A Closer Look at Histamine in Drosophila
Abstract
:1. Histamine in Drosophila
2. Histamine Receptors in Drosophila
3. Histamine Metabolism
3.1. Synthesis
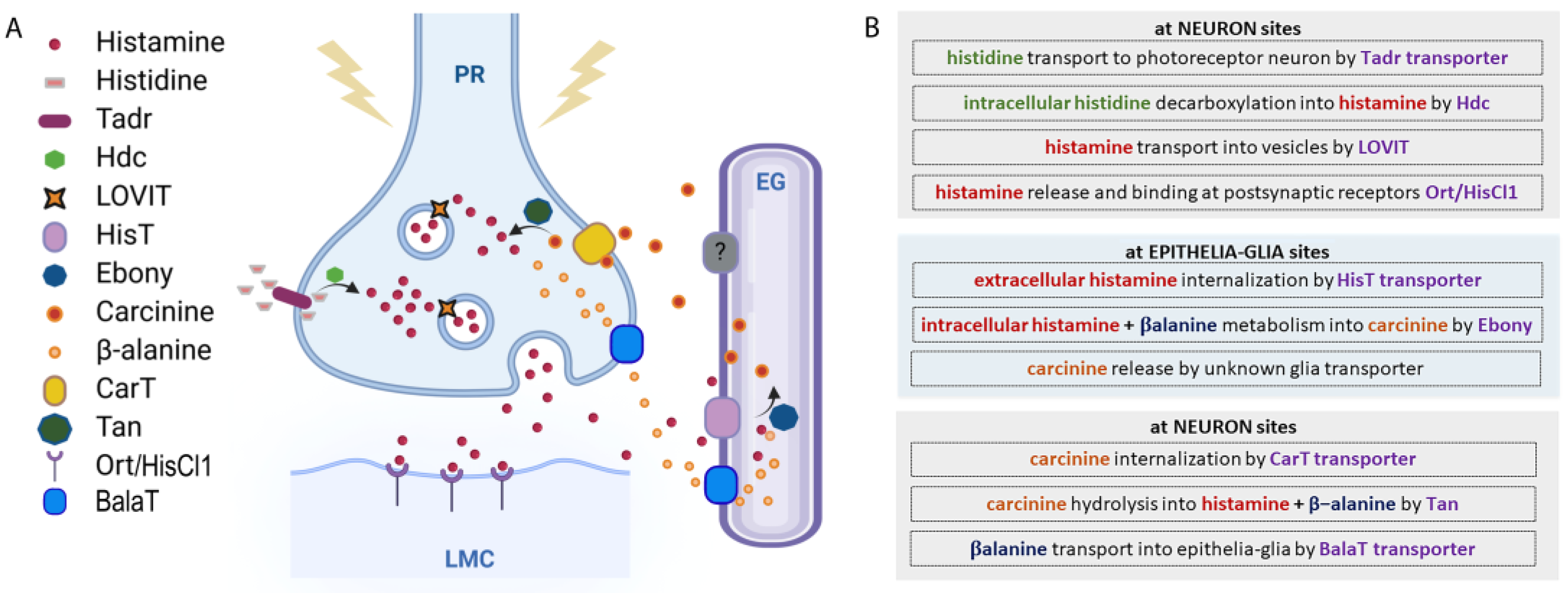
3.2. Storage, Release, Reuptake
3.3. Degradation
4. Histamine Functions
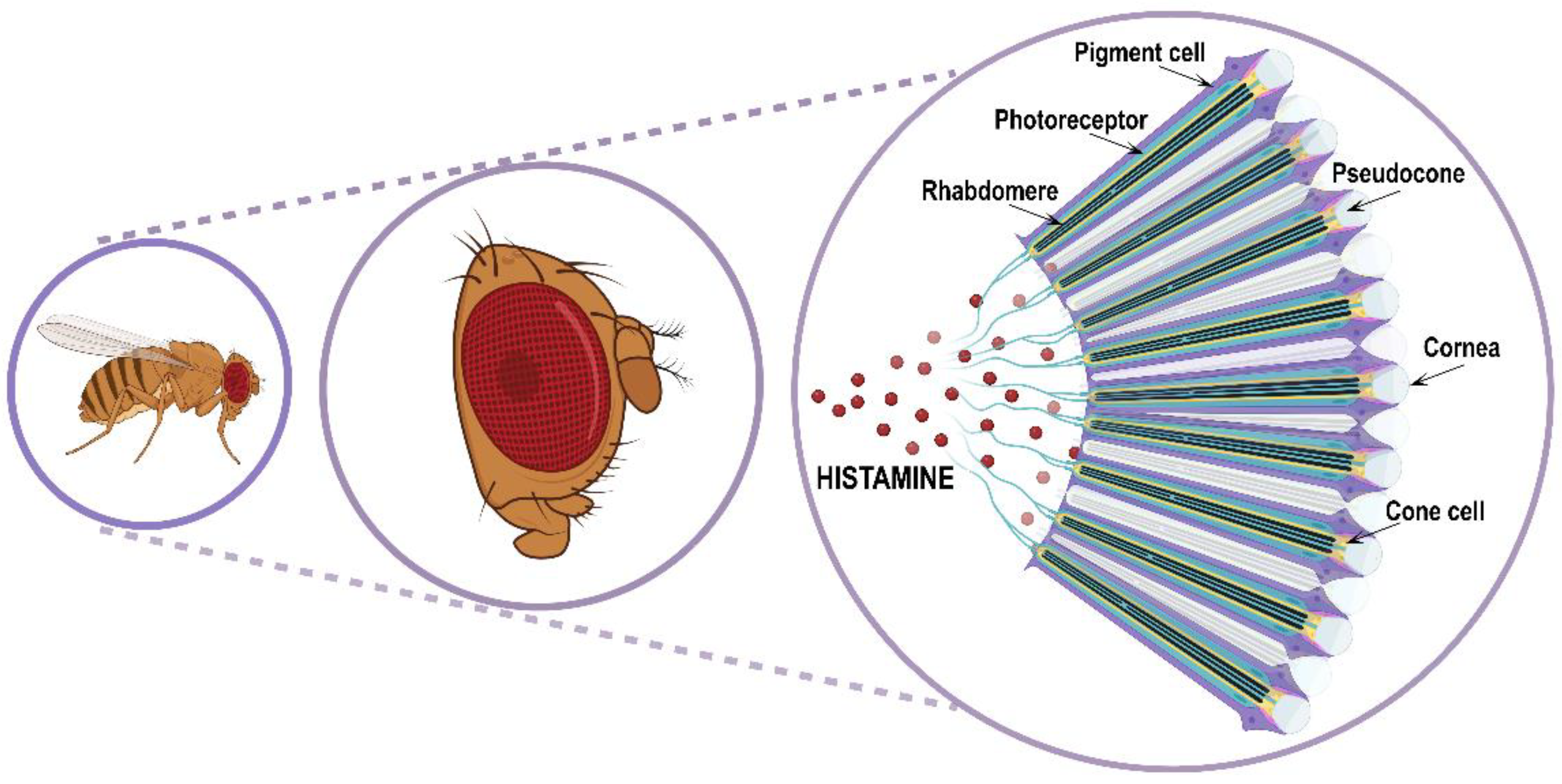
4.1. Visual Transmission
4.2. Wake–Sleep Cycle
4.3. Temperature Preference
4.4. Courtship Behavior
4.5. Mechanosensory Transmission
5. Histamine Sensing
6. What Next
Author Contributions
Funding
Conflicts of Interest
References
- Dale, H.H.; Laidlaw, P.P. The physiological action of beta-iminazolylethylamine. J. Physiol. 1910, 41, 318–344. [Google Scholar] [CrossRef]
- Haas, H.; Panula, P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat. Rev. Neurosci. 2003, 4, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Haas, H.L.; Sergeeva, O.A.; Selbach, O. Histamine in the nervous system. Physiol. Rev. 2008, 88, 1183–1241. [Google Scholar] [CrossRef] [PubMed]
- Buchner, E.; Buchner, S.; Burg, M.G.; Hofbauer, A.; Pak, W.L.; Pollack, I. Histamine is a major mechanosensory neurotransmitter candidate in Drosophila melanogaster. Cell Tissue Res. 1993, 273, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I.; Hofbauer, A. Histamine-like immunoreactivity in the visual system and brain of Drosophila melanogaster. Cell Tissue Res. 1991, 266, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Hamasaka, Y.; Nässel, D.R. Mapping of serotonin, dopamine, and histamine in relation to different clock neurons in the brain of Drosophila. J. Comp. Neurol. 2006, 494, 314–330. [Google Scholar] [CrossRef]
- Nässel, D.R.; Elekes, K. Aminergic neurons in the brain of blowflies and Drosophila: Dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res. 1992, 267, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Nässel, D.R. Histamine in the brain of insects: A review. Microsc. Res. Tech. 1999, 44, 121–136. [Google Scholar] [CrossRef]
- Python, F.; Stocker, R.F. Immunoreactivity against choline acetyltransferase, gamma-aminobutyric acid, histamine, octopamine, and serotonin in the larval chemosensory system of Dosophila melanogaster. J. Comp. Neurol. 2002, 453, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Monastirioti, M. Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc. Res. Tech. 1999, 45, 106–121. [Google Scholar] [CrossRef]
- Nässel, D.R.; Pirvola, U.; Panula, P. Histaminelike immunoreactive neurons innervating putative neurohaemal areas and central neuropil in the thoraco-abdominal ganglia of the flies Drosophila and Calliphora. J. Comp. Neurol. 1990, 297, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Alejevski, F.; Saint-Charles, A.; Michard-Vanhée, C.; Martin, B.; Galant, S.; Vasiliauskas, D.; Rouyer, F. The HisCl1 histamine receptor acts in photoreceptors to synchronize Drosophila behavioral rhythms with light-dark cycles. Nat. Commun. 2019, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Gengs, C.; Leung, H.T.; Skingsley, D.R.; Iovchev, M.I.; Yin, Z.; Semenov, E.P.; Burg, M.G.; Hardie, R.C.; Pak, W.L. The target of Drosophila photoreceptor synaptic transmission is a histamine-gated chloride channel encoded by ort (hclA). J. Biol. Chem. 2002, 277, 42113–42120. [Google Scholar] [CrossRef] [PubMed]
