Leukocytes within Autologous Blood Concentrates Have No Impact on the Growth and Proliferation of Human Primary Osteoblasts: An In Vitro Study
Abstract
:1. Introduction
2. Results
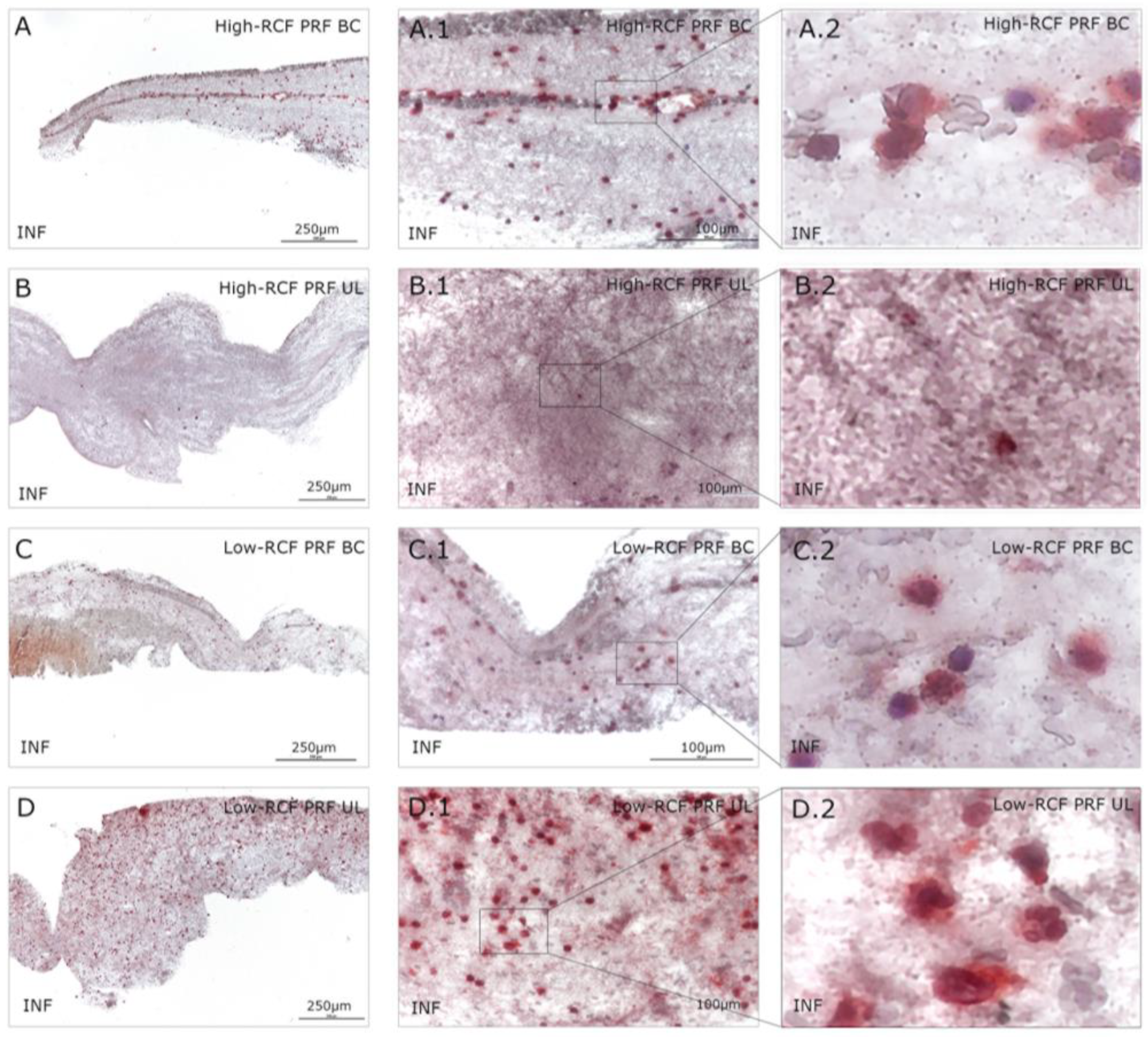
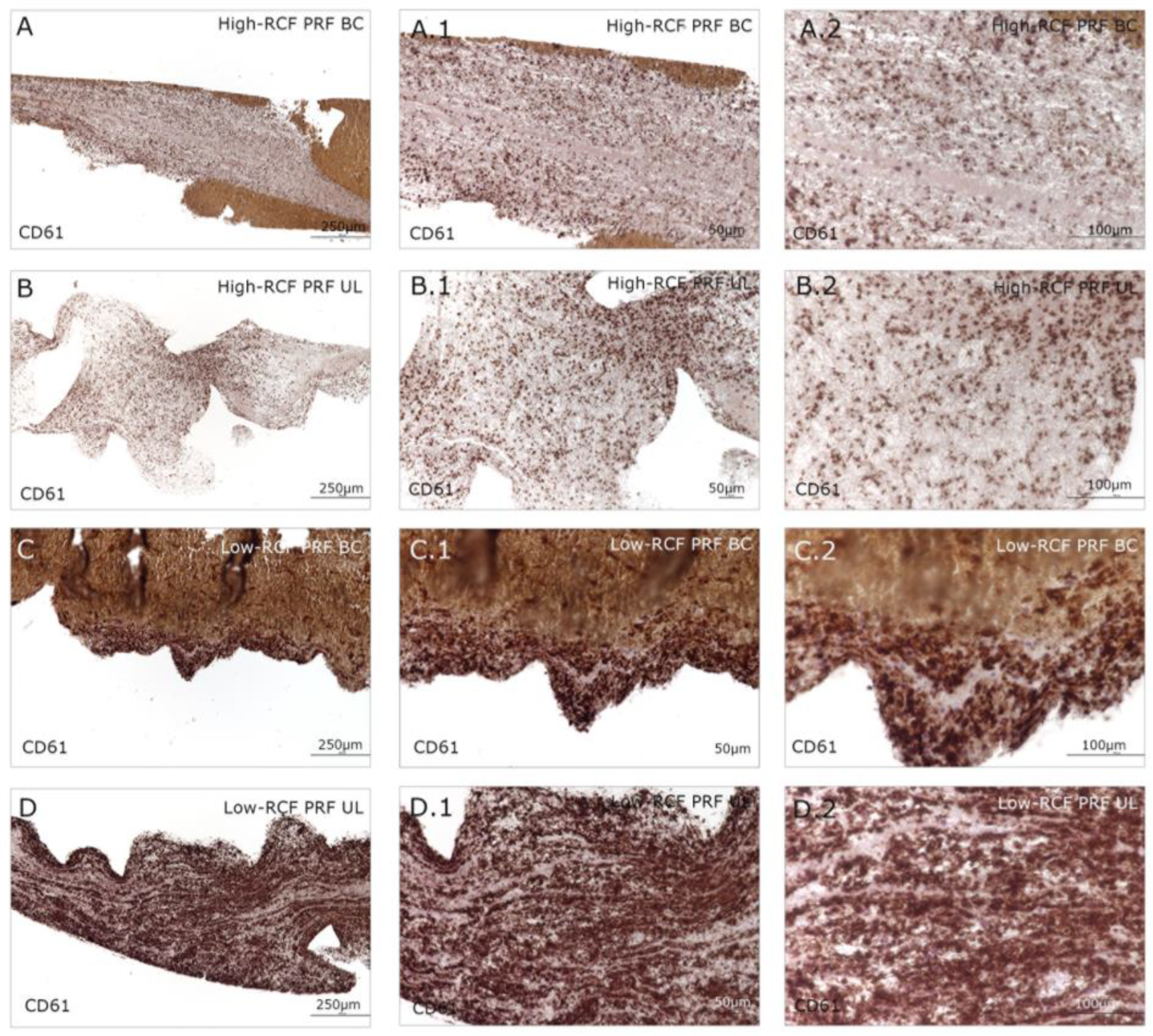
2.1. Cellular Characterization of Different Fractions of High- and Low-RCF PRF
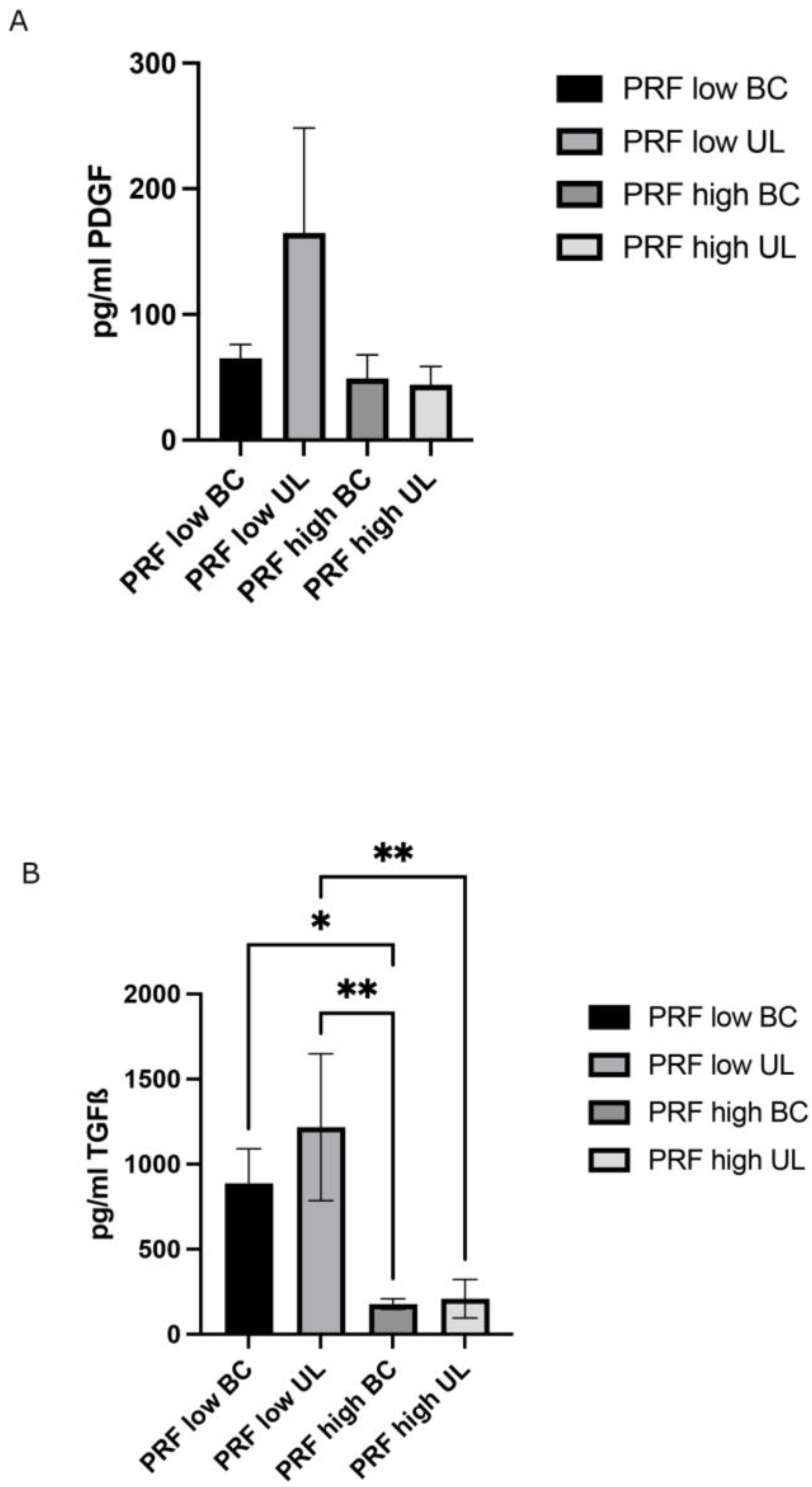
2.2. Determination of Growth Factor Release of Different PRF Fractions/Layers
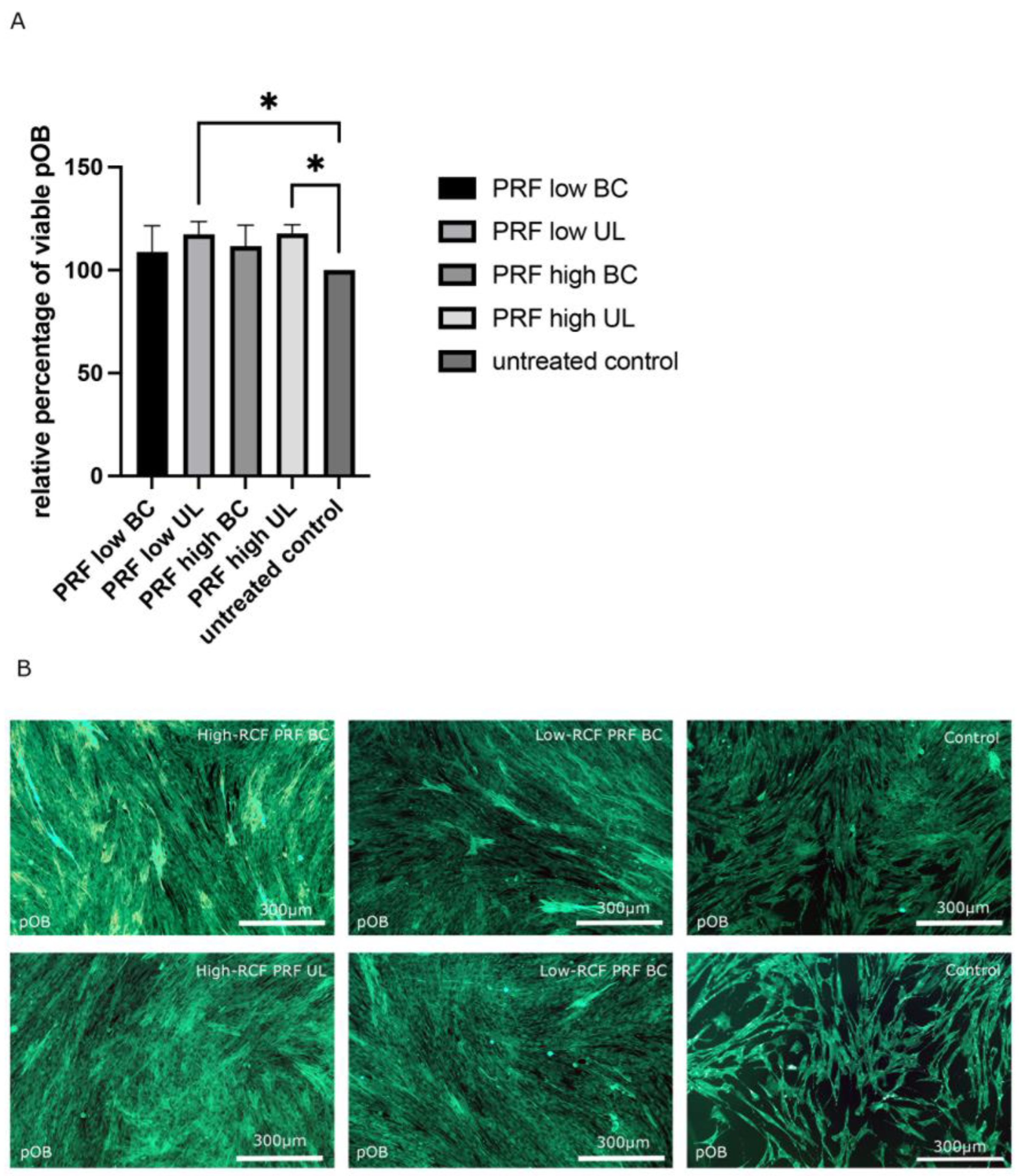
2.3. Comparative Bioactivity Analysis of Different PRF Fractions on pOBs
3. Discussion
4. Materials and Methods
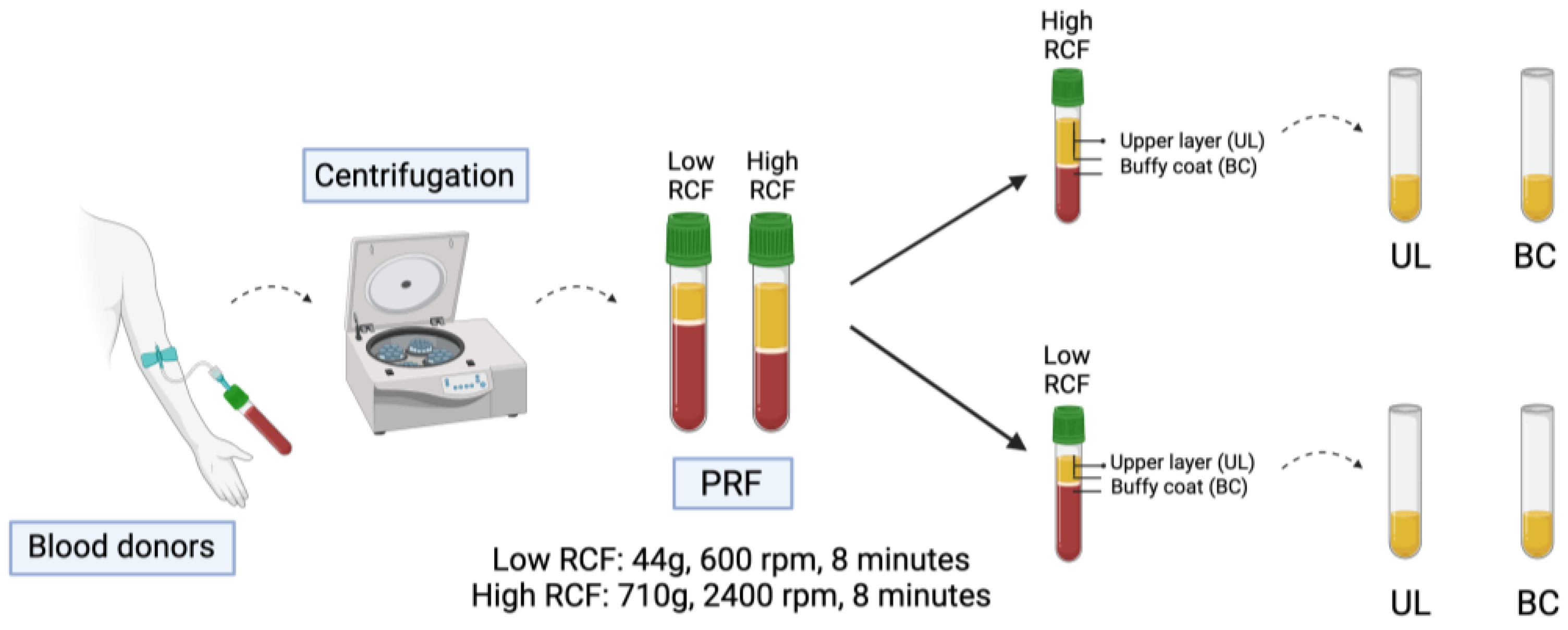
4.1. Preparation and Separation of the Blood Components
4.2. Isolation of Human Primary Osteoblasts (pOBs)
4.3. TranswellTM Experiments and Indirect PRF Application
4.4. Cell Viability Assay (MTS)
4.5. Paraffin Sectioning
4.6. Histology and Immunohistochemistry
4.7. Immunofluorescence Staining
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damsaz, M.; Castagnoli, C.Z.; Eshghpour, M.; Alamdari, D.H.; Alamdari, A.H.; Noujeim, Z.E.F.; Haidar, Z.S. Evidence-Based Clinical Efficacy of Leukocyte and Platelet-Rich Fibrin in Maxillary Sinus Floor Lift, Graft and Surgical Augmentation Procedures. Front. Surg. 2020, 7, 537138. [Google Scholar] [CrossRef] [PubMed]
