Recent Advances in the Use of Stem Cells in Tissue Engineering and Adjunct Therapies for Tendon Reconstruction and Future Perspectives
Abstract
1. Introduction
2. Stem Cells and Their Properties
2.1. Types and Sources of Stem Cells
2.1.1. Tendon Progenitor Stem Cells (TSPCs)
2.1.2. Adipose Tissue-Derived Stem Cells (ASCs)
2.1.3. Bone Marrow-Derived Mesenchymal Stem (BMSCs)
2.1.4. Embryonic Stem Cells (ESCs) and Pluripotent Stem Cells
3. The Role of Growth Factors, Transcription Factors and Other Biological Mediators in Stem Cell Differentiation and Tendon Regeneration
3.1. Growth Factors and Bioactive Proteins in Tendon Regeneration
3.2. Transforming Growth Factor Beta (TGF-β)
3.3. Growth Differentiation Factors (GDFs)
3.4. Connective Tissue Growth Factor CTGF
3.5. Fibroblast Growth Factor (FGFs)
3.6. Insulin-Like Growth Factor (IGF-1)
3.7. Yes-Associated Protein (YAP)
3.8. Wnt Ligands
3.9. Genetic Modification
3.10. Biomaterials
3.10.1. Effects of 3D Biomatrices on Cell Properties
3.10.2. The Influence of Topographical Structure
3.10.3. Polycaprolactone (PCL) as a Matrix
3.11. Mechanotransduction Stimulation
3.12. Pioglitazone and Its Effect on Stem Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int. J. Mol. Sci. 2019, 20, 3002. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Spanoudes, K.; Holladay, C.; Pandit, A.; Zeugolis, D. Progress in cell-based therapies for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Lomas, A.; Ryan, C.; Sorushanova, A.; Shologu, N.; Sideri, A.; Tsioli, V.; Fthenakis, G.; Tzora, A.; Skoufos, I.; Quinlan, L.; et al. The past, present and future in scaffold-based tendon treatments. Adv. Drug Deliv. Rev. 2015, 84, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Snedeker, J.G.; Foolen, J. Tendon injury and repair—A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017, 63, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Tempfer, H.; Traweger, A. Tendon Vasculature in Health and Disease. Front. Physiol. 2015, 6, 330. [Google Scholar] [CrossRef] [PubMed]
- Evrova, O.; Buschmann, J. In vitro and in vivo effects of PDGF-BB delivery strategies on tendon healing: A review. Eur. Cells Mater. 2017, 34, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Meier Bürgisser, G.; Calcagni, M.; Bachmann, E.; Fessel, G.; Snedeker, J.G.; Giovanoli, P.; Buschmann, J. Rabbit Achilles tendon full transection model—Wound healing, adhesion formation and biomechanics at 3, 6 and 12 weeks post-surgery. Biol. Open 2016, 5, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Galatz, L.M.; Gerstenfeld, L.; Heber-Katz, E.; Rodeo, S.A. Tendon regeneration and scar formation: The concept of scarless healing. J. Orthop. Res. 2015, 33, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Cheng, R.; das Neves, J.; Tang, J.; Xiao, J.; Ni, Q.; Liu, X.; Pan, G.; Li, D.; Cui, W.; et al. Advances in biomaterials for preventing tissue adhesion. J. Control. Release 2017, 261, 318–336. [Google Scholar] [CrossRef]
- Korntner, S.; Zeugolis, D.I. Wound healing and fibrosis—State of play. Adv. Drug Deliv. Rev. 2019, 146, 1–2. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.; Yildirimer, L.; Xu, T.; Zhao, X. Advanced technology-driven therapeutic interventions for prevention of tendon adhesion: Design, intrinsic and extrinsic factor considerations. Acta Biomater. 2021, 124, 15–32. [Google Scholar] [CrossRef]
- Li, Z.J.; Yang, Q.Q.; Zhou, Y.L. Basic Research on Tendon Repair: Strategies, Evaluation, and Development. Front. Med. 2021, 8, 664909. [Google Scholar] [CrossRef] [PubMed]
- Legrand, A.; Kaufman, Y.; Long, C.; Fox, P.M. Molecular Biology of Flexor Tendon Healing in Relation to Reduction of Tendon Adhesions. J. Hand Surg. 2017, 42, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, S.; Liu, S.; Chen, S.; Lin, Z.Y.; Pan, G.; He, F.; Li, F.; Fan, C.; Cui, W. Optimization of intrinsic and extrinsic tendon healing through controllable water-soluble mitomycin-C release from electrospun fibers by mediating adhesion-related gene expression. Biomaterials 2015, 61, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Tingart, M.; Maffulli, N. Progress with stem cell therapies for tendon tissue regeneration. Expert Opin. Biol. Ther. 2020, 20, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P. Stem cell technology for tendon regeneration: Current status, challenges, and future research directions. Stem Cells Cloning Adv. Appl. 2015, 8, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Harvey, T.; Flamenco, S.; Fan, C.-M. A Tppp3+Pdgfra+ tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nature 2019, 21, 1490–1503. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Mukherjee, S. Clinical Practice of Umbilical Cord Blood Stem Cells in Transplantation and Regenerative Medicine—Prodigious Promise for Imminent Times. Recent Pat. Biotechnol. 2022, 16, 16–34. [Google Scholar] [CrossRef]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental stem cell and dental tissue regeneration. Front. Med. 2019, 13, 152–159. [Google Scholar] [CrossRef]
- Lyu, K.; Liu, T.; Chen, Y.; Lu, J.; Jiang, L.; Liu, X.; Liu, X.; Li, Y.; Li, S. A “cell-free treatment” for tendon injuries: Adipose stem cell-derived exosomes. Eur. J. Med. Res. 2022, 27, 75. [Google Scholar] [CrossRef] [PubMed]
