Injury of Macrophages Induced by Clostridium perfringens Type C Exotoxins
Abstract
:1. Introduction
2. Results
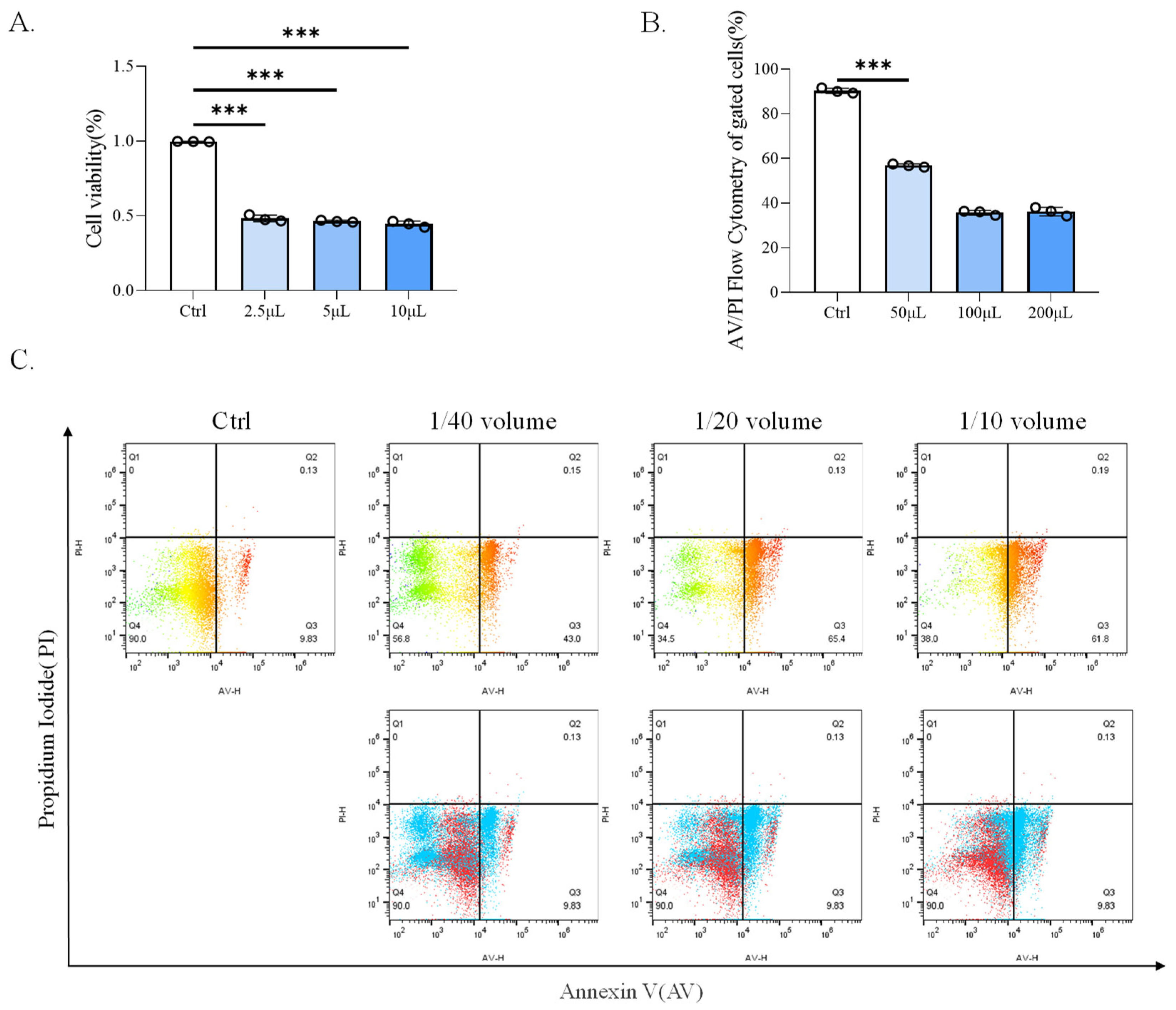
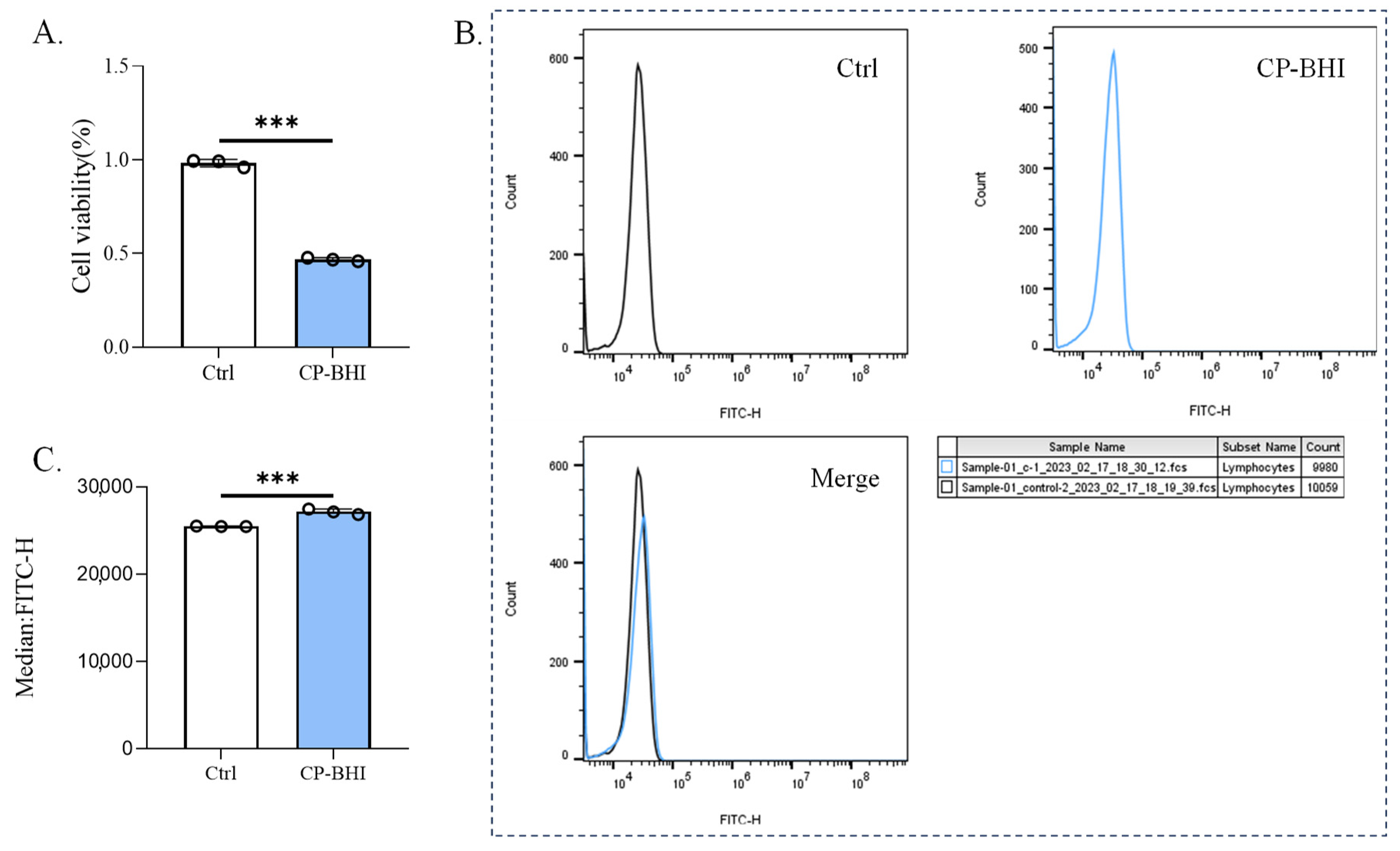
2.1. CP-BHI Induced the Death of Macrophages
2.2. CP-BHI Induced ROS Production in Macrophages
2.3. CP-BHI Decreased the Expression of Antioxidant-Related Factors in Macrophages
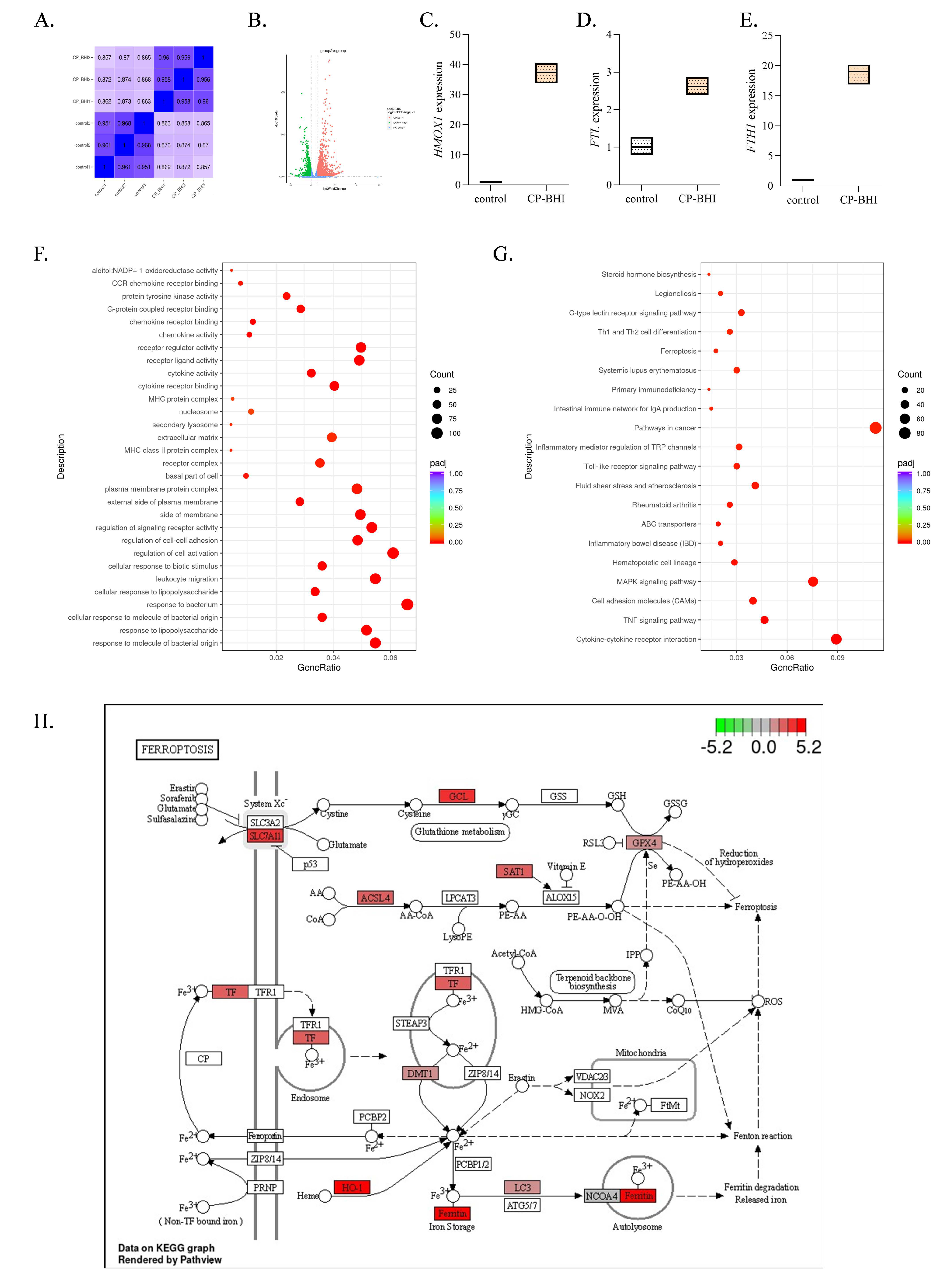
2.4. Transcriptome Analysis of THP-1 Cells Treated with CP-BHI Revealed Differential Expression of Ferroptosis-Related Genes
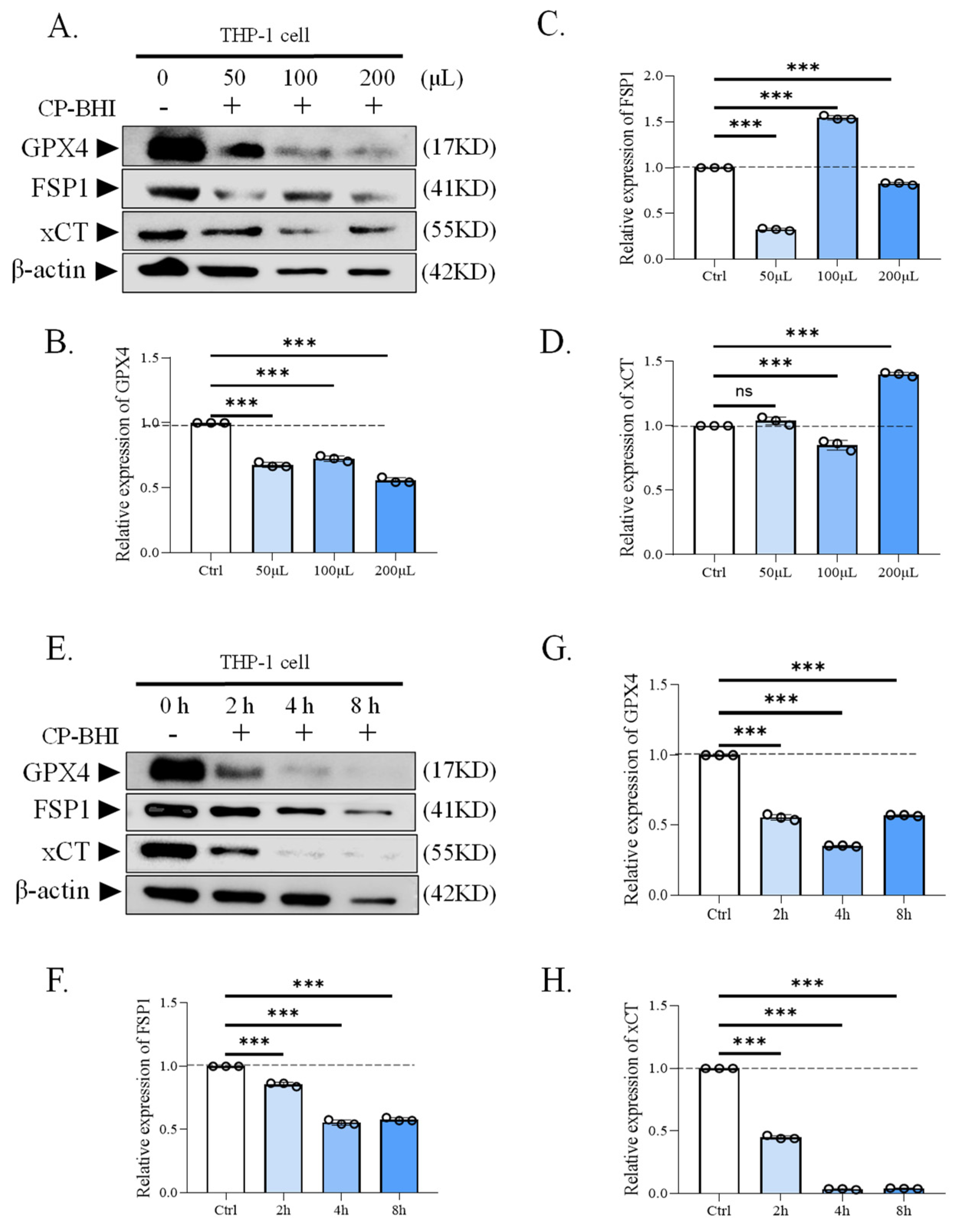
2.5. CP-BHI Increased the Expression of Ferroptosis-Related Protein in Macrophages
2.6. CP-BHI Increased the Level of Lipid Peroxidation in Macrophages
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Cell Lines, and Reagents
4.2. Cell Culture and Treatment
4.3. Cell Viability
4.4. Measurement of Reactive Oxygen Species (ROS)
4.5. Transcriptome Sequencing Analysis
4.6. Immunoblotting
4.7. Immunofluorescent Staining
4.8. Detection of Lipid Peroxidation Level
4.9. Detection of GSH and MDA Level
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; McClane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G.; et al. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe 2018, 53, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Huang, X.; Yan, Z.; Gao, X.; Wang, P.; Yang, Q.; Wang, W.; Xie, K.; Gun, S. Identification and Characterization of MAPK Signaling Pathway Genes and Associated lncRNAs in the Ileum of Piglets Infected by Clostridium perfringens Type C. Biomed. Res. Int. 2020, 2020, 8496872. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.E.; Rozgonyi, F. Pathogenesis, clinical characteristics, diagnostics and treatment of bacterial foodborne diseases. Orvosi Hetil. 2020, 161, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Elbestawy, A.R.; El-Shall, N.A.; Saad, A.M.; Salem, H.M.; El-Tahan, A.M.; Khafaga, A.F.; Taha, A.E.; AbuQamar, S.F.; et al. Necrotic enteritis in broiler chickens: Disease characteristics and prevention using organic antibiotic alternatives—A comprehensive review. Poult. Sci. 2022, 101, 101590. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, J.; Wang, X.; Robinson, K.; Whitmore, M.A.; Stewart, S.N.; Zhao, J.; Zhang, G. Identification of an Intestinal Microbiota Signature Associated With the Severity of Necrotic Enteritis. Front. Microbiol. 2021, 12, 703693. [Google Scholar] [CrossRef]
- Wade, B.; Keyburn, A.L.; Seemann, T.; Rood, J.I.; Moore, R.J. Binding of Clostridium perfringens to collagen correlates with the ability to cause necrotic enteritis in chickens. Vet. Microbiol. 2015, 180, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Lillehoj, H.S.; Sun, Z.; Lee, Y.; Zhao, H.; Xianyu, Z.; Yan, X.; Wang, Y.; Lin, S.; Liu, L.; et al. Characterization of Virulent netB+/tpeL+Clostridium perfringens Strains from Necrotic Enteritis-Affected Broiler Chicken Farms. Avian Dis. 2019, 63, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Jiang, X. The Chemistry and Biology of Ferroptosis. Cell Chem. Biol. 2020, 27, 365–375. [Google Scholar] [CrossRef]
- Conrad, M.; Kagan, V.E.; Bayir, H.; Pagnussat, G.C.; Head, B.; Traber, M.G.; Stockwell, B.R. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev. 2018, 32, 602–619. [Google Scholar] [CrossRef]
- Rood, J.I.; Cole, S.T. Molecular genetics and pathogenesis of Clostridium perfringens. Microbiol. Rev. 1991, 55, 621–648. [Google Scholar] [CrossRef] [PubMed]
- Brynestad, S.; Granum, P.E. Clostridium perfringens and foodborne infections. Int. J. Food Microbiol. 2002, 74, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, M.; Matsushita, O.; Minami, J.; Sakamoto, H.; Nakano, M.; Okabe, A. Role of alpha-toxin in Clostridium perfringens infection determined by using recombinants of C. perfringens and Bacillus subtilis. Infect. Immun. 1994, 62, 5032–5039. [Google Scholar] [CrossRef] [PubMed]
- Flores-Díaz, M.; Thelestam, M.; Clark, G.C.; Titball, R.W.; Alape-Girón, A. Effects of Clostridium perfringens phospholipase C in mammalian cells. Anaerobe 2004, 10, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Popescu, F.; Wyder, M.; Gurtner, C.; Frey, J.; Cooke, R.A.; Greenhill, A.R.; Posthaus, H. Susceptibility of primary human endothelial cells to C. perfringens beta-toxin suggesting similar pathogenesis in human and porcine necrotizing enteritis. Vet. Microbiol. 