- Malcangi, G.; Patano, A.; Palmieri, G.; Di Pede, C.; Latini, G.; Inchingolo, A.D.; Hazballa, D.; de Ruvo, E.; Garofoli, G.; Inchingolo, F.; et al. Maxillary Sinus Augmentation Using Autologous Platelet Concentrates (Platelet-Rich Plasma, Platelet-Rich Fibrin, and Concentrated Growth Factor) Combined with Bone Graft: A Systematic Review. Cells 2023, 12, 1797. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Siawasch, S.; Temmerman, A.; Cortellini, S.; Dhondt, R.; Teughels, W.; Castro, A.B. Do autologous platelet concentrates (APCs) have a role in intra-oral bone regeneration? A critical review of clinical guidelines on decision-making process. Periodontology 2000 2023, 93, 254–269. [Google Scholar] [CrossRef]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Anitua, E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int. J. Oral Maxillofac. Implant. 1999, 14, 529–535. [Google Scholar]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e37–e44. [Google Scholar] [CrossRef]
- He, L.; Lin, Y.; Hu, X.; Zhang, Y.; Wu, H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 707–713. [Google Scholar] [CrossRef]
- Nemeth, A.; Gurgel, B.V.; Lowenstein, A.; Juliasse, L.; Siroma, R.S.; Zhu, Z.; Awad Shibli, J.; Mourão, C.F. Does Liquid/Injectable Platelet-Rich Fibrin Help in the Arthrocentesis Treatment of Temporomandibular Joint Disorder Compared to Other Infusion Options? A Systematic Review of Randomized Clinical Trials. Bioengineering 2024, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e45–e50. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e51–e55. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e56–e60. