossRef]
- Ueda, Y.; Shimizu, Y.; Shimizu, N.; Ishitani, T.; Ohshima, T. Involvement of sonic hedgehog and notch signaling in regenerative neurogenesis in adult zebrafish optic tectum after stab injury. J. Comp. Neurol. 2018, 526, 2360–2372. [Google Scholar] [CrossRef]
- Jorstad, N.L.; Wilken, M.S.; Grimes, W.N.; Wohl, S.G.; VandenBosch, L.S.; Yoshimatsu, T.; Wong, R.O.; Rieke, F.; Reh, T.A. Stimulation of functional neuronal re-generation from Müller glia in adult mice. Nature 2017, 548, 103–107. [Google Scholar] [CrossRef]
- Wegner, M. All purpose Sox: The many roles of Sox proteins in gene expression. Int. J. Biochem. Cell Biol. 2010, 42, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Hindley, C.; McDowell, G.; Deibler, R.; Jones, A.; Kirschner, M.; Guillemot, F.; Philpott, A. Cell cycle-regulated multi-site phosphorylation of Neu-rogenin 2 coordinates cell cycling with differentiation during neurogenesis. Development 2011, 138, 4267–4277. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kawasaki, T. Histone acetyltransferase EP300 regulates the proliferation and differentiation of neural stem cells during adult neurogenesis and regenerative neurogenesis in the zebrafish optic tectum. Neurosci. Lett. 2021, 756, 135978. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of Reactive Oxygen Species and Neurodegeneration by the PGC-1 Transcriptional Coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Pilaz, L.; Silver, D.L. Post-transcriptional regulation in corticogenesis: How RNA-binding proteins help build the brain. Wiley Interdiscip. Rev. RNA 2015, 6, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, P.; Thomas, A.K.; Papadimitriou, C.; Cosacak, M.I.; Kizil, C. The zebrafish reveals dependence of adult neurogenesis on increased Vascularization. J. Neurosci. 2020, 40, 5989–6003. [Google Scholar]
- Bhattarai, P.; Thomas, K.A.; Cosacak, M.I.; Papadimitriou, C.; Mashkaryan, V.; Froc, C.; Reinhardt, S.; Kurth, T.; Dahl, A.; Zhang, Y.; et al. IL4/STAT6 Signaling Activates Neural Stem Cell Proliferation and Neurogenesis upon Amyloid-b42 Aggregation in Adult Zebrafish Brain. Cell. Rep. 2016, 17, 941–948. [Google Scholar] [CrossRef]
- Bhattarai, P.; Thomas, A.K.; Zhang, Y.; Kizil, C. The effects of aging on amyloid-beta42-induced neurodegeneration and regeneration in adult zebrafish brain. Neurogenesis 2017, 4, e1322666. [Google Scholar] [CrossRef]
- Kyritsis, N.; Kizil, C.; Zocher, S.; Kroehne, V.; Kaslin, J.; Freudenreich, D.; Iltzsche, A.; Brand, M. Acute Inflammation Initiates the Regenerative Response in the Adult Zebrafish Brain. Science 2012, 338, 1353–1356. [Google Scholar] [CrossRef]
- Baldwin, K.T.; Carbajal, K.S.; Segal, B.M.; Giger, R.J. Neuroinflammation triggered by β-glucan/dectin-1 signaling enables CNS axon regeneration. Proc. Natl. Acad. Sci. USA 2015, 112, 2581–2586. [Google Scholar] [CrossRef]
- Ohnmacht, J.; Yang, Y.-J.; Maurer, G.W.; Barreiro-Iglesias, A.; Tsarouchas, T.M.; Wehner, D.; Sieger, D.; Becker, C.G.; Becker, T. Spinal motor neurons are regenerated after mechanical lesion and genetic ablation in larval zebrafish. Development 2016, 143, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Goss, R.J. The evolution of regeneration: Adaptive or inherent? J. Theor. Biol. 1991, 152, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Antos, C.L.; Tanaka, E.M. Vertebrates That Regenerate as Models For Guiding Stem Cels. Cell Biol. Stem Cells 2010, 695, 184–214. [Google Scholar]