- Hardie, R.C. A histamine-activated chloride channel involved in neurotransmission at a photoreceptor synapse. Nature 1989, 339, 704–706. [Google Scholar] [CrossRef]
- Gisselmann, G.; Pusch, H.; Hovemann, B.T.; Hatt, H. Two cDNAs coding for histamine-gated ion channels in D. melanogaster. Nat. Neurosci. 2002, 5, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hirschberg, B.; Yuan, J.; Wang, A.P.; Hunt, D.C.; Ludmerer, S.W.; Schmatz, D.M.; Cully, D.F. Identification of two novel Drosophila melanogaster histamine-gated chloride channel subunits expressed in the eye. J. Biol. Chem. 2002, 277, 2000–2005. [Google Scholar] [CrossRef]
- Stuart, A.E.; Borycz, J.; Meinertzhagen, I.A. The dynamics of signaling at the histaminergic photoreceptor synapse of arthropods. Prog. Neurobiol. 2007, 82, 202–227. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, A.; Segaran, A.; Liu, C.H.; Nikolaev, A.; Rister, J.; Thum, A.S.; Roeder, T.; Semenov, E.; Juusola, M.; Hardie, R.C. Distinct roles for two histamine receptors (hclA and hclB) at the Drosophila photoreceptor synapse. J. Neurosci. 2008, 28, 7250–7259. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.J.; Cirelli, C.; Greenspan, R.J.; Tononi, G. Correlates of sleep and waking in Drosophila melanogaster. Science 2000, 287, 1834–1837. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Z. The roles of histamine and its receptor ligands in central nervous system disorders: An update. Pharmacol. Ther. 2017, 175, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Volonté, C.; Apolloni, S.; Sabatelli, M. Histamine beyond its effects on allergy: Potential therapeutic benefits for the treatment of Amyotrophic Lateral Sclerosis (ALS). Pharmacol. Ther. 2019, 202, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Volonté, C.; Apolloni, S.; Amadio, S. The Histamine and Multiple Sclerosis Alliance: Pleiotropic Actions and Functional Validation. Curr. Top. Behav. Neurosci. 2022, 59, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Chen, Z. Central histaminergic signalling, neural excitability and epilepsy. Br. J. Pharmacol. 2022, 179, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Y.; Liang, J.; Finkelman, F.D. Molecular Regulation of Histamine Synthesis. Front. Immunol. 2018, 9, 1392. [Google Scholar] [CrossRef] [PubMed]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef] [PubMed]