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Perez, K.; Dym, H. Clinical Uses of Platelet-Rich Fibrin in Oral and Maxillofacial Surgery. Dent. Clin. N. Am. 2020, 64, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wan, Y.; Lin, Y.; Jiang, H. Platelet-rich fibrin and concentrated growth factors as novel platelet concentrates for chronic hard-to-heal skin ulcers: A systematic review and Meta-analysis of randomized controlled trials. J. Dermatol. Treat. 2022, 33, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Vangansewinkel, T.; Gervois, P.; Merckx, G.; Hilkens, P.; Quirynen, M.; Lambrichts, I.; Bronckaers, A. Angiogenic Properties of L’eukocyte- and Platelet-Rich Fibrin’. Sci. Rep. 2018, 8, 14632. [Google Scholar] [CrossRef]
- Cabaro, S.; D’Esposito, V.; Gasparro, R.; Borriello, F.; Granata, F.; Mosca, G.; Passaretti, F.; Sammartino, J.C.; Beguinot, F.; Sammartino, G.; et al. White cell and platelet content affects the release of bioactive factors in different blood-derived scaffolds. Platelets 2018, 29, 463–467. [Google Scholar] [CrossRef]
- Moraschini, V.; Miron, R.J.; Mourão, C.; Louro, R.S.; Sculean, A.; da Fonseca, L.A.M.; Calasans Maia, M.D.; Shibli, J.A. Antimicrobial effect of platelet-rich fibrin: A systematic review of in vitro evidence-based studies. Periodontology 2000 2023. [Google Scholar] [CrossRef]
- Choukroun, J.; Ghanaati, S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: The first introduction to the low speed centrifugation concept. Eur. J. Trauma Emerg. Surg. Off. Publ. Eur. Trauma Soc. 2018, 44, 87–95. [Google Scholar] [CrossRef]
