Transient Receptor Potential Ankyrin 1 Ion Channel Is Expressed in Osteosarcoma and Its Activation Reduces Viability
Abstract
:1. Introduction
2. Results
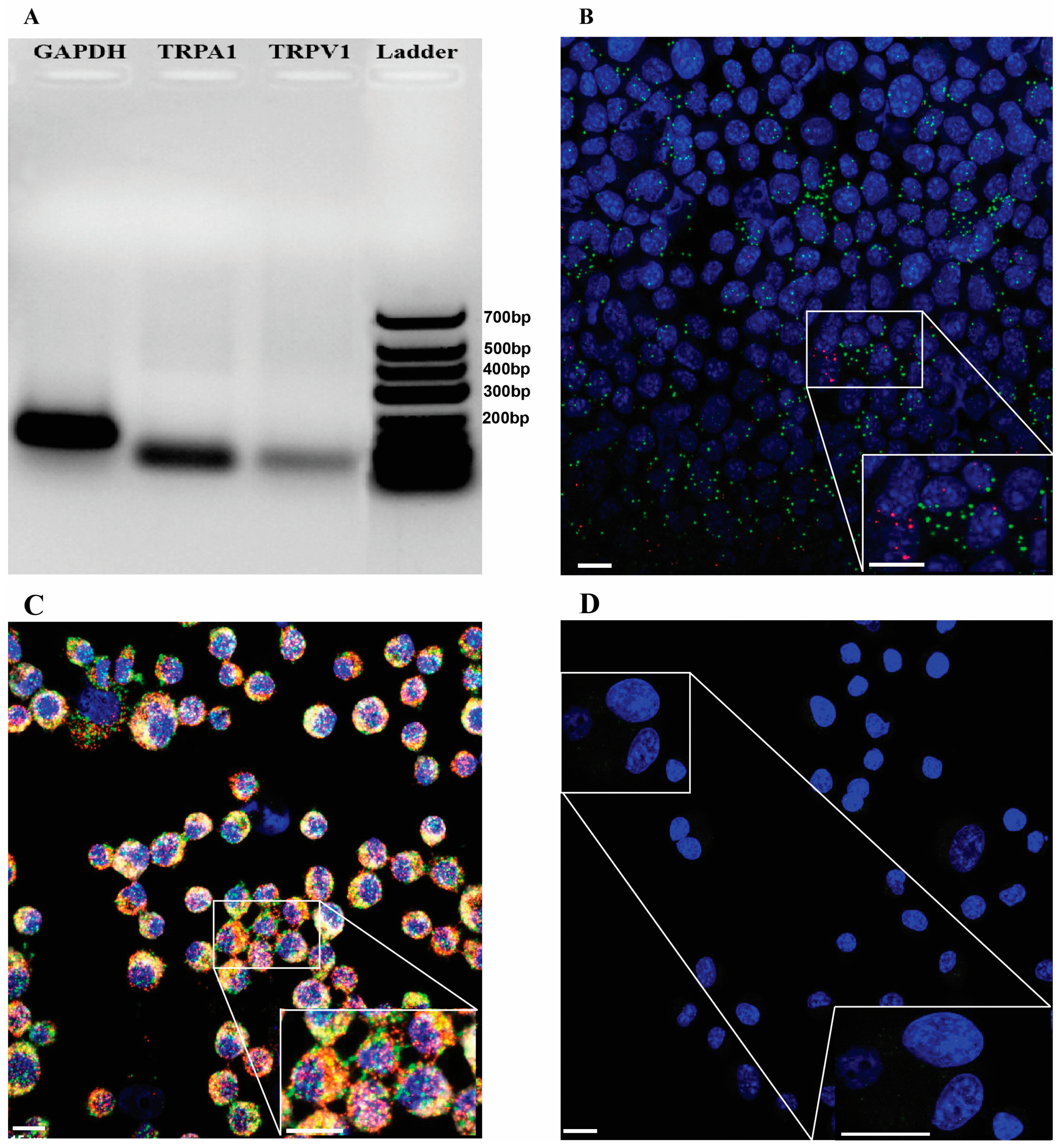
2.1. TRPA1/Trpa1 and TRPV1/Trpv1 mRNAs Are Expressed in Human and Mouse OS Tissues and in K7M2 Cells
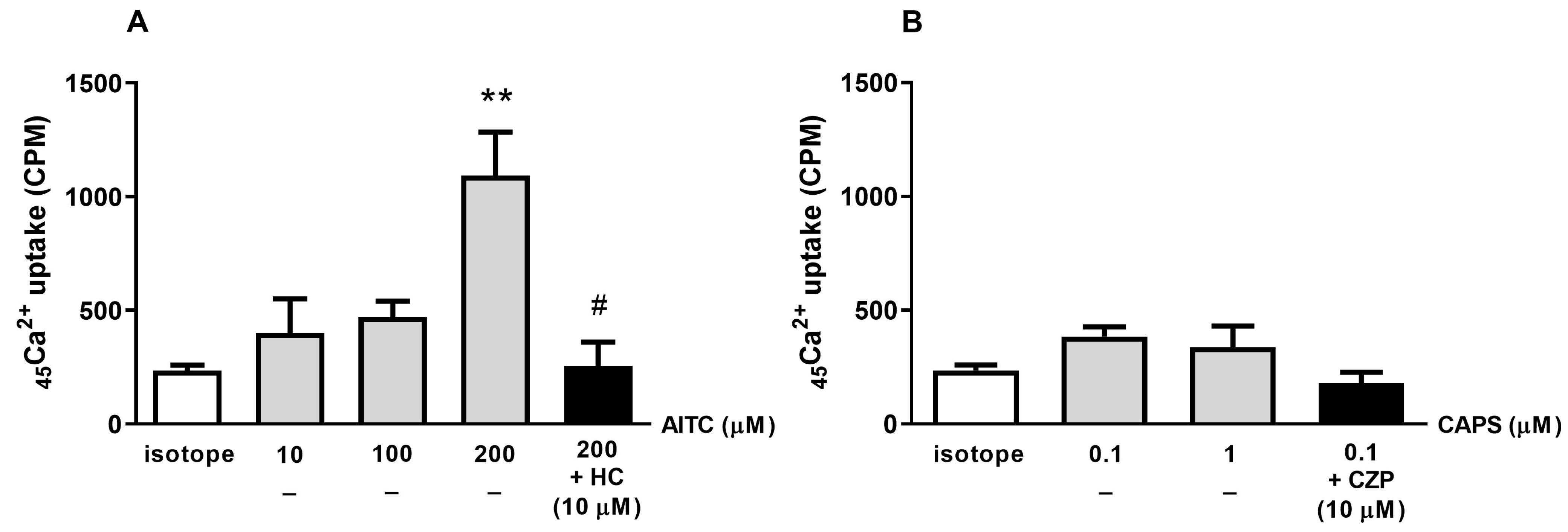
2.2. The TRPA1 Agonist AITC Induces Radioactive 45Ca2+ Uptake in K7M2 Cells
2.3. TRPA1 and TRPV1 Agonists Reduce K7M2 Cell Viability
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Human Samples
4.3. Cell Lines
4.4. RNA Isolation and PCR Gel Electrophoresis
4.5. Tissue Collection and Sample Preparation for RNAscope Study
4.6. K7M2 Cells’ Preparation for RNAscope
4.7. TRPA1/Trpa1 and TRPV1/Trpv1 RNAscope In Situ Hybridization
4.8. Radioactive 45Ca2+ Uptake Experiments on K7M2 Cells
4.9. ATP Luminescent K7M2 Cell Viability Assay
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AITC | Allyl isothiocyanate |
| AMPK | AMP-activated protein kinase |
| CAPS | Capsaicin |
| CHO | Chinese hamster ovary |
| CZP | Capsazepine |
| Cy3 | Cyanine 3 |
| Cy5 | Cyanine 5 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | Dimethyl sulfoxide |
| FBS | Fetal bovine serum |
| Gapdh | Mouse glyceraldehyde 3-phosphate dehydrogenase gene |
| H&E | Hematoxylin-eosin |
| HC | HC-030031 |
| MAPK/ERK | Mitogen-activated protein kinase/extracellular signal regulated kinase |
| OS | Osteosarcoma |
| RLU | Relative light units |
| RT | Room temperature |
| TRP | Transient receptor potential |
| TRPA1/TRPA1/Trpa1 | Transient receptor potential ankyrin 1 (Protein/human gene/mouse gene) |
| TRPV1/TRPV1/Trpv1 | Transient receptor potential vanilloid 1 (Protein/human gene/mouse gene) |
References
- Ottaviani, G.; Jaffe, N. The Epidemiology of Osteosarcoma. In Pediatric and Adolescent Osteosarcoma; Jaffe, N., Bruland, O.S., Bielack, S., Eds.; Springer: Boston, MA, USA, 2009; pp. 3–13. [Google Scholar]
- Sarhadi, V.K.; Daddali, R.; Seppänen-Kaijansinkko, R. Mesenchymal Stem Cells and Extracellular Vesicles in Osteosarcoma Pathogenesis and Therapy. Int. J. Mol. Sci. 2021, 22, 11035. [Google Scholar] [CrossRef] [PubMed]
- Durfee, R.A.; Mohammed, M.; Luu, H.H. Review of Osteosarcoma and Current Management. Rheumatol. Ther. 2016, 3, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Fallah, H.P.; Ahuja, E.; Lin, H.; Qi, J.; He, Q.; Gao, S.; An, H.; Zhang, J.; Xie, Y.; Liang, D. A Review on the Role of TRP Channels and Their Potential as Drug Targets_An Insight Into the TRP Channel Drug Discovery Methodologies. Front. Pharmacol. 2022, 13, 914499. [Google Scholar] [CrossRef] [PubMed]