- Verrey, F.; Closs, E.I.; Wagner, C.A.; Palacin, M.; Endou, H.; Kanai, Y. CATs and HATs: The SLC7 family of amino acid transporters. Pflugers Arch. 2004, 447, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Peng, L.; Wang, T. Tadr is an axonal histidine transporter required for visual neurotransmission in Drosophila. eLife 2022, 11, e75821. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; Reddig, K.; Li, H.S. Long-distance mechanism of neurotransmitter recycling mediated by glial network facilitates visual function in Drosophila. Proc. Natl. Acad. Sci. USA 2014, 111, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Burg, M.G.; Sarthy, P.V.; Koliantz, G.; Pak, W.L. Genetic and molecular identification of a Drosophila histidine decarboxylase gene required in photoreceptor transmitter synthesis. EMBO J. 1993, 12, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Melzig, J.; Buchner, S.; Wiebel, F.; Wolf, R.; Burg, M.; Pak, W.L.; Buchner, E. Genetic depletion of histamine from the nervous system of Drosophila eliminates specific visual and mechanosensory behavior. J. Comp. Physiol. A 1996, 179, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Melzig, J.; Burg, M.; Gruhn, M.; Pak, W.L.; Buchner, E. Selective histamine uptake rescues photo- and mechanoreceptor function of histidine decarboxylase-deficient Drosophila mutant. J. Neurosci. 1998, 18, 7160–7166. [Google Scholar] [CrossRef] [PubMed]
- Dau, A.; Friederich, U.; Dongre, S.; Li, X.; Bollepalli, M.K.; Hardie, R.C.; Juusola, M. Evidence for Dynamic Network Regulation of Drosophila Photoreceptor Function from Mutants Lacking the Neurotransmitter Histamine. Front. Neural Circuits 2016, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, T. LOVIT Is a Putative Vesicular Histamine Transporter Required in Drosophila for Vision. Cell Rep. 2019, 27, 1327–1333.e1323. [Google Scholar] [CrossRef]
- Rosikon, K.D.; Bone, M.C.; Lawal, H.O. Regulation and modulation of biogenic amine neurotransmission in Drosophila and Caenorhabditis elegans. Front. Physiol. 2023, 14, 970405. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.K.; Weihe, E.; Eiden, L.E. Localization and expression of VMAT2 aross mammalian species: A translational guide for its visualization and targeting in health and disease. Adv. Pharmacol. 2013, 68, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Han, Y.; Liang, Y.; Peng, L.; Wang, T. HisT is a specific histamine transporter that contributes to histamine recycling in glia. Sci. Adv. 2022, 8, eabq1780. [Google Scholar] [CrossRef] [PubMed]
- Slamet Soetanto, T.; Liu, S.; Sahid, M.N.A.; Toyama, K.; Maeyama, K.; Mogi, M. Histamine uptake mediated by plasma membrane monoamine transporter and organic cation transporters in rat mast cell lines. Eur. J. Pharmacol. 2019, 849, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.D.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Borycz, J.; Borycz, J.A.; Loubani, M.; Meinertzhagen, I.A. tan and ebony genes regulate a novel pathway for transmitter metabolism at fly photoreceptor terminals. J. Neurosci. 2002, 22, 10549–10557. [Google Scholar] [CrossRef] [PubMed]
