Marrubiin Inhibits Peritoneal Inflammatory Response Induced by Carrageenan Application in C57 Mice
Abstract
:1. Introduction
2. Results
2.1. Isolation of MAR
2.2. In Vivo Anti-Inflammatory Activity
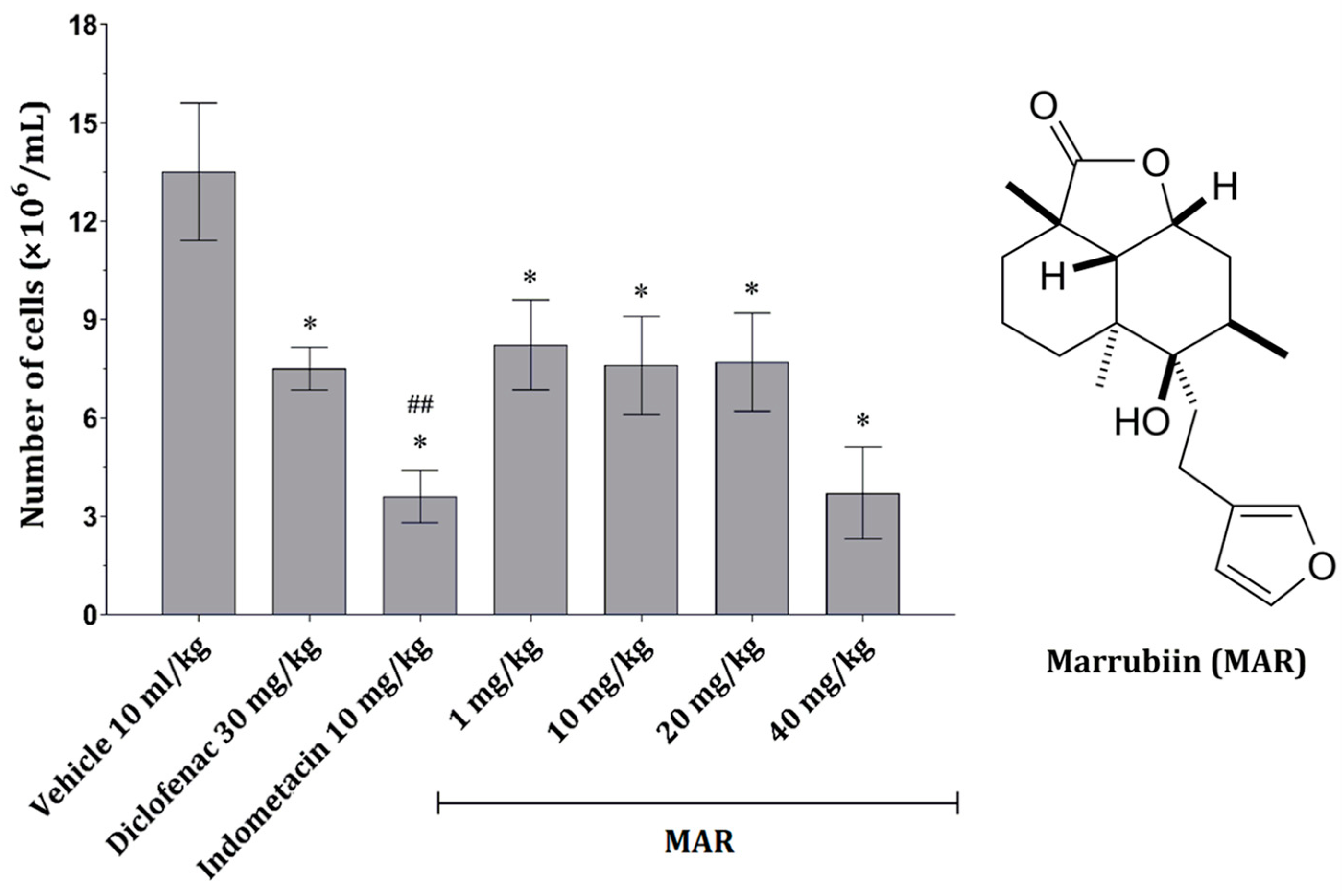
2.2.1. MAR Dose-Dependently Decreases the Number of Peritoneal Inflammatory Cells
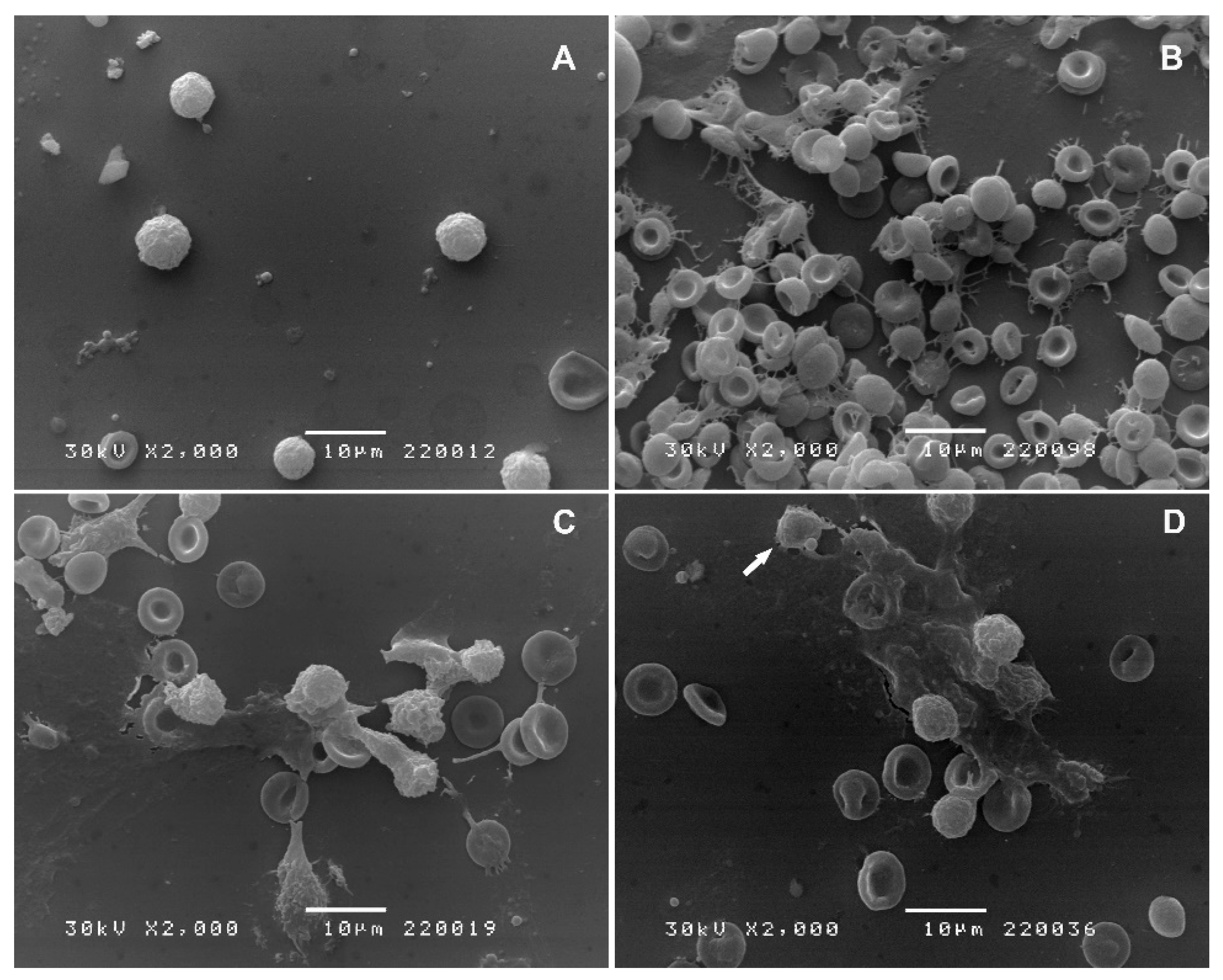
2.2.2. Scanning Electron Microscopy (SEM)
2.2.3. MAR Dose-Dependently Reduces the Inflammatory Parameters in Exudate Fluid
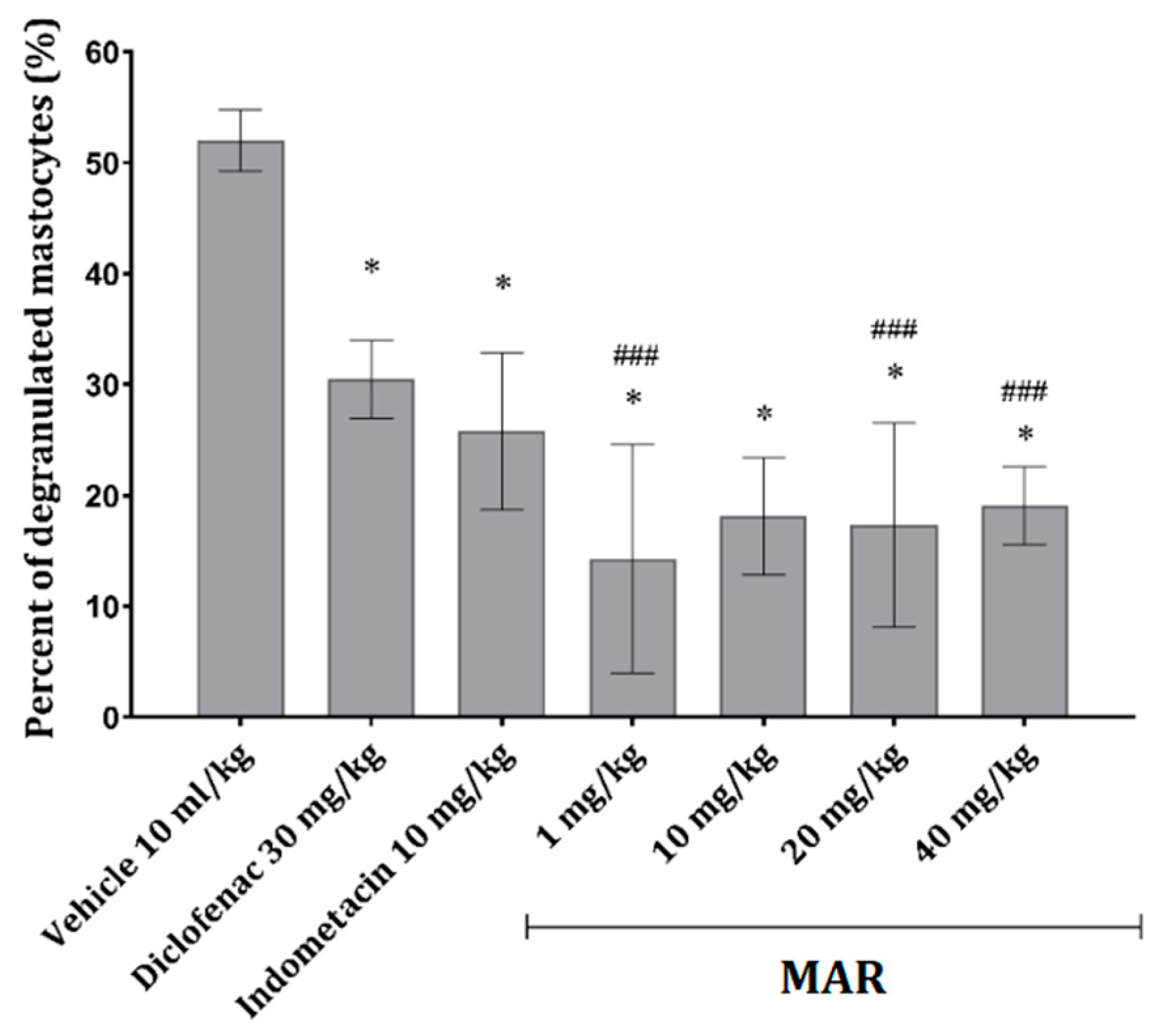
2.2.4. MAR Prevents Mast Cell Degranulation in the Mesenterial Tissue of Animals Treated with Carrageenan
2.3. Time Lapse Study—Effect of MAR on Inflammatory-Cell Viability
3. Discussion
4. Materials and Methods
4.1. General
4.2. Plant Material
4.3. Isolation of MAR
4.4. NMR Spectroscopy
4.5. Animals and Housing
4.6. In Vivo Anti-Inflammatory Activity Determination