- Fels, B.; Bulk, E.; Pethő, Z.; Schwab, A. The Role of TRP Channels in the Metastatic Cascade. Pharmaceuticals 2018, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A Gatekeeper for Inflammation. Annu. Rev. Physiol. 2013, 75, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, F.; Şelescu, T.; Domocoş, D.; Măruţescu, L.; Chiritoiu, G.; Chelaru, N.-R.; Dima, S.; Mihăilescu, D.; Babes, A.; Cucu, D. Functional expression of the transient receptor potential ankyrin type 1 channel in pancreatic adenocarcinoma cells. Sci. Rep. 2021, 11, 2018. [Google Scholar] [CrossRef] [PubMed]
- Francesconi, O.; Corzana, F.; Kontogianni, G.I.; Pesciullesi, G.; Gualdani, R.; Supuran, C.T.; Angeli, A.; Kavasi, R.M.; Chatzinikolaidou, M.; Nativi, C. Lipoyl-Based Antagonists of Transient Receptor Potential Cation A (TRPA1) Downregulate Osteosarcoma Cell Migration and Expression of Pro-Inflammatory Cytokines. ACS Pharmacol. Transl. Sci. 2022, 5, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Stock, K.; Kumar, J.; Synowitz, M.; Petrosino, S.; Imperatore, R.; Smith, E.S.J.; Wend, P.; Purfürst, B.; Nuber, U.A.; Gurok, U.; et al. Neural precursor cells induce cell death of high-grade astrocytomas via stimulation of TRPV1. Nat. Med. 2012, 18, 1232. [Google Scholar] [CrossRef] [PubMed]
- Hartel, M.; Di Mola, F.F.; Selvaggi, F.; Mascetta, G.; Wente, M.N.; Felix, K.; Giese, N.A.; Hinz, U.; Sebastiano, D.; Friess, H. PANCREATIC CANCER Vanilloids in pancreatic cancer: Potential for chemotherapy and pain management. Gut 2005, 55, 519–528. [Google Scholar] [CrossRef]
- Weber, L.V.; Al-Refae, K.; Wölk, G.; Bonatz, G.; Altmüller, J.; Becker, C.; Gisselmann, G.; Hatt, H. Breast Cancer-Targets and Therapy Dovepress Expression and functionality of TRPV1 in breast cancer cells. Breast Cancer 2016, 8, 243–252. [Google Scholar] [CrossRef]
- Xu, S.; Cheng, X.; Wu, J.; Wang, Y.; Wang, X.; Wu, L.; Yu, H.; Bao, J.; Zhang, L. Capsaicin restores sodium iodine symporter-mediated radioiodine uptake through bypassing canonical TSH‒TSHR pathway in anaplastic thyroid carcinoma cells. J. Mol. Cell Biol. 2022, 13, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Mistretta, F.; Buffi, N.M.; Lughezzani, G.; Lista, G.; Larcher, A.; Fossati, N.; Abrate, A.; Dell’Oglio, P.; Montorsi, F.; Guazzoni, G.; et al. Bladder Cancer and Urothelial Impairment: The Role of TRPV1 as Potential Drug Target. BioMed Res. Int. 2014, 2014, 987149. [Google Scholar] [CrossRef] [PubMed]