- Richardt, A.; Rybak, J.; Störtkuhl, K.F.; Meinertzhagen, I.A.; Hovemann, B.T. Ebony protein in the Drosophila nervous system: Optic neuropile expression in glial cells. J. Comp. Neurol. 2002, 452, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Richardt, A.; Kemme, T.; Wagner, S.; Schwarzer, D.; Marahiel, M.A.; Hovemann, B.T. Ebony, a novel nonribosomal peptide synthetase for beta-alanine conjugation with biogenic amines in Drosophila. J. Biol. Chem. 2003, 278, 41160–41166. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; Luan, Z.; Guo, P.; Li, H.S. Drosophila Vision Depends on Carcinine Uptake by an Organic Cation Transporter. Cell Rep. 2016, 14, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; An, F.; Borycz, J.A.; Borycz, J.; Meinertzhagen, I.A.; Wang, T. Histamine Recycling Is Mediated by CarT, a Carcinine Transporter in Drosophila Photoreceptors. PLoS Genet. 2015, 11, e1005764. [Google Scholar] [CrossRef] [PubMed]
- Stenesen, D.; Moehlman, A.T.; Krämer, H. The carcinine transporter CarT is required in Drosophila photoreceptor neurons to sustain histamine recycling. eLife 2015, 4, e10972. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xiong, L.; Xu, Y.; Tian, T.; Wang, T. The β-alanine transporter BalaT is required for visual neurotransmission in. eLife 2017, 6, e29146. [Google Scholar] [CrossRef] [PubMed]
- Provensi, G.; Costa, A.; Izquierdo, I.; Blandina, P.; Passani, M.B. Brain histamine modulates recognition memory: Possible implications in major cognitive disorders. Br. J. Pharmacol. 2020, 177, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T. Histamine as an Alert Signal in the Brain. Curr. Top. Behav. Neurosci. 2022, 59, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, L. Histamine in the Crosstalk Between Innate Immune Cells and Neurons: Relevance for Brain Homeostasis and Disease. Curr. Top. Behav. Neurosci. 2022, 59, 261–288. [Google Scholar] [CrossRef]
- Sarthy, P.V. Histamine: A neurotransmitter candidate for Drosophila photoreceptors. J. Neurochem. 1991, 57, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.E. From fruit flies to barnacles, histamine is the neurotransmitter of arthropod photoreceptors. Neuron 1999, 22, 431–433. [Google Scholar] [CrossRef] [PubMed]
- El Kholy, S.; Al Naggar, Y. Insights into the mechanism of histamine synthesis and recycling disruption induced by exposure to CdO NPs in the fruit fly (Drosophila melanogaster). Environ. Sci. Pollut. Res. Int. 2023, 30, 83376–83387. [Google Scholar] [CrossRef]