- Festa, E.; Fretz, J.; Berry, R.; Schmidt, B.; Rodeheffer, M.; Horowitz, M.; Horsley, V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 2011, 146, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Yea, J.-H.; Kim, Y.; Jo, C.H. Comparison of mesenchymal stem cells from bone marrow, umbilical cord blood, and umbilical cord tissue in regeneration of a full-thickness tendon defect in vitro and in vivo. Biochem. Biophys. Rep. 2023, 34, 101486. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.-M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Durgam, S.S.; Stewart, A.A.; Sivaguru, M.; Johnson, A.J.W.; Stewart, M.C. Tendon-derived progenitor cells improve healing of collagenase-induced flexor tendinitis. J. Orthop. Res. 2016, 34, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Clegg, P.D.; Comerford, E.J.; Canty-Laird, E.G. A comparison of the stem cell characteristics of murine tenocytes and tendon-derived stem cells. BMC Musculoskelet. Disord. 2018, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Lui, P.P.Y.; Rui, Y.F. Effect of in vitro passaging on the stem cell-related properties of tendon-derived stem cells-implications in tissue engineering. Stem Cells Dev. 2012, 21, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pongkitwitoon, S.; Lu, H.; Lee, C.; Gelberman, R.; Thomopoulos, S. CTGF induces tenogenic differentiation and proliferation of adipose-derived stromal cells. J. Orthop. Res. 2019, 37, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Wang, W.; Wang, B.; Zhang, P.; Zhou, G.; Zhang, W.J.; Cao, Y.; Liu, W. Repair of Achilles tendon defect with autologous ASCs engineered tendon in a rabbit model. Biomaterials 2014, 35, 8801–8809. [Google Scholar] [CrossRef]
- Zarychta-Wiśniewska, W.; Burdzinska, A.; Kulesza, A.; Gala, K.; Kaleta, B.; Zielniok, K.; Siennicka, K.; Sabat, M.; Paczek, L. Bmp-12 activates tenogenic pathway in human adipose stem cells and affects their immunomodulatory and secretory properties. BMC Cell Biol. 2017, 18, 13. [Google Scholar] [CrossRef]
- Mora, M.V.; Antuña, S.A.; Arranz, M.G.; Carrascal, M.T.; Barco, R. Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury 2014, 45, S22–S27. [Google Scholar] [CrossRef] [PubMed]
- Uysal, A.C.; Mizuno, H. Tendon regeneration and repair with adipose derived stem cells. Curr. Stem Cell Res. Ther. 2010, 5, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, X.; Lu, A.; Tu, M.; Huang, W.; Huang, P. BMP14 induces tenogenic differentiation of bone marrow mesenchymal stem cells in vitro. Exp. Ther. Med. 2018, 16, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Renzi, S.; Riccò, S.; Dotti, S.; Sesso, L.; Grolli, S.; Cornali, M.; Carlin, S.; Patruno, M.; Cinotti, S.; Ferrari, M. Autologous bone marrow mesenchymal stromal cells for regeneration of injured equine ligaments and tendons: A clinical report. Res. Vet. Sci. 2013, 95, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Jana, S.; Tsao, C.T.; Zhang, M. Tenogenic differentiation of human bone marrow stem cells via a combinatory effect of aligned chitosan-poly-caprolactone nanofibers and TGF-β3. J. Mater. Chem. B 2013, 1, 6516–6524. [Google Scholar] [CrossRef] [PubMed]
- Shojaee, A.; Parham, A. Strategies of tenogenic differentiation of equine stem cells for tendon repair: Current status and challenges. Stem Cell Res. Ther. 2019, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Rodas, G.; Rius-Tarruella, J.; Alomar, X.; Balius, R.; Ruíz-Cotorro, Á.; Masci, L.; Maffulli, N.; Orozco, L. Safety and Efficacy of Bone Marrow-Derived Mesenchymal Stem Cells for Chronic Patellar Tendinopathy (With Gap > 3 mm) in Patients: 12-Month Follow-up Results of a Phase 1/2 Clinical Trial. Orthop. J. Sports Med. 2023, 11, 23259671231184400. [Google Scholar] [CrossRef]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Dale, T.P.; Mazher, S.; Webb, W.R.; Zhou, J.; Maffulli, N.; Chen, G.-Q.; El Haj, A.J.; Forsyth, N.R. Tenogenic Differentiation of Human Embryonic Stem Cells. Tissue Eng. Part A 2018, 24, 361–368. [Google Scholar] [CrossRef]
- Brovkina, O.; Dashinimaev, E. Advances and complications of regenerative medicine in diabetes therapy. PeerJ 2020, 8, e9746. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, A.; Richardson, D.W. Regulation of the tenogenic gene expression in equine tenocyte-derived induced pluripotent stem cells by mechanical loading and Mohawk. Stem Cell Res. 2019, 39, 101489. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Y.; Liu, E.; Sun, Y.; Luo, Z.; Xu, Z.; Liu, W.; Zhong, L.; Lv, Y.; Wang, A.; et al. Human iPSC-derived neural crest stem cells promote tendon repair in a rat patellar tendon window defect model. Tissue Eng. Part A 2013, 19, 2439–2451. [Google Scholar] [CrossRef] [PubMed]
- Romanazzo, S.; Nemec, S.; Roohani, I. iPSC Bioprinting: Where are We at? Materials 2019, 12, 2453. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Zhou, Y.; Wang, W.; Zhang, J.; Wang, J.H.-C. Mechanical Overloading Induced-Activation of mTOR Signaling in Tendon Stem/Progenitor Cells Contributes to Tendinopathy Development. Front. Cell Dev. Biol. 2021, 9, 687856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.H.-C. Prostaglandin E2 (PGE2) exerts biphasic effects on human tendon stem cells. PLoS ONE 2014, 9, e87706. [Google Scholar] [CrossRef]
- Havis, E.; Bonnin, M.-A.; Olivera-Martinez, I.; Nazaret, N.; Ruggiu, M.; Weibel, J.; Durand, C.; Guerquin, M.-J.; Bonod-Bidaud, C.; Ruggiero, F.; et al. Transcriptomic analysis of mouse limb tendon cells during development. Development 2014, 141, 3683–3696. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Zhu, S.; Lu, P.; Zhu, T.; Gong, X.; Zhang, Z.; Hu, J.; Yin, Z.; Heng, B.C.; et al. Mohawk promotes the tenogenesis of mesenchymal stem cells through activation of the TGFβ signaling pathway. Stem Cells 2015, 33, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Theodossiou, S.K.; Tokle, J.; Schiele, N.R. TGFβ2-induced tenogenesis impacts cadherin and connexin cell-cell junction proteins in mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2019, 508, 889–893. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, S.Y.; Yang, E.-J.; Kim, H.Y.; Jo, I.; Shin, S.