- Ceci, M.; Mariano, V.; Romano, N. Zebrafish as a translational regeneration model to study the activation of neural stem cells and role of their environment. Prog. Neurobiol. 2018, 30, 45–66. [Google Scholar] [CrossRef]
- Becker, T.; Becker, C.G. Regenerative neurogenesis: The integration of developmental, physiological and immune signals. Development 2022, 149, dev199907. [Google Scholar] [CrossRef]
- Ghosh, S.; Hui, S.P. Regeneration of Zebrafish CNS: Adult Neurogenesis. Neural Plast. 2016, 2016, 5815439. [Google Scholar] [CrossRef]
- Becker, C.G.; Becker, T. Adult zebrafish as a model for successful central nervous system regeneration. Restor. Neurol. Neurosci. 2008, 26, 71–80. [Google Scholar] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef]
- Fischer, A.J.; Bosse, J.L.; El-Hodiri, H.M. Reprint of: The ciliary marginal zone (CMZ) in development and regeneration of the vertebrate eye. Exp. Eye Res. 2014, 123, 115–120. [Google Scholar] [CrossRef]
- Berg, D.A.; Kirkham, M.; Beljajeva, A.; Knapp, D.; Habermann, B.; Ryge, J.; Tanaka, E.M.; Simon, A. Efficient regeneration by activation of neurogenesis in homeostatically quiescent regions of the adult vertebrate brain. Development 2010, 137, 4127–4134. [Google Scholar] [CrossRef]
- A Raymond, P.; Barthel, L.K.; Bernardos, R.L.; Perkowski, J.J. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev. Biol. 2006, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, M.; Barthel, L.K.; Raymond, P.A. A self-renewing division of zebrafish Müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development 2013, 140, 4510–4521. [Google Scholar] [CrossRef] [PubMed]
- Reimer, M.M.; Sörensen, I.; Kuscha, V.; Frank, R.E.; Liu, C.; Becker, C.G.; Becker, T. Motor Neuron Regeneration in Adult Zebrafish. J. Neurosci. 2008, 28, 8510–8516. [Google Scholar] [CrossRef] [PubMed]
- Goldshmit, Y.; Sztal, T.E.; Jusuf, P.R.; Hall, T.E.; Nguyen-Chi, M.; Currie, P.D. Fgf-Dependent Glial Cell Bridges Facilitate Spinal Cord Regeneration in Zebrafish. J. Neurosci. 2012, 32, 7477–7492. [Google Scholar] [CrossRef] [PubMed]
- Kuscha, V.; Frazer, S.L.; Dias, T.B.; Hibi, M.; Becker, T.; Becker, C.G. Lesion-induced generation of interneuron cell types in specific dorsoventral domains in the spinal cord of adult zebrafish. J. Comp. Neurol. 2012, 520, 3604–3616. [Google Scholar] [CrossRef]
- Kuscha, V.; Barreiro-Iglesias, A.; Becker, C.G.; Becker, T. Plasticity of tyrosine hydroxylase and serotonergic systems in the regenerating spinal cord of adult zebrafish. J. Comp. Neurol. 2011, 520, 933–951. [Google Scholar] [CrossRef]
- Lange, C.; Garcia, M.T.; Decimo, I.; Bifari, F.; Eelen, G.; Quaegebeur, A.; Boon, R.; Zhao, H.; Boeckx, B.; Chang, J.; et al. Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis. EMBO J. 2016, 35, 924–941. [Google Scholar] [CrossRef]
- Bagnoli, S.; Tozzini, E.T.; Cellerino, A. Whole-Brain Clearing and Immunofluorescence in Nothobranchius furzeri. Cold Spring Harb. Protoc. 2023, 2023, 698–704. [Google Scholar] [CrossRef]
- Parish, C.L.; Beljajeva, A.; Arenas, E.; Simon, A. Midbrain dopaminergic neurogenesis and behavioural recovery in a salamander lesion-induced regeneration model. Development 2007, 134, 2881–2887. [Google Scholar] [CrossRef]
- Kirkham, M.; Berg, D.A.; Simon, A. Microglia activation during neuroregeneration in the adult vertebrate brain. Neurosci. Lett. 2011, 497, 11–16. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Chever, O.; Dossi, E.; Pannasch, U.; Derangeon, M.; Rouach, N. Astroglial networks promote neuronal coordina-tion. Sci. Signal. 2016, 9, ra6. [Google Scholar] [CrossRef]