- Oh, Y.; Jang, D.; Sonn, J.Y.; Choe, J. Histamine-HisCl1 receptor axis regulates wake-promoting signals in Drosophila melanogaster. PLoS ONE 2013, 8, e68269. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.T.; Bang, S.; Paik, D.; Kang, J.; Hwang, S.; Jeon, K.; Chun, B.; Hyun, S.; Lee, Y.; Kim, J. Histamine and its receptors modulate temperature-preference behaviors in Drosophila. J. Neurosci. 2006, 26, 7245–7256. [Google Scholar] [CrossRef] [PubMed]
- Luu, P.; Zaki, S.A.; Tran, D.H.; French, R.L. A Novel Gene Controlling the Timing of Courtship Initiation in Drosophila melanogaster. Genetics 2016, 202, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.S.J.; Boone, K.N.; Bennett, M.M.; Salman, F.; Ralston, J.D.; Hatch, K.; Allen, R.F.; Phelps, A.M.; Cook, A.P.; Phelps, J.S.; et al. Organization of an ascending circuit that conveys flight motor state in Drosophila. Curr. Biol. 2024, 34, 1059–1075.e1055. [Google Scholar] [CrossRef] [PubMed]
- Bollepogu Raja, K.K.; Yeung, K.; Shim, Y.K.; Li, Y.; Chen, R.; Mardon, G. A single cell genomics atlas of the Drosophila larval eye reveals distinct photoreceptor developmental timelines. Nat. Commun. 2023, 14, 7205. [Google Scholar] [CrossRef] [PubMed]
- Borst, A.; Drews, M.; Meier, M. The neural network behind the eyes of a fly. Curr. Opin. Physiol. 2020, 16, 33–42. [Google Scholar] [CrossRef]
- Courgeon, M.; Desplan, C. Coordination of neural patterning in the Drosophila visual system. Curr. Opin. Neurobiol. 2019, 56, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Millard, S.S.; Pecot, M.Y. Strategies for assembling columns and layers in the Drosophila visual system. Neural Dev. 2018, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.; Konstantinides, N.; Pinto-Teixeira, F.; Desplan, C. Generation and Evolution of Neural Cell Types and Circuits: Insights from the Drosophila Visual System. Annu. Rev. Genet. 2017, 51, 501–527. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.P. Building an ommatidium one cell at a time. Dev. Dyn. 2012, 241, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Ready, D.F.; Hanson, T.E.; Benzer, S. Development of the Drosophila retina, a neurocrystalline lattice. Dev. Biol. 1976, 53, 217–240. [Google Scholar] [CrossRef]
- Charlton-Perkins, M.A.; Friedrich, M.; Cook, T.A. Semper’s cells in the insect compound eye: Insights into ocular form and function. Dev. Biol. 2021, 479, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Senthilan, P.R.; Helfrich-Förster, C. Rhodopsin 7-The unusual Rhodopsin in Drosophila. PeerJ 2016, 4, e2427. [Google Scholar] [CrossRef] [PubMed]
- Tuthill, J.C.; Nern, A.; Holtz, S.L.; Rubin, G.M.; Reiser, M.B. Contributions of the 12 neuron classes in the fly lamina to motion vision. Neuron 2013, 79, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ramos, T.B.; Marshall, O.J.; Doe, C.Q. Notch signaling and Bsh homeodomain activity are integrated to diversify Drosophila lamina neuron types. eLife 2024, 12, RP90136. [Google Scholar] [CrossRef]