2011, 153, 173–177. [Google Scholar] [CrossRef]
- Autheman, D.; Wyder, M.; Popoff, M.; D’Herde, K.; Christen, S.; Posthaus, H. Clostridium perfringens beta-toxin induces necrostatin-inhibitable, calpain-dependent necrosis in primary porcine endothelial cells. PLoS ONE 2013, 8, e64644. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, Q.; Huang, X.; Yan, Z.; Zhang, S.; Luo, R.; Wang, P.; Wang, W.; Xie, K.; Jiang, T.; et al. Effects of Clostridium perfringens beta2 toxin on apoptosis, inflammation, and barrier function of intestinal porcine epithelial cells. Microb. Pathog. 2020, 147, 104379. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Qiao, X.; Ma, Y.; Han, M.; Jia, S.; Huang, X.; Han, B.; Wang, L.; Li, Y.; Xu, Y. Protection Efficacy of Oral Bait Probiotic Vaccine Constitutively Expressing Tetravalent Toxoids against Clostridium perfringens Exotoxins in Livestock (Rabbits). Vaccines 2020, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Shibutani, M.; Seike, S.; Yonezaki, M.; Takagishi, T.; Oda, M.; Kobayashi, K.; Sakurai, J. The p38 MAPK and JNK pathways protect host cells against Clostridium perfringens beta-toxin. Infect. Immun. 2013, 81, 3703–3708. [Google Scholar] [CrossRef] [PubMed]