- Takahashi, S. Metabolic compartmentalization between astroglia and neurons in physiological and pathophysiological condi-tions of the neurovascular unit. Neuropathology 2020, 40, 121–137. [Google Scholar] [CrossRef]
- Gerasimov, E.; Erofeev, A.; Borodinova, A.; Bolshakova, A.; Balaban, P.; Bezprozvanny, I.; Vlasova, O.L. Optogenetic Activation of Astrocytes—Effects on Neuronal Network Function. Int. J. Mol. Sci. 2021, 22, 9613. [Google Scholar] [CrossRef]
- Mu, Y.; Bennett, D.V.; Rubinov, M.; Narayan, S.; Yang, C.-T.; Tanimoto, M.; Mensh, B.D.; Looger, L.L.; Ahrens, M.B. Glia Accumulate Evidence that Actions Are Futile and Suppress Unsuccessful Behavior. Cell 2019, 178, 27–43.e19. [Google Scholar] [CrossRef]
- Ma, Z.; Stork, T.; Bergles, D.E.; Freeman, M.R. Neuromodulators signal through astrocytes to alter neural circuit activity and behavior. Nature 2016, 539, 428–432. [Google Scholar] [CrossRef]
- Sekiguchi, A.; Sugiura, K.; Kikuchi, S.; Karaki, S.; Tominaga, M. Roles of astrocytes, microglia, and neurons in the behavioral and motor responses to peripheral nerve injury in the primates. Prog. Neurobiol. 2016, 144, 53–80. [Google Scholar]
- Gu, Y.; Zhang, W.; Wang, L.; Zhang, M.; Chen, H.; Zhang, H. Interferon gamma induced by high mobility group box 1 in the retina leads to behavioral alterations in mouse. Neuroscience 2019, 420, 174–186. [Google Scholar]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–71.e14. [Google Scholar] [CrossRef] [PubMed]
- Corkrum, M.; Covelo, A.; Lines, J.; Bellocchio, L.; Pisansky, M.T. Dopaminergic dynamics underlying sex-specific cocaine re-ward. Nature 2020, 580, 237–241. [Google Scholar]
- Bojarskaite, L.; Bjørnstad, D.M.; Pettersen, K.H.; Cunen, C.; Hanisch, U.K.; Sundstrøm, L.E. GABA-induced excitation of NG2-expressing glia in the spinal trigeminal nucleus. Cereb. Cortex. 2019, 29, 3969–3985. [Google Scholar]
- Oe, Y.; Tominaga, M.; Muraoka, D.; Tokunaga, M.; Sakamoto, M. Sensory stimulation shifts recruitment between sensory and gustatory subdivisions in the nucleus of the solitary tract. Cell Rep. 2020, 30, 3720–3731. [Google Scholar]
- Brancaccio, M.; Patton, A.P.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron 2017, 93, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- Pabst, M.; Braganza, O.; Dannenberg, H.; Hu, W.; Pothmann, L.; Rosen, J.; Mody, I.; van Loo, K.; Deisseroth, K.; Becker, A.J.; et al. Astrocyte Intermediaries of Septal Cholinergic Modulation in the Hippocampus. Neuron 2016, 90, 853–865. [Google Scholar] [CrossRef]
- Sun, L.O.; Brady, C.M.; Cahill, H.; Al-Khindi, T.; Sakata, R.C. Functional assembly of mammalian presynaptic active zones by ELKS. Neuron 2013, 75, 108–120. [Google Scholar]
- Fellin, T.; Halassa, M.M.; Terunuma, M.; Succol, F.; Takano, H. Endogenous non-neuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 16261–16266. [Google Scholar]
- Pryazhnikov, E.; Khiroug, L. Sub-micromolar increase in [Ca2+]i triggers delayed exocytosis of ATP in cultured astrocytes. Glia 2008, 56, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Henneberger, C.; Papouin, T.; Oliet, S.H.R.; Rusakov, D.A. Long-term potentiation depends on release of d-serine from astrocytes. Nature 2010, 463, 232–236. [Google Scholar] [CrossRef]
- Lee, M.R.; Ruby, N.F.; Heller, H.C. D-serine: A potential neuromodulator in sleep regulation. Sleep 2010, 33, 413–414. [Google Scholar]
- Lyons, D.A.; Talbot, W.S. Glial Cell Development and Function in Zebrafish. Cold Spring Harb. Perspect. Biol. 2014, 7, a020586. [Google Scholar] [CrossRef]