4.6.1. Carrageenan-Induced Peritonitis Induction
- Control group—vehicle (olive oil) at a dose of 10 mL/kg
- Positive control group—indometacin at a dose of 10 mg/kg
- Positive control group—diclofenac at a dose of 30 mg/kg
- Experimental group—MAR at a dose of 1 mg/kg
- Experimental group—MAR at a dose of 10 mg/kg
- Experimental group—MAR at a dose of 20 mg/kg
- Experimental group—MAR at a dose of 40 mg/kg
4.6.2. Peritoneal Exudate Cell Count and Differential Staining
4.6.3. Scanning Electron Microscopy of PECs
4.6.4. Biochemical Analysis of the Peritoneal Exudate Fluid
4.6.5. Mesenterial Tissue Staining for Mastocyte Degranulation
4.7. In Vitro Effects of MAR on Inflammatory Cell Viability—Time Lap Study
4.8. Lipoxygenase Inhibition—In Vitro Study
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Popoola, O.K.; Elbagory, A.M.; Ameer, F.; Hussein, A.A. Marrubiin. Molecules 2013, 18, 9049–9060. [Google Scholar] [CrossRef] [PubMed]
- Piozzi, F.; Bruno, M.; Rosselli, S.; Maggio, A. The Diterpenoids of the Genus Marrubium (Lamiaceae). Nat. Prod. Commun. 2006, 1, 585–592. [Google Scholar] [CrossRef]
- Appleton, R.A.; Fulke, J.W.B.; Henderson, M.S.; McCrindle, R. The stereochemistry of marrubiin. J. Chem. Soc. C 1967, 1943–1947. [Google Scholar] [CrossRef]
- Zaabat, N.; Hay, A.E.; Michalet, S.; Darbour, N.; Bayet, C.; Skandrani, I.; Chekir-Ghedira, L.; Akkal, S.; Dijoux-Franca, M.G. Antioxidant and antigenotoxic properties of compounds isolated from Marrubium deserti de Noé. Food Chem. Toxicol. 2011, 49, 3328–3335. [Google Scholar] [CrossRef] [PubMed]
- Stulzer, H.K.; Tagliari, M.P.; Zampirolo, J.A.; Cechinel-Filho, V.; Schlemper, V. Antioedematogenic effect of marrubiin obtained from Marrubium vulgare. J. Ethnopharmacol. 2006, 108, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Meyre-Silva, C.; Yunes, R.A.; Schlemper, V.; Campos-Buzzi, F.; Cechinel-Filho, V. Analgesic potential of marrubiin derivatives, a bioactive diterpene present in Marrubium vulgare (Lamiaceae). Il Farmaco 2005, 60, 321–326. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, R.A.; Cechinel-Filho, V.; Oliveira, A.E.; Schlemper, V. Analysis of the antinociceptive properties of marrubiin isolated from Marrubium vulgare. Phytomedicine 2000, 7, 111–115. [Google Scholar] [CrossRef]
- Mnonopi, N.; Levendal, R.A.; Mzilikazi, N.; Frost, C.L. Marrubiin, a constituent of Leonotis leonurus, alleviates diabetic symptoms. Phytomedicine 2012, 19, 488–493. [Google Scholar] [CrossRef]
- SwissADME. Available online: http://www.swissadme.ch/ (accessed on 23 January 2024).