- Amantini, C.; Mosca, M.; Nabissi, M.; Lucciarini, R.; Caprodossi, S.; Arcella, A.; Giangaspero, F.; Santoni, G. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J. Neurochem. 2007, 102, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Dai, X.; Wang, P.; Tao, Y.; Chai, D. Capsaicin induces cytotoxicity in human osteosarcoma MG63 cells through TRPV1-dependent and -independent pathways. Cell Cycle 2019, 18, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Zhang, L.; Barritt, G. TRP channels in cancer. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2007, 1772, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Lefranc, F. Transient Receptor Potential (TRP) Ion Channels Involved in Malignant Glioma Cell Death and Therapeutic Perspectives. Front. Cell Dev. Biol. 2021, 9, 618961. [Google Scholar] [CrossRef] [PubMed]
- Silverman, H.A.; Chen, A.; Kravatz, N.L.; Chavan, S.S.; Chang, E.H. Involvement of Neural Transient Receptor Potential Channels in Peripheral Inflammation. Front. Immunol. 2020, 11, 590261. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Luostarinen, S.; Hämäläinen, M.; Hatano, N.; Muraki, K.; Moilanen, E. The inflammatory regulation of TRPA1 expression in human A549 lung epithelial cells. Pulm. Pharmacol. Ther. 2021, 70, 102059. [Google Scholar] [CrossRef] [PubMed]
- Kemény, Á.; Kodji, X.; Horváth, S.; Komlódi, R.; Szőke, É.; Sándor, Z.; Perkecz, A.; Gyömörei, C.; Sétáló, G.; Kelemen, B.; et al. TRPA1 Acts in a Protective Manner in Imiquimod-Induced Psoriasiform Dermatitis in Mice. J. Investig. Dermatol. 2018, 138, 1774–1784. [Google Scholar] [CrossRef]
- Park, M.; Naidoo, A.A.; Burns, A.; Choi, J.K.; Gatfield, K.M.; Vidgeon-Hart, M.; Bae, I.H.; Lee, C.S.; Choi, G.; Powell, A.J.; et al. Do TRPV1 antagonists increase the risk for skin tumourigenesis? A collaborative in vitro and in vivo assessment. Cell Biol. Toxicol. 2018, 34, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Kiss, F.; Kormos, V.; Szőke, É.; Kecskés, A.; Tóth, N.; Steib, A.; Szállási, Á.; Scheich, B.; Gaszner, B.; Kun, J.; et al. Functional Transient Receptor Potential Ankyrin 1 and Vanilloid 1 Ion Channels Are Overexpressed in Human Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 1921. [Google Scholar] [CrossRef] [PubMed]