- Niklaus, S.; Ulbricht, E.; Kröger, S. Differential regulation of glial glutamate transporters and receptors after rat facial nerve axotomy. Glia 2017, 65, 964–977. [Google Scholar]
- Rothstein, J.D.; Dykes-Hoberg, M.; Pardo, C.A.; Bristol, L.A.; Jin, L.; Kuncl, R.W.; Kanai, Y.; Hediger, M.A.; Wang, Y.; Schielke, J.P.; et al. Knockout of Glutamate Transporters Reveals a Major Role for Astroglial Transport in Excitotoxicity and Clearance of Glutamate. Neuron 1996, 16, 675–686. [Google Scholar] [CrossRef]
- Tanaka, K.; Watase, K.; Manabe, T.; Yamada, K.; Watanabe, M.; Takahashi, K.; Iwama, H.; Nishikawa, T.; Ichihara, N.; Kikuchi, T.; et al. Epilepsy and Exacerbation of Brain Injury in Mice Lacking the Glutamate Transporter GLT-1. Science 1997, 276, 1699–1702. [Google Scholar] [CrossRef] [PubMed]
- Verdugo, C.D.; Myren-Svelstad, S.; Aydin, E.; Van Hoeymissen, E.; Deneubourg, C.; Vanderhaeghe, S.; Vancraeynest, J.; Pelgrims, R.; Cosacak, M.I.; Muto, A.; et al. Glia-neuron interactions underlie state transitions to generalized seizures. Nat. Commun. 2019, 10, 3830. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Bykova, M.E.; Shamshurina, E.V.; Varaksin, A.A. Transduction of Brain Neurons in Juvenile Chum Salmon (Oncorhynchus keta) with Recombinant Adeno-Associated Hippocampal Virus Injected into the Cerebellum during Long-Term Monitoring. Int. J. Mol. Sci. 2022, 23, 4947. [Google Scholar] [CrossRef]
- Semyanov, A. What the folk is the glial syncytium? Front. Neurosci. 2019, 13, 922. [Google Scholar]
- Wallraff, A.; Köhling, R.; Heinemann, U.; Theis, M.; Willecke, K.; Steinhäuser, C. The Impact of Astrocytic Gap Junctional Coupling on Potassium Buffering in the Hippocampus. J. Neurosci. 2006, 26, 5438–5447. [Google Scholar] [CrossRef]
- Buskila, Y.; Bellot-Saez, A.; Morley, J.W. Synaptic sources of endocannabinoid-independent retrograde signaling in the hip-pocampus. Neuron 2019, 103, 935–948. [Google Scholar]
- Mu, Y.; Oron, E.; He, S.; Evgrafov, O.; Zheng, C. Restoring serotonergic modulation of the dorsomedial prefrontal cortex rescues hypersociability in 22q11.2 deletion syndrome. Nat. Med. 2019, 25, 270–278. [Google Scholar]
- Baraban, M.; Koudelka, S.; Lyons, D.A. Ca2+ activity in young NG2 expressing glial cells in the hippocampus. Front. Cell. Neurosci. 2018, 12, 334. [Google Scholar]
- Kegel, L.; Aunis, D.; Poulain, B. Is astrocyte Ca2+ dynamics altered during exocytosis? Trends Neurosci. 2019, 42, 606–617. [Google Scholar]
- Sugitani, K.; Mokuya, T.; Homma, S.; Maeda, M.; Konno, A.; Ogai, K. Specific Activation of Yamanaka Factors via HSF1 Signaling in the Early Stage of Zebrafish Optic Nerve Regeneration. Int. J. Mol. Sci. 2023, 24, 3253. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pushchina, E.V.; Kapustyanov, I.A.; Kluka, G.G. Adult Neurogenesis of Teleost Fish Determines High Neuronal Plasticity and Regeneration. Int. J. Mol. Sci. 2024, 25, 3658. https://doi.org/10.3390/ijms25073658
Pushchina EV, Kapustyanov IA, Kluka GG. Adult Neurogenesis of Teleost Fish Determines High Neuronal Plasticity and Regeneration. International Journal of Molecular Sciences. 2024; 25(7):3658. https://doi.org/10.3390/ijms25073658
Chicago/Turabian StylePushchina, Evgeniya Vladislavovna, Ilya Alexandovich Kapustyanov, and Gleb Gennadievich Kluka. 2024. "Adult Neurogenesis of Teleost Fish Determines High Neuronal Plasticity and Regeneration" International Journal of Molecular Sciences 25, no. 7: 3658. https://doi.org/10.3390/ijms25073658