- Dos Santos, R.F.; Araújo Peres, J.A.; Queiroz, M.S. Advances in separation methods for the use of platelet-rich fibrin in tissue repair: An integrative review. Gen. Dent. 2023, 71, 65–69. [Google Scholar] [PubMed]
- Dos Santos, R.G.; Santos, G.S.; Alkass, N.; Chiesa, T.L.; Azzini, G.O.; da Fonseca, L.F.; Dos Santos, A.F.; Rodrigues, B.L.; Mosaner, T.; Lana, J.F. The regenerative mechanisms of platelet-rich plasma: A review. Cytokine 2021, 144, 155560. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, F.; Luo, R.; Duan, X. Platelet-Rich Plasma for Bone Fracture Treatment: A Systematic Review of Current Evidence in Preclinical and Clinical Studies. Front. Med. 2021, 8, 676033. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Kantarci, A.; Deady, J.; Hasturk, H.; Liu, H.; Alshahat, M.; Van Dyke, T.E. Platelet-rich plasma: Growth factors and pro- and anti-inflammatory properties. J. Periodontol. 2007, 78, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.; Wragg, N.M.; Shariatzadeh, M.; Wilson, S.L. The Use of Platelet-Rich Plasma (PRP) for the Management of Non-union Fractures. Curr. Osteoporos. Rep. 2021, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Qi, X.; Zhang, Y.; Sheng, J.; Xu, Z.; Tao, S.; Xie, X.; Li, X.; Zhang, C. Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in promoting repair of bone defects. J. Transl. Med. 2016, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, J.; Wu, J.; Du, X.; Jing, W.; Liu, L. Roles of PRF and IGF-1 in promoting alveolar osteoblast growth and proliferation and molecular mechanism. Int. J. Clin. Exp. Pathol. 2018, 11, 3294–3301. [Google Scholar] [PubMed]