- Yamakoshi, H.; Sawayama, Y.; Akahori, Y.; Kato, M.; Nakamura, S. Total syntheses of (+)-Marrubiin and (−)-Marrulibacetal. Org. Lett. 2016, 18, 3430–3433. [Google Scholar] [CrossRef]
- Lopes, A.H.; Silva, R.L.; Fonseca, M.D.; Gomes, F.I.; Maganin, A.G.; Ribeiro, L.S.; Marques, L.M.M.; Cunha, F.Q.; Alves-Filho, J.C.; Zamboni, D.S.; et al. Molecular basis of carrageenan-induced cytokines production in macrophages. Cell Commun. Signal. 2020, 18, 141. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, S.; Wang, Y.; Rahman, M.; Syk, I.; Zhang, E.; Thorlacius, H. Proinflammatory role of neutrophil extracellular traps in abdominal sepsis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L586–L596. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.H.; Wu, A.B.; Lee, C.J.; Chen, F.A.; Wang, C.C. Anti-inflammatory effects of indomethacin’s methyl ester derivative and induction of apoptosis in HL-60 Cells. Biol. Pharm. Bull. 2005, 28, 2206–2210. [Google Scholar] [CrossRef]
- Okur, M.E.; Karakaş, N.; Karadağ, A.E.; Yılmaz, R.; ve Demirci, F. In vitro cytotoxicity evaluation of Marrubium vulgare L. methanol extract. J. Pharm. Res. 2019, 23, 711–718. [Google Scholar] [CrossRef]
- Paunovic, V.; Kosic, M.; Djordjevic, S.; Zugic, A.; Djalinac, N.; Gasic, U.; Trajkovic, V. Marrubium vulgare ethanolic extract induces proliferation block, apoptosis, and cytoprotective autophagy in cancer cells in vitro. Cell. Mol. Biol. 2016, 62, 108–114. [Google Scholar] [CrossRef]
- Barth, C.R.; Funchal, G.A.; Luft, C.; de Oliveira, J.R.; Porto, B.N.; Donadio, M.V. Carrageenan-induced inflammation promotes ROS generation and neutrophil extracellular trap formation in a mouse model of peritonitis. Eur. J. Immunol. 2016, 46, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Galkina, S.I.; Fedorova, N.V.; Golenkina, E.A.; Stadnichuk, V.I.; Sudina, G.F. Cytonemes versus Neutrophil Extracellular Traps in the Fight of Neutrophils with Microbes. Int. J. Mol. Sci. 2020, 21, 586. [Google Scholar] [CrossRef]
- Radulović, N.S.; Randjelović, P.J.; Stojanović, N.M.; Cakić, N.D.; Bogdanović, G.A.; Živanović, A.V. Aboriginal bush foods: A major phloroglucinol from Crimson Bottlebrush flowers (Callistemon citrinus, Myrtaceae) displays strong antinociceptive and anti-inflammatory activity. Food Res. Int. 2015, 77, 280–289. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins Basic Pathology, 10th ed.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Hawkins, C.L.; Davies, M.J. Role of myeloperoxidase and oxidant formation in the extracellular environment in inflammation-induced tissue damage. Free Radic. Biol. Med. 2021, 172, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Shacter, E.; Lopez, R.L.; Pati, S. Inhibition of the myeloperoxidase-H2O2-Cl-system of neutrophils by indomethacin and other non-steroidal anti-inflammatory drugs. Biochem. Pharmacol. 1991, 41, 975–984. [Google Scholar] [CrossRef]
- Yousefi, K.; Hamedeyazdan, S.; Torbati, M.; Fathiazad, F. Chromatographic fingerprint analysis of marrubiin in Marrubium vulgare L. via HPTLC Technique. Adv. Pharm. Bull. 2016, 6, 131–136. [Google Scholar] [CrossRef]
- Rezgui, M.; Majdoub, N.; Mabrouk, B.; Baldisserotto, A.; Bino, A.; Ben Kaab, L.B.; Manfredini, S. Antioxidant and antifungal activities of marrubiin, extracts and essential oil from Marrubium vulgare L. against pathogenic dermatophyte strains. J. Mycol. Med. 2020, 30, 100927. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, E.K.S.; Kubota, K.; Luz, D.A.; Mendes, P.F.S.; Figueiredo, P.L.B.; Lima, R.R.; Maia, C.S.F.; Fontes-Júnior, E.A. Aniba canelilla (Kunth) Mez (Lauraceae) Essential Oil: Effects on Oxidative Stress and Vascular Permeability. Antioxidants 2022, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Oshima, G.; Kunimoto, M.; Nakagawa, Y. Appearance of extracellular glutathione peroxidase (eGPx) in the ascite fluid of casein-elicited rats. Biol. Pharm. Bull. 2000, 23, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Laparra-Llopis, J.M.; Schneider, C.; Espín, J.C. Targeting Mammalian 5-Lipoxygenase by Dietary Phenolics as an Anti-Inflammatory Mechanism: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7937. [Google Scholar] [CrossRef] [PubMed]
- SwissTargetPrediction. Available online: http://www.swisstargetprediction.ch/ (accessed on 23 January 2024).