- Takemura, S.Y.; Xu, C.S.; Lu, Z.; Rivlin, P.K.; Parag, T.; Olbris, D.J.; Plaza, S.; Zhao, T.; Katz, W.T.; Umayam, L.; et al. Synaptic circuits and their variations within different columns in the visual system of Drosophila. Proc. Natl. Acad. Sci. USA 2015, 112, 13711–13716. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Ito, K.; Bacon, J.P.; Strausfeld, N.J. Optic glomeruli and their inputs in Drosophila share an organizational ground pattern with the antennal lobes. J. Neurosci. 2012, 32, 6061–6071. [Google Scholar] [CrossRef]
- Wu, M.; Nern, A.; Williamson, W.R.; Morimoto, M.M.; Reiser, M.B.; Card, G.M.; Rubin, G.M. Visual projection neurons in the Drosophila lobula link feature detection to distinct behavioral programs. eLife 2016, 5, e21022. [Google Scholar] [CrossRef] [PubMed]
- Helfrich-Förster, C. Light input pathways to the circadian clock of insects with an emphasis on the fruit fly Drosophila melanogaster. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2020, 206, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Nériec, N.; Desplan, C. From the Eye to the Brain: Development of the Drosophila Visual System. Curr. Top. Dev. Biol. 2016, 116, 247–271. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.P.; Nern, A.; Picard, S.; Reiser, M.B.; Rubin, G.M.; Eddy, S.R.; Henry, G.L. A genetic, genomic, and computational resource for exploring neural circuit function. eLife 2020, 9, e50901. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Xu, S.; Li, Z.K.; Tang, M.; Mao, R.; Yang, T.; Ma, S.X.; Wang, P.H.; Li, M.T.; Sunilkumar, A.; et al. A single photoreceptor splits perception and entrainment by cotransmission. Nature 2023, 623, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ji, X.; Gu, Q.; Liao, B.; Dong, W.; Han, J. Parallel Synaptic Acetylcholine Signals Facilitate Large Monopolar Cell Repolarization and Modulate Visual Behavior in. J. Neurosci. 2021, 41, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, G.M.; Damulewicz, M.; Cusumano, P. Better Sleep at Night: How Light Influences Sleep in Drosophila. Front. Physiol. 2020, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Davis, R.L. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: Anatomical and physiological heterogeneity. Front. Neural Circuits 2009, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Schlichting, M.; Menegazzi, P.; Lelito, K.R.; Yao, Z.; Buhl, E.; Dalla Benetta, E.; Bahle, A.; Denike, J.; Hodge, J.J.; Helfrich-Förster, C.; et al. A Neural Network Underlying Circadian Entrainment and Photoperiodic Adjustment of Sleep and Activity in Drosophila. J. Neurosci. 2016, 36, 9084–9096. [Google Scholar] [CrossRef] [PubMed]