- Al-Maawi, S.; Dohle, E.; Kretschmer, W.; Rutkowski, J.; Sader, R.; Ghanaati, S. A Standardized g-Force Allows the Preparation of Similar Platelet-Rich Fibrin Qualities Regardless of Rotor Angle. Tissue Eng. Part A 2022, 28, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Rauch, I.; Müller, M.; Decker, T. The regulation of inflammation by interferons and their STATs. Jakstat 2013, 2, e23820. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Saxena, Y.; Routh, S.; Mukhopadhaya, A. Immunoporosis: Role of Innate Immune Cells in Osteoporosis. Front. Immunol. 2021, 12, 687037. [Google Scholar] [CrossRef] [PubMed]
- Baca-Gonzalez, L.; Serrano Zamora, R.; Rancan, L.; González Fernández-Tresguerres, F.; Fernández-Tresguerres, I.; López-Pintor, R.M.; López-Quiles, J.; Leco, I.; Torres, J. Plasma rich in growth factors (PRGF) and leukocyte-platelet rich fibrin (L-PRF): Comparative release of growth factors and biological effect on osteoblasts. Int. J. Implant. Dent. 2022, 8, 39. [Google Scholar] [CrossRef]
- Castro, A.B.; Cortellini, S.; Temmerman, A.; Li, X.; Pinto, N.; Teughels, W.; Quirynen, M. Characterization of the Leukocyte- and Platelet-Rich Fibrin Block: Release of Growth Factors, Cellular Content, and Structure. Int. J. Oral Maxillofac. Implant. 2019, 34, 855–864. [Google Scholar] [CrossRef]