- Göger, F.; Özek, G.; Tekin, M.; Yur, S.; Özek, T. Phytochemical Profiling and Evaluation of Marrubium sivasense Aytaç, Akgül & Ekici for Antioxidant Activity and Inhibition Effects on α-Amylase, Lipoxygenase, Xanthine Oxidase and Tyrosinase Enzymes. JOTCSA 2009, 6, 281–292. [Google Scholar]
- Phosrithong, N.; Nuchtavorn, N. Antioxidant and anti-inflammatory activites of Clerodendrum leaf extracts collected in Thailand. Eur. J. Intern. Med. 2016, 8, 281–285. [Google Scholar] [CrossRef]
- Galili, U.; Prokocimer, M.; Izak, G. The in vitro sensitivity of leukemic and normal leukocytes to hydrocortisone induced cytolysis. Blood 1980, 56, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, G.; Lavoie-Lamoureux, A.; Beauchamp, G.; Lavoie, J.-P. Neutrophils are not less sensitive than other blood leukocytes to the genomic effects of glucocorticoids. PLoS ONE 2012, 7, e44606. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Mladenović, M.Z.; Maslovarić, A.; Stojiljković, N.I.; Randjelović, P.J.; Radulović, N.S. Lemon balm (Melissa officinalis L.) essential oil and citronellal modulate anxiety-related symptoms—In vitro and in vivo studies. J. Ethnopharmacol. 2022, 10, 114788. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Mitić, K.V.; Randjelović, P.; Stevanović, M.; Stojiljković, N.; Ilić, S.; Tričković Vukić, D.; Sokolović, D.; Jevtović-Stoimenov, T.; Radulović, N.S. Thymol regulates the functions of immune cells in the rat peritoneal cavity after L-arginine-induced pancreatitis. Life Sci. 2021, 280, 119704. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Randjelović, P.J.; Mladenović, M.Z.; Ilić, I.R.; Petrović, V.; Stojiljković, N.; Ilić, S.; Radulović, N.S. Toxic essential oils, part VI: Acute oral toxicity of lemon balm (Melissa officinalis L.) essential oil in BALB/c mice. Food Chem. Toxicol. 2019, 133, 110794. [Google Scholar] [CrossRef] [PubMed]
- Ničković, V.P.; Novaković, T.; Lazarević, S.; Šulović, L.; Živković, Z.; Živković, J.; Mladenović, B.; Stojanović, N.M.; Petrović, V.; Sokolović, D.T. Pre-vs. post-treatment with melatonin in CCl4-induced liver damage: Oxidative stress inferred from biochemical and pathohistological studies. Life Sci. 2018, 202, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Pini, J.J.P.B.; Vugman, I. Degranulation of rat mesentery mast cells by antihistamines: Influence of ionization. Agents Actions 1978, 8, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Filipović, S.I.; Stojanović, N.M.; Mitić, K.V.; Ranđelović, P.J.; Radulović, N.S. Revisiting the Effect of 3 Sesquiterpenoids from Conocephalum conicum (Snake Liverwort) on Rat Spleen Lymphocyte Viability and Membrane Functioning. Nat. Prod. Commun. 2022, 17, 8. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.V. In Vitro anti-inflammatory properties of selected green leafy vegetables. Biomedicines 2018, 6, 107. [Google Scholar] [CrossRef]