- Bunkar, N.; Sharma, J.; Chouksey, A.; Kumari, R.; Gupta, P.K.; Tiwari, R.; Lodhi, L.; Srivastava, R.K.; Bhargava, A.; Mishra, P.K. Clostridium perfringens phospholipase C impairs innate immune response by inducing integrated stress response and mitochondrial-induced epigenetic modifications. Cell. Signal. 2020, 75, 109776. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, X.; Xie, K.; Zhang, J.; Yang, J.; Yan, Z.; Gun, S. Decreased S100A9 expression alleviates Clostridium perfringens beta2 toxin-induced inflammatory injury in IPEC-J2 cells. PeerJ 2023, 11, e14722. [Google Scholar] [CrossRef] [PubMed]
- Motafeghi, F.; Mortazavi, P.; Mahdavi, M.; Shokrzadeh, M. Cellular effects of epsilon toxin on the cell viability and oxidative stress of normal and lung cancer cells. Microb. Pathog. 2022, 169, 105649. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Meng, L.; Cao, S.; Liu, S.; Wu, J.; Li, Q.; Huang, W.; Zhang, L. PPARα alleviates iron overload-induced ferroptosis in mouse liver. EMBO Rep. 2022, 23, e52280. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.; Seibt, T.; et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-K.; Chen, S.-E.; Chang, L.-C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2018, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Rui, T.; Wang, H.; Li, Q.; Cheng, Y.; Gao, Y.; Fang, X.; Ma, X.; Chen, G.; Gao, C.; Gu, Z.; et al. Deletion of Ferritin H in neurons counteracts the protective effect of melatonin against traumatic brain injury-induced ferroptosis. J. Pineal Res. 2021, 70, e12704. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. Autophagy promotes ferroptosis by degradation of Ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Monian, P.; Pan, Q.; Zhang, W.; Xiang, J.; Jiang, X. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Schorpp, K.; Jin, J.; Yozwiak, C.E.; Hoffstrom, B.G.; Decker, A.M.; Rajbhandari, P.; Stokes, M.E.; Bender, H.G.; Csuka, J.M.; et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep. 2020, 30, 3411–3423. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, D.; Ding, Y.; Song, F.; Li, Y.; Zeng, J.; Wang, Y. Injury of Macrophages Induced by Clostridium perfringens Type C Exotoxins. Int. J. Mol. Sci. 2024, 25, 3718. https://doi.org/10.3390/ijms25073718
Zhang S, Wang D, Ding Y, Song F, Li Y, Zeng J, Wang Y. Injury of Macrophages Induced by Clostridium perfringens Type C Exotoxins. International Journal of Molecular Sciences. 2024; 25(7):3718. https://doi.org/10.3390/ijms25073718
Chicago/Turabian StyleZhang, Siyu, Dong Wang, Yawen Ding, Fuyang Song, Yong Li, Jin Zeng, and Yujiong Wang. 2024. "Injury of Macrophages Induced by Clostridium perfringens Type C Exotoxins" International Journal of Molecular Sciences 25, no. 7: 3718. https://doi.org/10.3390/ijms25073718