-J. Expression of tenocyte lineage-related factors from tonsil-derived mesenchymal stem cells. Tissue Eng. Regen. Med. 2016, 13, 162–170. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Parks, W.C.; Rifkin, D.B.; Derwin, K.A. Mechanisms of tendon injury and repair. J. Orthop. Res. 2015, 33, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Katzel, E.B.; Wolenski, M.; Loiselle, A.E.; Basile, P.; Flick, L.M.; Langstein, H.N.; Hilton, M.J.; Awad, H.A.; Hammert, W.C.; O’Keefe, R.J. Impact of Smad3 loss of function on scarring and adhesion formation during tendon healing. J. Orthop. Res. 2011, 29, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Most, D.; Stelnicki, E.; Siebert, J.W.; Longaker, M.T.; Hui, K.; Lineaweaver, W.C. Gene expression of transforming growth factor beta-1 in rabbit zone II flexor tendon wound healing: Evidence for dual mechanisms of repair. Plast. Reconstr. Surg. 1997, 100, 937–944. [Google Scholar] [CrossRef]
- Kuo, C.K.; Petersen, B.C.; Tuan, R.S. Spatiotemporal protein distribution of TGF-betas, their receptors, and extracellular matrix molecules during embryonic tendon development. Dev. Dyn. 2008, 237, 1477–1489. [Google Scholar] [CrossRef]
- Pryce, B.A.; Watson, S.S.; Murchison, N.D.; Staverosky, J.A.; Dünker, N.; Schweitzer, R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development 2009, 136, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Finley, V.G.; Kuo, C.K. Embryonic mechanical and soluble cues regulate tendon progenitor cell gene expression as a function of developmental stage and anatomical origin. J. Biomech. 2014, 47, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Berthet, E.; Chen, C.; Butcher, K.; Schneider, R.A.; Alliston, T.; Amirtharajah, M. Smad3 binds scleraxis and mohawk and regulates tendon matrix organization. J. Orthop. Res. 2013, 31, 1475–1483. [Google Scholar] [CrossRef]
- Massagué, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef]
- Jiang, K.; Chun, G.; Wang, Z.; DU, Q.; Wang, A.; Xiong, Y. Effect of transforming growth factor-β3 on the expression of Smad3 and Smad7 in tenocytes. Mol. Med. Rep. 2016, 13, 3567–3573. [Google Scholar] [CrossRef]
- Maeda, T.; Sakabe, T.; Sunaga, A.; Sakai, K.; Rivera, A.L.; Keene, D.R.; Sasaki, T.; Stavnezer, E.; Iannotti, J.; Schweitzer, R.; et al. Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr. Biol. 2011, 21, 933–941. [Google Scholar] [CrossRef]
- Han, B.; Jones, I.A.; Yang, Z.; Fang, W.; Vangsness, C.T. Repair of Rotator Cuff Tendon Defects in Aged Rats Using a Growth Factor Injectable Gel Scaffold. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-H.; Chen, D.-Q.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Vaziri, N.D.; Zhao, Y.-Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. TGF-β1 Signaling and Tissue Fibrosis. Cold Spring Harb. Perspect. Biol. 2018, 10, a022293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Z.; Liu, Y.; Wang, Z.; Kang, Y. Inhibition of Smad3 promotes the healing of rotator cuff injury in a rat model. J. Orthop. Res. 2021, 39, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Mao, W.F.; Zhou, Y.L.; Wang, X.T.; Liu, P.Y.; Tang, J.B. Adeno-associated virus-2-mediated TGF-β1 microRNA transfection inhibits adhesion formation after digital flexor tendon injury. Gene Ther. 2016, 23, 167–175. [Google Scholar] [CrossRef]
- Tsubone, T.; Moran, S.L.; Amadio, P.C.; Zhao, C.; An, K.-N. Expression of growth factors in canine flexor tendon after laceration in vivo. Ann. Plast. Surg. 2004, 53, 393–397. [Google Scholar] [CrossRef]
- Chan, K.M.; Fu, S.C.; Wong, Y.P.; Hui, W.C.; Cheuk, Y.C.; Wong, M.W. Expression of transforming growth factor beta isoforms and their roles in tendon healing. Wound Repair Regen. 2008, 16, 399–407. [Google Scholar] [CrossRef]
- Chang, J.; Thunder, R.; Most, D.; Longaker, M.T.; Lineaweaver, W.C. Studies in flexor tendon wound healing: Neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast. Reconstr. Surg. 2000, 105, 148–155. [Google Scholar] [CrossRef]
- Kapacee, Z.; Yeung, C.-Y.C.; Lu, Y.; Crabtree, D.; Holmes, D.F.; Kadler, K.E. Synthesis of embryonic tendon-like tissue by human marrow stromal/mesenchymal stem cells requires a three-dimensional environment and transforming growth factor β3. Matrix Biol. 2010, 29, 668–677. [Google Scholar] [CrossRef]
- Manning, C.N.; Kim, H.M.; Sakiyama-Elbert, S.; Galatz, L.M.; Havlioglu, N.; Thomopoulos, S. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J. Orthop. Res. 2011, 29, 1099–1105. [Google Scholar] [CrossRef]
- Hou, Y.; Mao, Z.; Wei, X.; Lin, L.; Chen, L.; Wang, H.; Fu, X.; Zhang, J.Y.; YU, C. Effects of transforming growth factor-beta1 and vascular endothelial growth factor 165 gene transfer on Achilles tendon healing. Matrix Biol. 2009, 28, 324–335. [Google Scholar] [CrossRef]
- Kaji, D.A.; Tan, Z.; Johnson, G.L.; Huang, W.; Vasquez, K.; Lehoczky, J.A.; Levi, B.; Cheah, K.S.E.; Huang, A.H. Cellular Plasticity in Musculoskeletal Development, Regeneration, and Disease. J. Orthop. Res. 2020, 38, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.H.A.I.; Ming, N.I.; Rui, Y.F.; Zhang, K.Y.; Zhang, Q.; Xu, L.L.; Chang, K.M.; Li, G.; Wang, Y. Effect of growth and differentiation factor 6 on the tenogenic differentiation of bone mar-row-derived mesenchymal stem cells. Chin. Med. J. 2013, 126, 1509–1516. [Google Scholar]
- Francis-West, P.H.; Parish, J.; Lee, K.; Archer, C.W. BMP/GDF-signalling interactions during synovial joint development. Cell Tissue Res. 1999, 296, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, P.; Fahlgren, A.; Aspenberg, P. Mechanical load and BMP signaling during tendon repair: A role for follistatin? Clin. Orthop. Relat. Res. 2008, 466, 1592–1597. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Würgler-Hauri, C.C.; Dourte, L.M.; Baradet, T.C.; Williams, G.R.; Soslowsky, L.J. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J. Shoulder Elb. Surg. 2007, 16, S198–S203. [Google Scholar] [CrossRef]