- Axelrod, B.; Cheesbrough, T.M.; Laakso, S. Lipoxygenase from soybeans: EC 1.13. 11.12 Linoleate: Oxygen oxidoreductase. Meth. Enzymol. 1981, 71, 441–451. [Google Scholar] [CrossRef]
| Dose/Parameter | Proteins (μg/mL) | MPO (OD × 1000) | GSH (nmol/mg of Proteins) | GPx (nmol/min/mg of Proteins) |
|---|---|---|---|---|
| Vehicle 10 mL/kg | 77 ± 5 | 128 ± 14 | 8.4 ± 4.1 | 4.0 ± 0.1 |
| Diclofenac 5 mg/kg | 45 ± 10 * | 76 ± 14 * | 13 ± 3 | 6.6 ± 1.2 * |
| Indometacin 10 mg/kg | 40 ± 7 * | 84 ± 13 * | 6.8 ± 2.2 | 2.6 ± 0.4 * |
| MAR 1 mg/kg | 55 ± 17 ** | 81 ± 16 * | 13 ± 6 | 6.1 ± 0.4 * |
| MAR 10 mg/kg | 58 ± 4 ** | 86 ± 17 ** | 12 ± 5 | 6.8 ± 0.2 * |
| MAR 20 mg/kg | 55 ± 2 * | 90 ± 15 ** | 9.7 ± 2.8 | 5.2 ± 0.3 ** |
| MAR 40 mg/kg | 47 ± 4 * | 84 ± 7 * | 13 ± 2 | 5.5 ± 0.5 * |
| Time (h)/ Concentration (M) | MAR | ||||
|---|---|---|---|---|---|
| 10−4 | 10−5 | 10−6 | 10−7 | 10−8 | |
| Granulocyte viability compared with the control (%) | |||||
| 1 | 101 ± 6 | 120 ± 10 | 113 ± 11 | 118 ± 9 | 107 ± 6 |
| 2 | 82 ± 8 ** | 106 ± 4 | 108 ± 6 | 98 ± 3 | 97 ± 3 |
| 3 | 96 ± 5 | 91 ± 5 | 101 ± 7 | 98 ± 6 | 94 ± 6 |
| 4 | 80 ± 9 ** | 96 ± 6 | 92 ± 9 | 111 ± 12 | 98 ± 6 |
| 5 | 69 ± 5 * | 72 ± 12 * | 70 ± 14 * | 78 ± 9 * | 79 ± 10 * |
| PMNCs viability compared with the control (%) | |||||
| 1 | 115 ± 9 | 97 ± 5 | 109 ± 6 | 112 ± 8 | 103 ± 7 |
| 2 | 105 ± 8 | 112 ± 8 | 115 ± 9 | 100 ± 5 | 99 ± 2 |
| 3 | 63 ± 11 * | 59 ± 12 * | 67 ± 17 * | 75 ± 12 * | 93 ± 11 |
| 4 | 54 ± 16 * | 55 ± 11 * | 62 ± 12 * | 70 ± 4 * | 88 ± 5 ** |
| 5 | 46 ± 10 * | 50 ± 13 * | 53 ± 11 * | 61 ± 7 * | 80 ± 2 ** |
| Spleen lymphocyte viability compared with the control (%) | |||||
| 1 | 96 ± 5 | 95 ± 4 | 102 ± 7 | 108 ± 66 | 102 ± 5 |
| 2 | 102 ± 4 | 95 ± 7 | 106 ± 5 | 97 ± 6 | 96 ± 4 |
| 3 | 98 ± 4 | 93 ± 5 | 94 ± 4 | 87 ± 10 | 105 ± 2 |
| 4 | 90 ± 2 ** | 102 ± 8 | 98 ± 4 | 114 ± 9 | 101 ± 4 |
| 5 | 79 ± 7 * | 92 ± 7 | 103 ± 4 | 102 ± 5 | 95 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radulović, N.S.; Đorđević Zlatković, M.R.; Stojanović, N.M.; Nešić, M.S.; Zlatković, D.B.; Potić Floranović, M.S.; Tričković Vukić, D.S.; Randjelovic, P.J. Marrubiin Inhibits Peritoneal Inflammatory Response Induced by Carrageenan Application in C57 Mice. Int. J. Mol. Sci. 2024, 25, 4496. https://doi.org/10.3390/ijms25084496
Radulović NS, Đorđević Zlatković MR, Stojanović NM, Nešić MS, Zlatković DB, Potić Floranović MS, Tričković Vukić DS, Randjelovic PJ. Marrubiin Inhibits Peritoneal Inflammatory Response Induced by Carrageenan Application in C57 Mice. International Journal of Molecular Sciences. 2024; 25(8):4496. https://doi.org/10.3390/ijms25084496
Chicago/Turabian StyleRadulović, Niko S., Miljana R. Đorđević Zlatković, Nikola M. Stojanović, Milan S. Nešić, Dragan B. Zlatković, Milena S. Potić Floranović, Dragana S. Tričković Vukić, and Pavle J. Randjelovic. 2024. "Marrubiin Inhibits Peritoneal Inflammatory Response Induced by Carrageenan Application in C57 Mice" International Journal of Molecular Sciences 25, no. 8: 4496. https://doi.org/10.3390/ijms25084496