- Ashour, S.H.; Mudalal, M.; Al-Aroomi, O.A.; Al-Attab, R.; Li, W.; Yin, L. The Effects of Injectable Platelet-Rich Fibrin and Advanced-Platelet Rich Fibrin on Gingival Fibroblast Cell Vitality, Proliferation, Differentiation. Tissue Eng. Regen. Med. 2023, 20, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Santhanakrishnan, M. Mechanical, chemical, structural analysis and comparative release of PDGF-AA from L-PRF, A-PRF and T-PRF—An in vitro study. Biomater. Res. 2020, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Katagiri, H.; Kono, M.; Schaller, B.; Zhang, Y.; Sculean, A.; Miron, R.J. Improved growth factor delivery and cellular activity using concentrated platelet-rich fibrin (C-PRF) when compared with traditional injectable (i-PRF) protocols. Clin. Oral Investig. 2020, 24, 4373–4383. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, T.; Zeng, H.; Qiu, Y.; Wei, Y.; Cheng, Y.; Wang, Y.; Zhang, X.; Ke, J.; Miron, R.; et al. Liquid platelet-rich fibrin produced via horizontal centrifugation decreases the inflammatory response and promotes chondrocyte regeneration in vitro. Front. Bioeng. Biotechnol. 2023, 11, 1301430. [Google Scholar] [CrossRef] [PubMed]
- Steller, D.; Scheibert, A.; Sturmheit, T.; Rose, D.; Hakim, S.G. Impact of PRP and PRF on Viability of Zoledronic Acid Treated Osteoblasts in 2D and 3D Cell Culture. Anticancer Res. 2022, 42, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Steller, D.; Herbst, N.; Pries, R.; Juhl, D.; Hakim, S.G. Positive impact of Platelet-rich plasma and Platelet-rich fibrin on viability, migration and proliferation of osteoblasts and fibroblasts treated with zoledronic acid. Sci. Rep. 2019, 9, 8310. [Google Scholar] [CrossRef] [PubMed]