- Uemura, K.; Hayashi, M.; Itsubo, T.; Oishi, A.; Iwakawa, H.; Komatsu, M.; Uchiyama, S.; Kato, H. Myostatin promotes tenogenic differentiation of C2C12 myoblast cells through Smad3. FEBS Open Bio 2017, 7, 522–532. [Google Scholar] [CrossRef]
- Le, W.; Yao, J. The Effect of Myostatin (GDF-8) on Proliferation and Tenocyte Differentiation of Rat Bone Marrow-Derived Mesenchymal Stem Cells. J. Hand Surg. Asian-Pac. Vol. 2017, 22, 200–207. [Google Scholar] [CrossRef]
- Ciardulli, M.C.; Marino, L.; Lamparelli, E.P.; Guida, M.; Forsyth, N.R.; Selleri, C.; Della Porta, G.; Maffulli, N. Dose-Response Tendon-Specific Markers Induction by Growth Differentiation Factor-5 in Human Bone Marrow and Umbilical Cord Mesenchymal Stem Cells. Int. J. Mol. Sci. 2020, 21, 5905. [Google Scholar] [CrossRef]
- Hasslund, S.; Dadali, T.; Ulrich-Vinther, M.; Søballe, K.; Schwarz, E.M.; Awad, H.A. Freeze-dried allograft-mediated gene or protein delivery of growth and differentiation factor 5 reduces reconstructed murine flexor tendon adhesions. J. Tissue Eng. 2014, 5, 2041731414528736. [Google Scholar] [CrossRef]
- Lee, C.H.; Shah, B.; Moioli, E.K.; Mao, J.J. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J. Clin. Investig. 2010, 120, 3340–3349. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, F.Y.; Tarafder, S.; Kao, K.; Jun, Y.; Yang, G.; Mao, J.J. Harnessing endogenous stem/progenitor cells for tendon regeneration. J. Clin. Investig. 2015, 125, 2690–2701. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tao, X.; Chen, L.; Han, W.; Zhou, Y.; Tang, K. CTGF positively regulates BMP12 induced tenogenic differentiation of tendon stem cells and signaling. Cell. Physiol. Biochem. 2015, 35, 1831–1845. [Google Scholar] [CrossRef]
- Maddaluno, L.; Urwyler, C.; Werner, S. Fibroblast growth factors: Key players in regeneration and tissue repair. Development 2017, 144, 4047–4060. [Google Scholar] [CrossRef] [PubMed]
- Lotz, S.; Goderie, S.; Tokas, N.; Hirsch, S.E.; Ahmad, F.; Corneo, B.; Le, S.; Banerjee, A.; Kane, R.S.; Stern, J.H.; et al. Sustained levels of FGF2 maintain undifferentiated stem cell cultures with biweekly feeding. PLoS ONE 2013, 8, e56289. [Google Scholar] [CrossRef] [PubMed]
- Solchaga, L.A.; Penick, K.; Goldberg, V.M.; Caplan, A.I.; Welter, J.F. Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells. Tissue Eng. Part A 2010, 16, 1009–1019. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Shimazu, A.; Miyazaki, K.; Pan, H.; Koike, C.; Yoshida, E.; Takagishi, K.; Kato, Y. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem. Biophys. Res. Commun. 2001, 288, 413–419. [Google Scholar] [CrossRef]
- Cai, T.-Y.; Zhu, W.; Chen, X.-S.; Zhou, S.-Y.; Jia, L.-S.; Sun, Y.-Q. Fibroblast growth factor 2 induces mesenchymal stem cells to differentiate into tenocytes through the MAPK pathway. Mol. Med. Rep. 2013, 8, 1323–1328. [Google Scholar] [CrossRef]
- Hankemeier, S.; Keus, M.; Zeichen, J.; Jagodzinski, M.; Barkhausen, T.; Bosch, U.; Krettek, C.; Van Griensven, M. Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: Potential implications for tissue engineering of tendons and ligaments. Tissue Eng. 2005, 11, 41–49. [Google Scholar] [CrossRef]
- Guo, D.; Li, H.; Liu, Y.; Yu, X.; Zhang, X.; Chu, W.; She, Y.; Wang, D.; Chen, G. Fibroblast growth factor-2 promotes the function of tendon-derived stem cells in Achilles tendon restoration in an Achilles tendon injury rat model. Biochem. Biophys. Res. Commun. 2020, 521, 91–97. [Google Scholar] [CrossRef]
- Tokunaga, T.; Shukunami, C.; Okamoto, N.; Taniwaki, T.; Oka, K.; Sakamoto, H.; Ide, J.; Mizuta, H.; Hiraki, Y. FGF-2 Stimulates the Growth of Tenogenic Progenitor Cells to Facilitate the Generation of Tenomodulin-Positive Tenocytes in a Rat Rotator Cuff Healing Model. Am. J. Sports Med. 2015, 43, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Pfäffle, R.; Kiess, W. GH and IGF-1 Replacement in Children. Handb. Exp. Pharmacol. 2020, 261, 67–86. [Google Scholar] [PubMed]
- Nicholls, A.R.; Holt, R.I. Growth Hormone and Insulin-Like Growth Factor-1. Front. Horm. Res. 2016, 47, 101–114. [Google Scholar] [PubMed]
- Werner, H.; Weinstein, D.; Bentov, I. Similarities and differences between insulin and IGF-I: Structures, receptors, and signalling pathways. Arch. Physiol. Biochem. 2008, 114, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, F.; Barberi, L.; Dobrowolny, G.; Villa Nova Bacurau, A.; Nicoletti, C.; Rizzuto, E.; Rosenthal, N.; Scicchitano, B.M.; Musarò, A. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 2019, 18, e12954. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, F.; Yeung, E.W.; Li, Y. IGF-IEc expression, regulation and biological function in different tissues. Growth Horm. IGF Res. 2010, 20, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Tunç, B.S.; Toprak, F.; Toprak, S.F.; Sozer, S. In vitro investigation of growth factors including MGF and IGF-1 in neural stem cell activation, proliferation, and migration. Brain Res. 2021, 1759, 147366. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wu, K.; Zhang, B.; Song, G. Mechano growth factor E peptide promotes rat bone marrow-derived mesenchymal stem cell migration through CXCR4-ERK1/2. Growth Factors 2015, 33, 210–219. [Google Scholar] [PubMed]
- Trenker, R.; Jura, N. Receptor tyrosine kinase activation: From the ligand perspective. Curr. Opin. Cell Biol. 2020, 63, 174–185. [Google Scholar] [CrossRef]
- Guo, C.A.; Guo, S. Insulin receptor substrate signaling controls cardiac energy metabolism and heart failure. J. Endocrinol. 2017, 233, R131–R143. [Google Scholar] [CrossRef]