- Borst, A.; Haag, J.; Mauss, A.S. How fly neurons compute the direction of visual motion. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2020, 206, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Kirszenblat, L.; Yaun, R.; van Swinderen, B. Visual experience drives sleep need in Drosophila. Sleep 2019, 42, zsz102. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, M.M. Histamine in the regulation of wakefulness. Sleep. Med. Rev. 2011, 15, 65–74. [Google Scholar] [CrossRef] [PubMed]
- De Biase, S.; Pellitteri, G.; Gigli, G.L.; Valente, M. Evaluating pitolisant as a narcolepsy treatment option. Expert. Opin. Pharmacother. 2021, 22, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, N.; Okuampa, D.; Hansen, H.; Alvarez, M.; Cornett, E.M.; Kakazu, J.; Kaye, A.M.; Kaye, A.D. pitolisant, a novel histamine-3 receptor competitive antagonist, and inverse agonist, in the treatment of excessive daytime sleepiness in adult patients with narcolepsy. Health Psychol. Res. 2022, 10, 34222. [Google Scholar] [CrossRef]
- Ishizuka, T.; Sakamoto, Y.; Sakurai, T.; Yamatodani, A. Modafinil increases histamine release in the anterior hypothalamus of rats. Neurosci. Lett. 2003, 339, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, T.; Murotani, T.; Yamatodani, A. Modanifil activates the histaminergic system through the orexinergic neurons. Neurosci. Lett. 2010, 483, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Formica, F.; Pozzi, M.; Avantaggiato, P.; Molteni, E.; Arrigoni, F.; Giordano, F.; Clementi, E.; Strazzer, S. Disordered Consciousness or Disordered Wakefulness? The Importance of Prolonged Polysomnography for the Diagnosis, Drug Therapy, and Rehabilitation of an Unresponsive Patient With Brain Injury. J. Clin. Sleep. Med. 2017, 13, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Dhamapurkar, S.K.; Wilson, B.A.; Rose, A.; Watson, P.; Shiel, A. Does Modafinil improve the level of consciousness for people with a prolonged disorder of consciousness? a retrospective pilot study. Disabil. Rehabil. 2017, 39, 2633–2639. [Google Scholar] [CrossRef]
- Li, K.; Gong, Z. Feeling Hot and Cold: Thermal Sensation in Drosophila. Neurosci. Bull. 2017, 33, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Schnizler, K.; Saeger, B.; Pfeffer, C.; Gerbaulet, A.; Ebbinghaus-Kintscher, U.; Methfessel, C.; Franken, E.M.; Raming, K.; Wetzel, C.H.; Saras, A.; et al. A novel chloride channel in Drosophila melanogaster is inhibited by protons. J. Biol. Chem. 2005, 280, 16254–16262. [Google Scholar] [CrossRef] [PubMed]
- Yusein, S.; Wolstenholme, A.; Semenov, E. Functional consequences of mutations in the Drosophila histamine receptor HCLB. J. Insect Physiol. 2010, 56, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Patella, P.; Wilson, R.I. Functional Maps of Mechanosensory Features in the Drosophila Brain. Curr. Biol. 2018, 28, 1189–1203.e1185. [Google Scholar] [CrossRef] [PubMed]