- Czifra, G.; Varga, A.; Nyeste, K.; Marincsák, R.; Tóth, B.I.; Kovács, I.; Kovács, L.; Bíró, T. Increased expressions of cannabinoid receptor-1 and transient receptor potential vanilloid-1 in human prostate carcinoma. J. Cancer Res. Clin. Oncol. 2009, 135, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Marincsák, R.; Tóth, B.; Czifra, G.; Márton, I.; Rédl, P.; Tar, I.; Tóth, L.; Kovács, L.; Bíró, T. Increased expression of TRPV1 in squamous cell carcinoma of the human tongue. Oral. Dis. 2009, 15, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, C.; Chiang, C.; Xiao, T.; Chen, Y.; Zhao, Y.; Zheng, D. The Impact of TRPV1 on Cancer Pathogenesis and Therapy: A Systematic Review. Int. J. Biol. Sci. 2021, 17, 2034–2049. [Google Scholar] [CrossRef] [PubMed]
- Kadio, B.; Yaya, S.; Basak, A.; Djè, K.; Gomes, J.; Mesenge, C. Calcium role in human carcinogenesis: A comprehensive analysis and critical review of literature. Cancer Metastasis Rev. 2016, 35, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.O.; Dalloneau, E.; Pérez-Berezo, M.-T.; Plata, C.; Wu, Y.; Guillon, A.; Morello, E.; Aimar, R.-F.; Potier-Cartereau, M.; Esnard, F.; et al. Transient Receptor Potential (TRP) Channels in Head-and-Neck Squamous Cell Carcinomas: Diagnostic, Prognostic, and Therapeutic Potentials. Int. J. Mol. Sci. 2020, 21, 6374. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, C.; Hu, H.; Zhang, B. Activated TRPA1 plays a therapeutic role in TMZ resistance in glioblastoma by altering mitochondrial dynamics. BMC Mol. Cell Biol. 2022, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Bo, P.; Lien, J.-C.; Chen, Y.-Y.; Yu, F.-S.; Lu, H.-F.; Yu, C.-S.; Chou, Y.-C.; Yu, C.-C.; Chung, J.-G. Allyl Isothiocyanate Induces Cell Toxicity by Multiple Pathways in Human Breast Cancer Cells. Am. J. Chin. Med. 2016, 44, 415–437. [Google Scholar] [CrossRef]
- Wei, Y.; Cai, J.; Zhu, R.; Xu, K.; Li, H.; Li, J. Function and therapeutic potential of transient receptor potential ankyrin 1 in fibrosis. Front. Pharmacol. 2022, 13, 1014041. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, W.; Ma, J.; Xu, P.; Zhang, W.; Guo, S.; Liu, L.; Ma, J.; Shi, Q.; Jian, Z.; et al. Downregulated TRPV1 Expression Contributes to Melanoma Growth via the Calcineurin-ATF3-p53 Pathway. J. Investig. Dermatol. 