- Hakuno, F.; Takahashi, S.-I. 40 years of IGF1: IGF1 receptor signaling pathways. J. Mol. Endocrinol. 2018, 61, T69–T86. [Google Scholar] [CrossRef] [PubMed]
- Józefiak, A.; Larska, M.; Pomorska-Mól, M.; Ruszkowski, J.J. The IGF-1 Signaling Pathway in Viral Infections. Viruses 2021, 13, 1488. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Thien, C.; Wang, C.; Ni, M.; Gao, J.; Wang, A.; Jiang, Q.; Tuan, R.S.; Zheng, Q.; Zheng, M.H. 3D uniaxial mechanical stimulation induces tenogenic differentiation of tendon-derived stem cells through a PI3K/AKT signaling pathway. FASEB J. 2018, 32, 4804–4814. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.C.X.; Kihara, A.H.; Goulart, V.A.; Tonelli, F.M.; Gomes, K.N.; Ulrich, H.; Resende, R.R. Calcium signaling and cell proliferation. Cell. Signal. 2015, 27, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Disser, N.P.; Sugg, K.B.; Talarek, J.R.; Sarver, D.C.; Rourke, B.J.; Mendias, C.L. Insulin-like growth factor 1 signaling in tenocytes is required for adult tendon growth. FASEB J. 2019, 33, 12680–12695. [Google Scholar] [CrossRef]
- Hernandez, D.M.; Kang, J.H.; Choudhury, M.; Andrianifahanana, M.; Yin, X.; Limper, A.H.; Leof, E.B. IPF pathogenesis is dependent upon TGFβ induction of IGF-1. FASEB J. 2020, 34, 5363–5388. [Google Scholar] [CrossRef] [PubMed]
- Banes, A.J.; Tsuzaki, M.; Hu, P.; Brigman, B.; Brown, T.; Almekinders, L.; Lawrence, W.; Fischer, T. PDGF-BB, IGF-I and mechanical load stimulate DNA synthesis in avian tendon fibroblasts in vitro. J. Biomech. 1995, 28, 1505–1513. [Google Scholar] [CrossRef]
- Kurtz, C.A.; Loebig, T.G.; Anderson, D.D.; DeMeo, P.J.; Campbell, P.G. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. Am. J. Sports Med. 1999, 27, 363–369. [Google Scholar] [CrossRef]
- Savaris, M.; dos Santos, V. Brandalise Influence of different sterilization processes on the properties of commercial poly(lactic acid). Mater. Sci. Eng. C 2016, 69, 661–667. [Google Scholar] [CrossRef]
- Mainil-Varlet, P.; Curtis, R.; Gogolewski, S. Effect ofin vivo andin vitro degradation on molecular and mechanical properties of various low-molecular-weight polylactides. J. Biomed. Mater. Res. 1997, 36, 360–380. [Google Scholar] [CrossRef]
- Moioli, E.K.; Hong, L.; Guardado, J.; Clark, P.A.; Mao, J.J. Sustained release of TGFbeta3 from PLGA microspheres and its effect on early osteogenic differentiation of human mesenchymal stem cells. Tissue Eng. 2006, 12, 537–546. [Google Scholar] [CrossRef]
- Simpson, H.L.; Jackson, N.C.; Shojaee-Moradie, F.; Jones, R.H.; Russell-Jones, D.L.; Sönksen, P.H.; Dunger, D.B.; Umpleby, A.M. Insulin-like growth factor I has a direct effect on glucose and protein metabolism, but no effect on lipid metabolism in type 1 diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 425–432. [Google Scholar] [CrossRef]
- Bianda, T.L.; Hussain, M.A.; Keller, A.; Glatz, Y.; Schmitz, O.; Christiansen, J.S.; Alberti, K.G.M.M.; Froesch, E.R. Insulin-like growth factor-I in man enhances lipid mobilization and oxidation induced by a growth hormone pulse. Diabetologia 1996, 39, 961–969. [Google Scholar] [CrossRef]
- Natoli, R.M.; Athanasiou, K.A. P188 reduces cell death and IGF-I reduces GAG release following single-impact loading of articular cartilage. J. Biomech. Eng. 2008, 130, 041012. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.; Rowan, A.D.; Cawston, T. Insulin-like growth factor 1 blocks collagen release and down regulates matrix metalloproteinase-1, -3, -8, and -13 mRNA expression in bovine nasal cartilage stimulated with oncostatin M in combination with interleukin 1alpha. Ann. Rheum. Dis. 2001, 60, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Chubinskaya, S.; Schoeberl, B.; Florine, E.; Kopesky, P.; Grodzinsky, A.J. Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: Relevance to post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.Y.; Kim, H.T. Chondrocyte apoptosis induced by collagen degradation: Inhibition by caspase inhibitors and IGF-1. J. Orthop. Res. 2004, 22, 140–144. [Google Scholar] [CrossRef]
- Dahlgren, L.A.; van der Meulen, M.C.H.; Bertram, J.E.A.; Starrak, G.S.; Nixon, A.J. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. J. Orthop. Res. 2002, 20, 910–919. [Google Scholar] [CrossRef]
- Lyras, D.N.; Kazakos, K.; Verettas, D.; Chronopoulos, E.; Folaranmi, S.; Agrogiannis, G. Effect of combined administration of transforming growth factor-b1 and insulin-like growth factor I on the mechanical properties of a patellar tendon defect model in rabbits. Acta Orthop. Belg. 2010, 76, 380–386. [Google Scholar]
- Andersson, T.; Eliasson, P.; Aspenberg, P. Growth hormone does not stimulate early healing in rat tendons. Int. J. Sports Med. 2012, 33, 240–243. [Google Scholar] [CrossRef]
- Hansen, M.; Boesen, A.; Holm, L.; Flyvbjerg, A.; Langberg, H.; Kjaer, M. Local administration of insulin-like growth factor-I (IGF-I) stimulates tendon collagen synthesis in humans. Scand. J. Med. Sci. Sports 2013, 23, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Doessing, S.; Heinemeier, K.M.; Holm, L.; Mackey, A.L.; Schjerling, P.; Rennie, M.; Smith, K.; Reitelseder, S.; Kappelgaard, A.-M.; Rasmussen, M.H.; et al. Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. J. Physiol. 2010, 588, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.L.; Hansen, M.; Turtumoygard, I.F.; Hoffner, R.; Schjerling, P.; Christensen, J.; Mendias, C.L.; Magnusson, P.S.; Kjaer, M. No Treatment Benefits of Local Administration of Insulin-like Growth Factor-1 in Addition to Heavy Slow Resistance Training in Tendinopathic Human Patellar Tendons: A Randomized, Double-Blind, Placebo-Controlled Trial with 1-Year Follow-up. Am. J. Sports Med. 2021, 49, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Notermans, T.; Hammerman, M.; Eliasson, P.; Isaksson, H. Tendon mechanobiology in small-animal experiments during post-transection healing. Eur. Cells Mater. 2021, 42, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Kominsky, D.J.; Campbell, E.L.; Colgan, S.P. Metabolic Shifts in Immunity and Inflammation. J. Immunol. 2010, 184, 4062–4068. [Google Scholar] [CrossRef] [PubMed]