- Hampel, S.; Eichler, K.; Yamada, D.; Bock, D.D.; Kamikouchi, A.; Seeds, A.M. Distinct subpopulations of mechanosensory chordotonal organ neurons elicit grooming of the fruit fly antennae. eLife 2020, 9, e59976. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.R.; Park, S.K.; Pikielny, C.W.; Steinbrecht, R.A. Gustatory organs of Drosophila melanogaster: Fine structure and expression of the putative odorant-binding protein PBPRP2. Cell Tissue Res. 2001, 304, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kwon, J.Y. A systematic analysis of Drosophila gustatory receptor gene expression in abdominal neurons which project to the central nervous system. Mol. Cells 2011, 32, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Dahanukar, A. Recent advances in the genetic basis of taste detection in Drosophila. Cell Mol. Life Sci. 2020, 77, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.; Lee, Y. Histamine gustatory aversion in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2021, 134, 103586. [Google Scholar] [CrossRef]
- Aryal, B.; Lee, Y. Histamine avoidance through three gustatory receptors in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2022, 144, 103760. [Google Scholar] [CrossRef] [PubMed]
- Tiligada, E.; Ennis, M. Histamine Receptors as Drug Targets; Humana: New York, NY, USA, 2017. [Google Scholar]
- Fiscon, G.; Conte, F.; Amadio, S.; Volonté, C.; Paci, P. Drug Repurposing: A Network-based Approach to Amyotrophic Lateral Sclerosis. Neurotherapeutics 2021, 18, 1678–1691. [Google Scholar] [CrossRef] [PubMed]
- Apolloni, S.; Amadio, S.; Fabbrizio, P.; Morello, G.; Spampinato, A.G.; Latagliata, E.C.; Salvatori, I.; Proietti, D.; Ferri, A.; Madaro, L.; et al. Histaminergic transmission slows progression of amyotrophic lateral sclerosis. J. Cachexia Sarcopenia Muscle 2019, 10, 872–893. [Google Scholar] [CrossRef]
- Volonté, C.; Morello, G.; Spampinato, A.G.; Amadio, S.; Apolloni, S.; D’Agata, V.; Cavallaro, S. Omics-based exploration and functional validation of neurotrophic factors and histamine as therapeutic targets in ALS. Ageing Res. Rev. 2020, 62, 101121. [Google Scholar] [CrossRef] [PubMed]
- Amadio, S.; Conte, F.; Esposito, G.; Fiscon, G.; Paci, P.; Volonté, C. Repurposing Histaminergic Drugs in Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 6347. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volonté, C.; Liguori, F.; Amadio, S. A Closer Look at Histamine in Drosophila. Int. J. Mol. Sci. 2024, 25, 4449. https://doi.org/10.3390/ijms25084449
Volonté C, Liguori F, Amadio S. A Closer Look at Histamine in Drosophila. International Journal of Molecular Sciences. 2024; 25(8):4449. https://doi.org/10.3390/ijms25084449
Chicago/Turabian StyleVolonté, Cinzia, Francesco Liguori, and Susanna Amadio. 2024. "A Closer Look at Histamine in Drosophila" International Journal of Molecular Sciences 25, no. 8: 4449. https://doi.org/10.3390/ijms25084449