2018, 138, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, A.G.; Sancho, R.; García-Limones, C.; Behrens, A.; Dijke, P.T.; Calzado, M.A.; Muñoz, E. Vanilloid receptor-1 regulates neurogenic inflammation in colon and protects mice from colon cancer. Cancer Res. 2012, 72, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Behray, M.; Wang, Q.; Wang, W.; Zhou, Z.; Chao, Y.; Bao, Y. Anti-cancer activities of allyl isothiocyanate and its conjugated silicon quantum dots. Sci. Rep. 2018, 8, 1084. [Google Scholar] [CrossRef]
- Al, S.; Gn, S.; Dm, S. Inhibition of bladder cancer cell proliferation by allyl isothiocyanate (mustard essential oil). Mutat. Res. 2015, 771, 29–35. [Google Scholar] [CrossRef]
- Qin, G.; Li, P.; Xue, Z. Effect of allyl isothiocyanate on the viability and apoptosis of the human cervical cancer HeLa cell line in vitro. Oncol. Lett. 2018, 15, 8756. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.G.B.; Faria, J.V.; Santos, J.P.S.D.; Faria, R.X. Capsaicin: TRPV1-independent mechanisms and novel therapeutic possibilities. Eur. J. Pharmacol. 2020, 887, 173356. [Google Scholar] [CrossRef]
- Virk, H.S.; Rekas, M.Z.; Biddle, M.S.; Wright, A.K.A.; Sousa, J.; Weston, C.A.; Chachi, L.; Roach, K.M.; Bradding, P. Validation of antibodies for the specific detection of human TRPA1. Sci. Rep. 2019, 9, 18500. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.M. Preparation of Cells for Microscopy using Cytospin. Methods Enzymol. 2013, 533, 235–240. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hudhud, L.; Rozmer, K.; Kecskés, A.; Pohóczky, K.; Bencze, N.; Buzás, K.; Szőke, É.; Helyes, Z. Transient Receptor Potential Ankyrin 1 Ion Channel Is Expressed in Osteosarcoma and Its Activation Reduces Viability. Int. J. Mol. Sci. 2024, 25, 3760. https://doi.org/10.3390/ijms25073760
Hudhud L, Rozmer K, Kecskés A, Pohóczky K, Bencze N, Buzás K, Szőke É, Helyes Z. Transient Receptor Potential Ankyrin 1 Ion Channel Is Expressed in Osteosarcoma and Its Activation Reduces Viability. International Journal of Molecular Sciences. 2024; 25(7):3760. https://doi.org/10.3390/ijms25073760
Chicago/Turabian StyleHudhud, Lina, Katalin Rozmer, Angéla Kecskés, Krisztina Pohóczky, Noémi Bencze, Krisztina Buzás, Éva Szőke, and Zsuzsanna Helyes. 2024. "Transient Receptor Potential Ankyrin 1 Ion Channel Is Expressed in Osteosarcoma and Its Activation Reduces Viability" International Journal of Molecular Sciences 25, no. 7: 3760. https://doi.org/10.3390/ijms25073760