- Vannella, K.M.; Wynn, T.A. Mechanisms of Organ Injury and Repair by Macrophages. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J. Insulin-like growth factor-I regulation of immune function: A potential therapeutic target in autoimmune diseases? Pharmacol. Rev. 2010, 62, 199–236. [Google Scholar] [CrossRef]
- Lyras, D.; Kazakos, K.; Agrogiannis, G.; Verettas, D.; Kokka, A.; Kiziridis, G.; Chronopoulos, E.; Tryfonidis, M. Experimental study of tendon healing early phase: Is IGF-1 expression influenced by platelet rich plasma gel? Orthop. Traumatol. Surg. Res. 2010, 96, 381–387. [Google Scholar] [CrossRef]
- Wong, C.-C.; Huang, Y.-M.; Chen, C.-H.; Lin, F.-H.; Yeh, Y.-Y.; Bai, M.-Y. Cytokine and Growth Factor Delivery from Implanted Platelet-Rich Fibrin Enhances Rabbit Achilles Tendon Healing. Int. J. Mol. Sci. 2020, 21, 3221. [Google Scholar] [CrossRef]
- Vaysman, M.; Alben, M.; Todd, M.; Ruotolo, C. Pharmacologic Enhancement of Rotator Cuff Repair: A Narrative Review. Orthop. Rev. 2022, 14, 37782. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, C.; Li, R.; Zhong, W.; Xu, G.; Zhang, W. Role of Yes-associated protein (YAP) in regulation of mesenchymal stem cell tenogenic differentiation. Histochem. J. 2022, 53, 273–283. [Google Scholar] [CrossRef]
- Miyabara, S.; Yuda, Y.; Kasashima, Y.; Kuwano, A.; Arai, K. Regulation of Tenomodulin Expression Via Wnt/β-catenin Signaling in Equine Bone Marrow-derived Mesenchymal Stem Cells. J. Equine Sci. 2014, 25, 7–13. [Google Scholar] [CrossRef][Green Version]
- Chen, M.; Li, Y.; Xiao, L.; Dai, G.; Lu, P.; Rui, Y. Noncanonical Wnt5a signaling regulates tendon stem/progenitor cells senes-cence. Stem Cell Res Ther. 2021, 12, 544. [Google Scholar] [CrossRef]
- Kuo, C.K.; Tuan, R.S. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng. Part A 2008, 14, 1615–1627. [Google Scholar] [CrossRef]
- Xu, K.; Sun, Y.; Al-Ani, M.K.; Wang, C.; Sha, Y.; Sung, K.P.; Dong, N.; Qiu, X.; Yang, L. Synergistic promoting effects of bone morphogenetic protein 12/connective tissue growth factor on functional differentiation of tendon derived stem cells and patellar tendon window defect regeneration. J. Biomech. 2018, 66, 95–102. [Google Scholar] [CrossRef]
- Wang, Q.-W.; Chen, Z.-L.; Piao, Y.-J. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J. Biosci. Bioeng. 2005, 100, 418–422. [Google Scholar] [CrossRef]
- Alberton, P.; Popov, C.; Prägert, M.; Kohler, J.; Shukunami, C.; Schieker, M.; Docheva, D. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev. 2012, 21, 846–858. [Google Scholar] [CrossRef]
- Otabe, K.; Nakahara, H.; Hasegawa, A.; Matsukawa, T.; Ayabe, F.; Onizuka, N.; Inui, M.; Takada, S.; Ito, Y.; Sekiya, I.; et al. Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. J. Orthop. Res. 2015, 33, 1–8. [Google Scholar] [CrossRef]
- Lejard, V.; Blais, F.; Guerquin, M.-J.; Bonnet, A.; Bonnin, M.-A.; Havis, E.; Malbouyres, M.; Bidaud, C.B.; Maro, G.; Gilardi-Hebenstreit, P.; et al. EGR1 and EGR2 involvement in vertebrate tendon differentiation. J. Biol. Chem. 2011, 286, 5855–5867. [Google Scholar] [CrossRef]
- Tao, X.; Liu, J.; Chen, L.; Zhou, Y.; Tang, K. EGR1 induces tenogenic differentiation of tendon stem cells and promotes rabbit rotator cuff repair. Cell. Physiol. Biochem. 2015, 35, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, G.-D.; Liu, J.-P.; Wang, H.-S.; Liu, X.-M.; Wang, Q.; Cai, X.-H. miR-135a modulates tendon stem/progenitor cell senescence via suppressing ROCK1. Bone 2015, 71, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, L.; Xu, J.; Yang, Z.; Wu, T.; Zhang, J.; Shi, L.; Zhu, D.; Zhang, J.; Li, G. MiR-378a suppresses tenogenic differentiation and tendon repair by targeting at TGF-β2. Cell Res. Ther. 2019, 10, 108. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, W.; Chen, J.; Qi, C.; Wang, T.; Zhang, J.; Qu, D.; Yu, T.; Zhang, Y. ADSCs Promote Tenocyte Proliferation by Reducing the Methylation Level of lncRNA Morf4l1 in Tendon Injury. Front. Chem. 2022, 10, 908312. [Google Scholar] [CrossRef] [PubMed]
- Loewer, S.; Cabili, M.N.; Guttman, M.; Loh, Y.H.; Thomas, K.; Park, I.H.; Garber, M.; Curran, M.; Onder, T.; Agarwal, S.; et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat. Genet. 2010, 42, 1113–1117. [Google Scholar]
- Wang, Y.; Xu, Z.; Jiang, J.; Xu, C.; Kang, J.; Xiao, L.; Wu, M.; Xiong, J.; Guo, X.; Liu, H. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev. Cell 2013, 25, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-F.; Liu, Y.; Fu, W.-M.; Xu, J.; Wang, B.; Sun, Y.-X.; Wu, T.-Y.; Xu, L.-L.; Chan, K.-M.; Zhang, J.-F.; et al. Long noncoding RNA H19 accelerates tenogenic differentiation and promotes tendon healing through targeting miR-29b-3p and activating TGF-β1 signaling. FASEB J. 2017, 31, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Strunz, F.; Yan, Z.; Lu, J.; Brochhausen, C.; Kiderlen, S.; Clausen-Schaumann, H.; Wang, X.; Gomes, M.E.; Alt, V.; et al. Corrigendum to “Three-dimensional self-assembling nanofiber matrix rejuvenates aged/degenerative human tendon stem/progenitor cells”. Biomaterials 2021, 269, 120617. [Google Scholar] [CrossRef] [PubMed]