- You, J.S.; Kim, S.G.; Oh, J.S.; Kim, J.S. Effects of Platelet-Derived Material (Platelet-Rich Fibrin) on Bone Regeneration. Implant Dent. 2019, 28, 244–255. [Google Scholar] [CrossRef]
- Yu, S.; Bd, Y.T.; Bd, Y.W.; Bd, M.F.; SL, B.M.; GT, B.M.; ZY, B.M.; Miron, R.J.; Zhang, Y.; Yang, Z.; et al. Early tissue and healing responses after maxillary sinus augmentation using horizontal platelet rich fibrin bone blocks. BMC Oral Health 2023, 23, 589. [Google Scholar] [CrossRef]
- Vahabi, S.; Yadegary, Z.; Karamshahi, M. Evaluating the adhesion of human gingival fibroblasts and MG-63 osteoblast-like cells to activated PRP-coated membranes. Cell Tissue Bank 2019, 20, 339–349. [Google Scholar] [CrossRef]
- de Almeida Barros Mourão, C.F.; Lourenço, E.S.; Nascimento, J.R.B.; Machado, R.C.M.; Rossi, A.M.; Leite, P.E.C.; Granjeiro, J.M.; Alves, G.G.; Calasans-Maia, M.D. Does the association of blood-derived growth factors to nanostructured carbonated hydroxyapatite contributes to the maxillary sinus floor elevation? A randomized clinical trial. Clin. Oral Investig. 2019, 23, 369–379. [Google Scholar] [CrossRef]
- Kang, F.; Yi, Q.; Gu, P.; Dong, Y.; Zhang, Z.; Zhang, L.; Bai, Y. Controlled growth factor delivery system with osteogenic-angiogenic coupling effect for bone regeneration. J. Orthop. Translat. 