- Paterson, Y.Z.; Cribbs, A.; Espenel, M.; Smith, E.J.; Henson, F.M.D.; Guest, D.J. Genome-wide transcriptome analysis reveals equine embryonic stem cell-derived tenocytes resemble fetal, not adult tenocytes. Stem Cell Res. Ther. 2020, 11, 184. [Google Scholar] [CrossRef]
- Yin, H.; Yan, Z.; Bauer, R.J.; Peng, J.; Schieker, M.; Nerlich, M.; Docheva, D. Functionalized thermosensitive hydrogel combined with tendon stem/progenitor cells as injectable cell delivery carrier for tendon tissue engineering. Biomed. Mater. 2018, 13, 034107. [Google Scholar] [CrossRef]
- Ning, L.J.; Zhang, Y.J.; Zhang, Y.J.; Zhu, M.; Ding, W.; Jiang, Y.L.; Zhang, Y.; Luo, J.C.; Qin, T.W. Enhancement of Migration and Tenogenic Differentiation of Macaca Mulatta Tendon-Derived Stem Cells by Decellularized Tendon Hydrogel. Front. Cell Dev. Biol. 2021, 9, 651583. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Rothrauff, B.B.; Lin, H.; Yu, S.; Tuan, R.S. Tendon-Derived Extracellular Matrix Enhances Transforming Growth Factor-β3-Induced Tenogenic Differentiation of Human Adipose-Derived Stem Cells. Tissue Eng. Part A 2017, 23, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Shi, Y.; Cao, Y.; Liu, W. Recent progress in stem cell differentiation directed by material and mechanical cues. Biomed. Mater. 2016, 11, 014109. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Zhang, E.; Yang, L.; Yuan, H.; Tu, W.; Zhang, H.; Yin, Z.; Shen, W.; Chen, X.; et al. An epigenetic bioactive composite scaffold with well-aligned nanofibers for functional tendon tissue engineering. Acta Biomater. 2018, 66, 141–156. [Google Scholar] [CrossRef]
- Yuan, H.; Li, X.; Lee, M.-S.; Zhang, Z.; Li, B.; Xuan, H.; Li, W.-J.; Zhang, Y. Collagen and chondroitin sulfate functionalized bioinspired fibers for tendon tissue engineering application. Int. J. Biol. Macromol. 2021, 170, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Spanoudes, K.; Gaspar, D.; Pandit, A.; Zeugolis, D.I. The biophysical, biochemical, and biological toolbox for tenogenic phenotype maintenance in vitro. Trends Biotechnol. 2014, 32, 474–482. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, H.; Chu, J.; Eglin, D.; Serra, T.; Docheva, D. An anisotropic nanocomposite hydrogel guides aligned orientation and enhances tenogenesis of human tendon stem/progenitor cells. Biomater. Sci. 2021, 9, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, J.; Wang, B.; Zhang, W.J.; Zhou, G.; Cao, Y.; Liu, W. The regulation of phenotype of cultured tenocytes by microgrooved surface structure. Biomaterials 2010, 31, 6952–6958. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, K.; Zhang, W.; Zhang, Z.; Zhou, G.; Cao, Y.; Liu, W. Microgrooved topographical surface directs tenogenic lineage specific differentiation of mouse tendon derived stem cells. Biomed. Mater. 2017, 12, 015013. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Rev. Adv. Mater. Sci. 2013, 34, 123–140. [Google Scholar]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, N. Preparation and characterization of poly(ε-caprolactone) scaffolds modified with cell-loaded fibrin gel. Int. J. Biol. Macromol. 2019, 125, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhou, B.; Ni, M.; Wang, M.; Ding, L.; Li, Y.; Liu, Y.; Zhang, W.; Li, G.; Wang, J.; et al. Nonwoven-based gelatin/polycaprolactone membrane loaded with ERK inhibitor U0126 for treatment of tendon defects. Stem Cell Res. Ther. 2022, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Beason, D.P.; Connizzo, B.K.; Dourte, L.M.; Mauck, R.L.; Soslowsky, L.J.; Steinberg, D.R.; Bernstein, J. Fiber-aligned polymer scaffolds for rotator cuff repair in a rat model. J. Shoulder Elb. Surg. 2012, 21, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Dudhia, J.; Dakin, S.G.; Snelling, S.J.B.; De Godoy, R.; Mouthuy, P.-A.; Smith, R.K.W.; Morrey, M.; Carr, A.J. Histopathological and immunohistochemical evaluation of cellular response to a woven and electrospun polydioxanone (PDO) and polycaprolactone (PCL) patch for tendon repair. Sci. Rep. 2020, 10, 4754. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Zhang, X.; Yang, L.; Zhang, J. Research Progress of Biodegradable Polymers in Repairing Achilles Tendon Injury. Front. Mater. 2022, 9, 815930. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, J.Y.; Cho, D.-W. Solid Free-form Fabrication Technology and Its Application to Bone Tissue Engineering. Int. J. Stem Cells 2010, 3, 85–95. [Google Scholar] [CrossRef]
- Steffens, D.; Rezende, R.A.; Santi, B.; Pereira, F.D.A.d.S.; Neto, P.I.; da Silva, J.V.L.; Pranke, P. 3D-Printed PCL Scaffolds for the Cultivation of Mesenchymal Stem Cells. J. Appl. Biomater. Funct. Mater. 2016, 14, 19–25. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Safinia, L.; Datan, N.; Höhse, M.; Mantalaris, A.; Bismarck, A. Towards a methodology for the effective surface modification of porous polymer scaffolds. Biomaterials 2005, 26, 7537–7547. [Google Scholar] [CrossRef]
- Lee, J.; Abdeen, A.A.; Zhang, D.; Kilian, K.A. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials 2013, 34, 8140–8148. [Google Scholar] [CrossRef]
- Wang, W.; Deng, D.; Li, J.; Liu, W. Elongated cell morphology and uniaxial mechanical stretch contribute to physical attributes of niche environment for MSC tenogenic differentiation. Cell Biol. Int. 2013, 37, 755–760. [Google Scholar] [CrossRef]
- Burk, J.; Plenge, A.; Brehm, W.; Heller, S.; Pfeiffer, B.; Kasper, C. Induction of Tenogenic Differentiation Mediated by Extracellular Tendon Matrix and Short-Term Cyclic Stretching. Stem Cells Int. 2016, 2016, 7342379. [Google Scholar] [CrossRef]
- Delakowski, A.J.; Posselt, J.D.; Wagner, C.T. Modular Bioreactor Design for Directed Tendon/Ligament Tissue Engineering. Bioengineering 2022, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.S.; Su, Y.-P.; Begun, D.L.; Miller, J.D.; Alford, A.I.; Goldstein, S.A. The effects of axial displacement on fracture callus morphology and MSC homing depend on the timing of application. Bone 2010, 47, 41–48. [Google Scholar] [CrossRef]
- Karimi, E.; Vahedi, N.; Sarbandi, R.R.; Parandakh, A.; Ganjoury, C.; Sigaroodi, F.; Najmoddin, N.; Tabatabaei, M.; Tafazzoli-Shadpour, M.; Ardeshirylajimi, A.; et al. Nanoscale vibration could promote tenogenic differentiation of umbilical cord mesenchymal stem cells. Vitr. Cell. Dev. Biol. Anim. 2023, 59, 401–409. [Google Scholar] [CrossRef]
- Nam, H.Y.; Pingguan-Murphy, B.; Abbas, A.A.; Merican, A.M.; Kamarul, T. Uniaxial Cyclic Tensile Stretching at 8% Strain Exclusively Promotes Tenogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cells Int. 2019, 2019, 9723025. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Suen, C.-W.; Zhang, J.-F.; Li, G. Current concepts on tenogenic differentiation and clinical applications. J. Orthop. Transl. 2017, 9, 28–42. [Google Scholar] [CrossRef]
- Subramanian, G.; Stasuk, A.; Elsaadany, M.; Yildirim-Ayan, E. Effect of Uniaxial Tensile Cyclic Loading Regimes on Matrix Organization and Tenogenic Differentiation of Adipose-Derived Stem Cells Encapsulated within 3D Collagen Scaffolds. Stem Cells Int. 2017, 2017, 6072406. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, S.K.; Kwon, Y.-W.; Chung, S.G.; Kim, S. Pioglitazone-Primed Mesenchymal Stem Cells Stimulate Cell Proliferation, Collagen Synthesis and Matrix Gene Expression in Tenocytes. Int. J. Mol. Sci. 2019, 20, 472. [Google Scholar] [CrossRef] [PubMed]
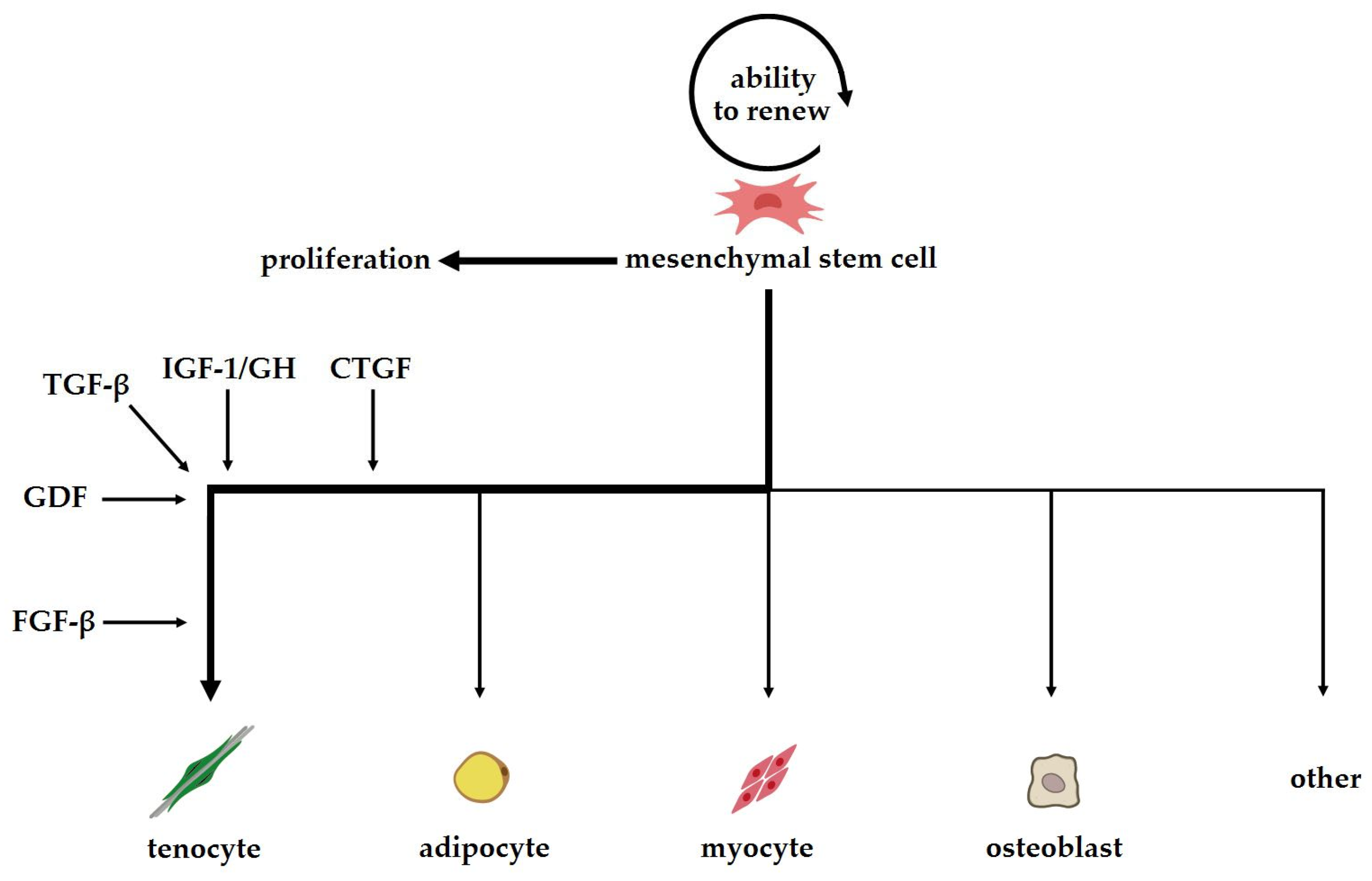
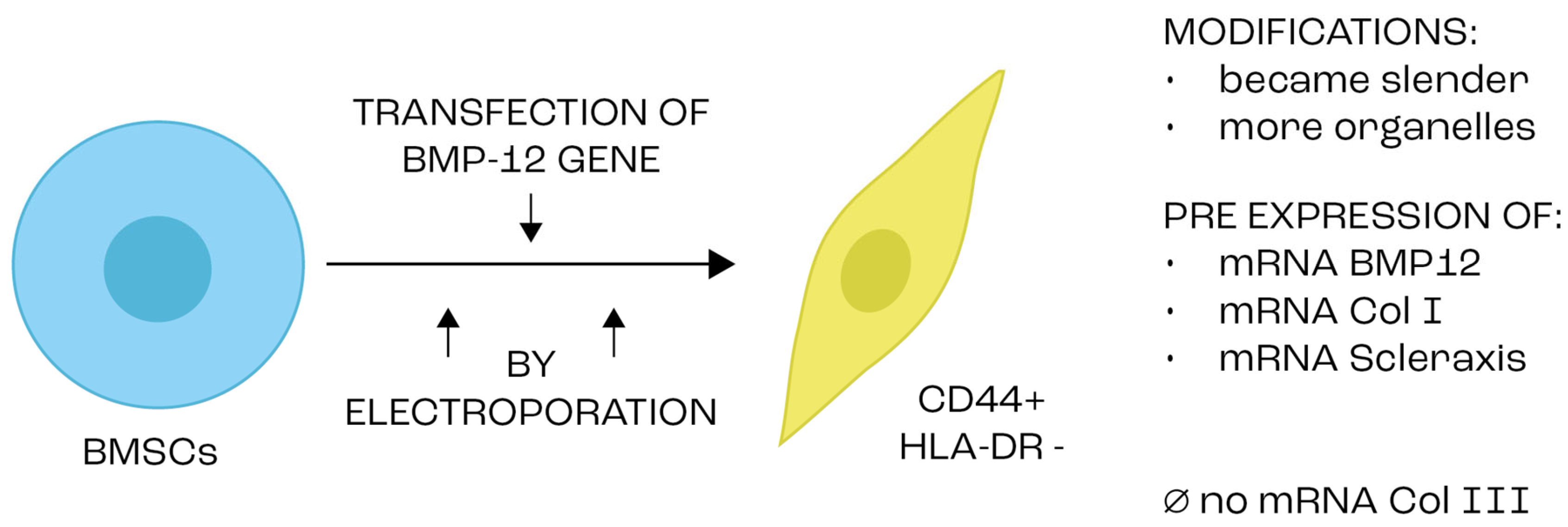
| Type of Stem Cells | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Tendon progenitor stem cells (TSPCs) | Differentiation into tissue similar in structure to tendons | Need to obtain a large volume of source material to obtain an adequate number of cells | [24,25,26,27] |
| Adipose tissue-derived stem cells (ASCs) | Easy to obtain through liposuction | Tendency to undergo adipogenesis Fat deposition during the process of tendon regeneration | [28,29,30,31,32] |
| Bone marrow-derived mesenchymal stem cells (BMSCs) | Easy to obtain | Occurrence of ossification in tendons | [33,34,35,36,37,38,39] |
| Embryonic stem cells (ESCs) | Ability to differentiate into all cell lines | Ethical concerns due to the way they are obtained Risk of neoplastic transformation | [40] |
| Induced pluripotent stem cells (iPSCs) | Ability to differentiate into all cell lines | Risk of neoplastic transformation | [42,43] |
| Category | Factors | Effect on the Cell/Tissue and Mode of Action | Refs. |
|---|---|---|---|
| Growth factors | Transforming growth factor beta (TGF-β) | Participates in inflammatory reactions, angiogenesis, collagen synthesis and fibrosis or excessive scarring Activates the Smad2/3 intracellular pathway | [51,52,53] |
| Connective tissue growth factor (CTGF) | Activates focal adhesion kinase (FAK)/extracellular signal-regulated kinase 1/2 (ERK1/2) pathway, promotes proliferation of damaged cells and stimulaties expression of tendon markers such as Scx and Tnmd | [28,81,82,83] | |
| Fibroblast growth factors (FGFs) | Activates the FGF/ERK/mitogen-activated protein kinase (MAPK) pathway affectes cell migration, proliferation and differentiation | [88] | |
| Growth differentiation factors (GDFs) | Participates in the development of the musculoskeletal system inhibits fibrosis | [73,74,80] | |
| Insulin-like growth factor 1 (IGF-1) | Activates the phosphoinositide 3-kinase (PI3K)/Akt, Ras-MAPK and phospholipase C (PLC) kinase pathways | [103,104,105] | |
| Proteins | Yes-associated protein (YAP) | Increases the production tendon-related proteins such as tenomodulin and tenascin C | [132] |
| Wnt ligands | Wnt ligands | Mediate tendon differentiation of mesenchymal stem cells induced by mechanical stimulation | [47,135] |
| Genetic modification | microRNA-135a (miR-135a) | Inhibits ROCK1 gene expression and stimulates tendon differentiation | [142] |
| RNA | Long non-coding RNA (lncRNA) H19 | Activates TGF-β1 signalling pathway Stimulates tendon differentiation and tendon repair. | [147] |
| 3D biomatrix | 3D biomatrix | Promotes cell proliferation and extracellular matrix remodelling | [148] |
| Drugs | Pioglitazone | Simulates peroxisome proliferator-activated receptor gamma (PPARγ), vascular endothelial growth factor (VEGF) and collagen secretion | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dec, P.; Żyłka, M.; Burszewski, P.; Modrzejewski, A.; Pawlik, A. Recent Advances in the Use of Stem Cells in Tissue Engineering and Adjunct Therapies for Tendon Reconstruction and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 4498. https://doi.org/10.3390/ijms25084498
Dec P, Żyłka M, Burszewski P, Modrzejewski A, Pawlik A. Recent Advances in the Use of Stem Cells in Tissue Engineering and Adjunct Therapies for Tendon Reconstruction and Future Perspectives. International Journal of Molecular Sciences. 2024; 25(8):4498. https://doi.org/10.3390/ijms25084498
Chicago/Turabian StyleDec, Paweł, Małgorzata Żyłka, Piotr Burszewski, Andrzej Modrzejewski, and Andrzej Pawlik. 2024. "Recent Advances in the Use of Stem Cells in Tissue Engineering and Adjunct Therapies for Tendon Reconstruction and Future Perspectives" International Journal of Molecular Sciences 25, no. 8: 4498. https://doi.org/10.3390/ijms25084498
APA StyleDec, P., Żyłka, M., Burszewski, P., Modrzejewski, A., & Pawlik, A. (2024). Recent Advances in the Use of Stem Cells in Tissue Engineering and Adjunct Therapies for Tendon Reconstruction and Future Perspectives. International Journal of Molecular Sciences, 25(8), 4498. https://doi.org/10.3390/ijms25084498