2021, 31, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Gheno, E.; Alves, G.G.; Ghiretti, R.; Mello-Machado, R.C.; Signore, A.; Lourenço, E.S.; Leite, P.E.C.; Mourão, C.; Sohn, D.S.; Calasans-Maia, M.D. “Sticky Bone” Preparation Device: A Pilot Study on the Release of Cytokines and Growth Factors. Materials 2022, 15, 1474. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, Z.; Mitev, V.; Stanimirov, P.; Isaeva, A.; Gateva, N.; Ishkitiev, N. Use of platelet concentrates in oral and maxillofacial surgery: An overview. Acta Odontol. Scand. 2017, 75, 1–11. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourão, C.F.; Dohle, E.; Bayrak, B.; Winter, A.; Sader, R.; Ghanaati, S. Leukocytes within Autologous Blood Concentrates Have No Impact on the Growth and Proliferation of Human Primary Osteoblasts: An In Vitro Study. Int. J. Mol. Sci. 2024, 25, 4542. https://doi.org/10.3390/ijms25084542
Mourão CF, Dohle E, Bayrak B, Winter A, Sader R, Ghanaati S. Leukocytes within Autologous Blood Concentrates Have No Impact on the Growth and Proliferation of Human Primary Osteoblasts: An In Vitro Study. International Journal of Molecular Sciences. 2024; 25(8):4542. https://doi.org/10.3390/ijms25084542
Chicago/Turabian StyleMourão, Carlos Fernando, Eva Dohle, Büşra Bayrak, Anne Winter, Robert Sader, and Shahram Ghanaati. 2024. "Leukocytes within Autologous Blood Concentrates Have No Impact on the Growth and Proliferation of Human Primary Osteoblasts: An In Vitro Study" International Journal of Molecular Sciences 25, no. 8: 4542. https://doi.org/10.3390/ijms25084542





