Syndecans, Exostosins and Sulfotransferases as Potential Synovial Inflammation Moderators in Patients with Hip Osteoarthritis
Abstract
:1. Introduction
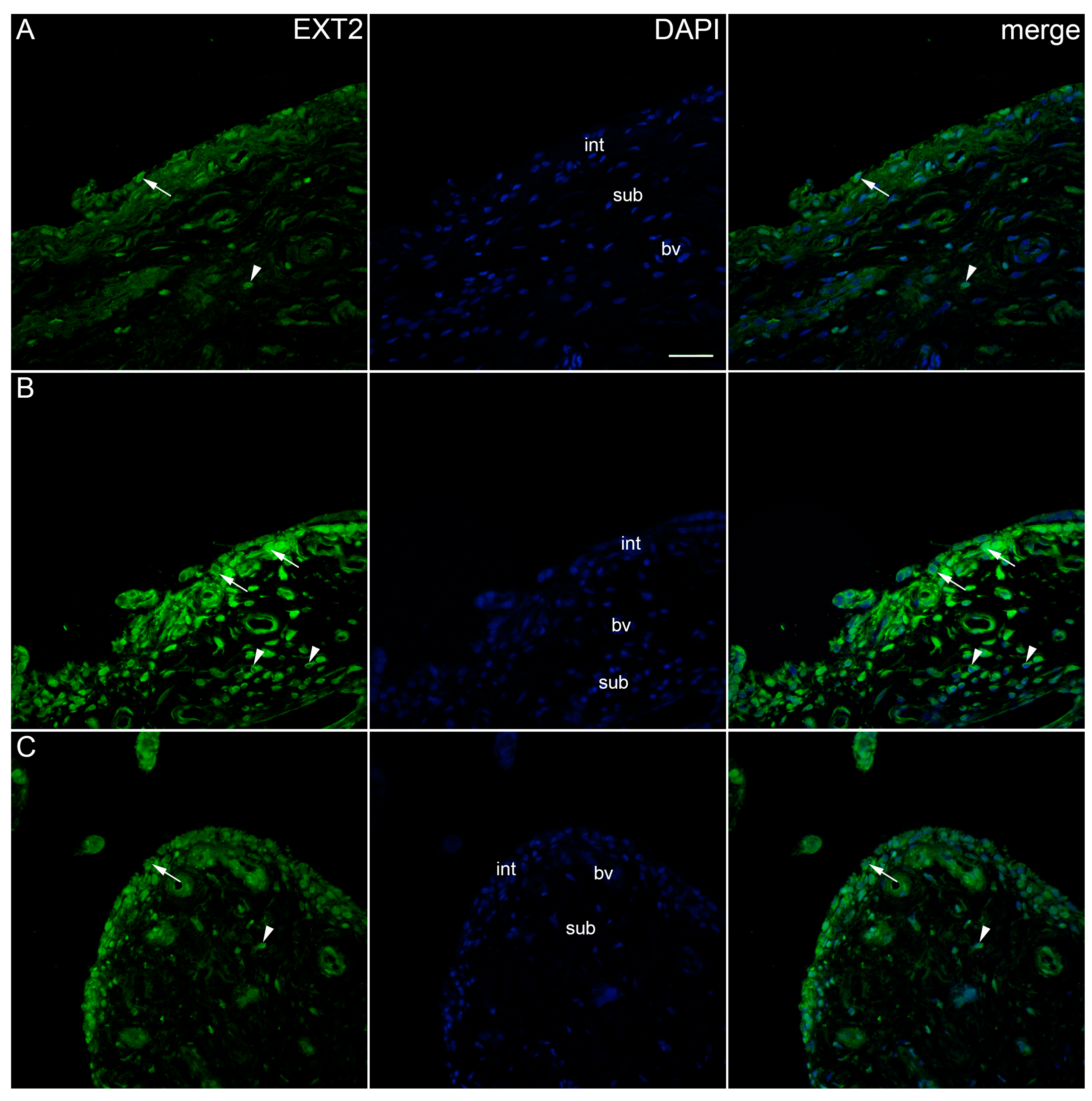
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Tissue Collection and Basic Staining Procedures
4.3. Immunofluorescence Staining
4.4. Data Acquisition and Quantitative Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and Burden of Osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Courties, A.; Berenbaum, F. Is hip osteoarthritis preventable? Jt. Bone Spine 2020, 87, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.H.; Rat, A.C.; Achit, H.; Ngueyon-Sime, W.; Gard, C.; Guillemin, F.; Jolly, D.; Fautrel, B. Health resource use and costs of symptomatic knee and/or hip osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1011–1017. [Google Scholar] [CrossRef]
- Vina, E.R.; Kwoh, C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 21, e508–e522. [Google Scholar]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The Global Burden of Hip and Knee Osteoarthritis: Estimates from the Global Burden of Disease 2010 Study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for Standardized Definitions of Osteoarthritis and Risk Stratification for Clinical Trials and Clinical Use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, S.; Roman-Blas, J.A.; Largo, R.; Herrero-Beaumont, G. Osteoarthritis: A Progressive Disease with Changing Phenotypes. Rheumatology 2014, 53, 1–3. [Google Scholar] [CrossRef]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of Criteria for the Classification and Reporting of Osteoarthritis: Classification of Osteoarthritis of the Knee. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, M. Synovial inflammation in osteoarthritis. A treatable target? Semin. Arthritis Rheum. 2024, 64, 152326. [Google Scholar] [CrossRef] [PubMed]
- Deligne, C.; Casulli, S.; Pigenet, A.; Bougault, C.; Campillo-Gimenez, L.; Nourissat, G.; Berenbaum, F.; Elbim, C.; Houard, X. Differential Expression of Interleukin-17 and Interleukin-22 in Inflamed and Non-Inflamed Synovium from Osteoarthritis Patients. Osteoarthr. Cartil. 2015, 23, 1843–1852. [Google Scholar] [CrossRef]
- Pessler, F.; Dai, L.; Diaz-Torne, C.; Gomez-Vaquero, C.; Paessler, M.E.; Zheng, D.-H.; Einhorn, E.; Range, U.; Scanzello, C.; Schumacher, H.R. The Synovitis of “Non-Inflammatory” Orthopaedic Arthropathies: A Quantitative Histological and Immunohistochemical Analysis. Ann. Rheum. Dis. 2008, 67, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Berenbaum, F. The Role of Synovitis in Pathophysiology and Clinical Symptoms of Osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, V.; Stellavato, A.; Russo, R.; Cimini, D.; Valletta, M.; Alfano, A.; Pedone, P.V.; Chambery, A.; Schiraldi, C. Molecular Fingerprint of Human Pathological Synoviocytes in Response to Extractive Sulfated and Biofermentative Unsulfated Chondroitins. Int. J. Mol. Sci. 2022, 23, 15865. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-Grade Inflammation as a Key Mediator of the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Oh, E.-S.; Couchman, J.R. Syndecans-2 and -4; Close Cousins, but Not Identical Twins. Mol. Cells 2004, 17, 181–187. [Google Scholar] [CrossRef]
- Pap, T.; Bertrand, J. Syndecans in Cartilage Breakdown and Synovial Inflammation. Nat. Rev. Rheumatol. 2013, 9, 43–55. [Google Scholar] [CrossRef]
- Xian, X.; Gopal, S.; Couchman, J.R. Syndecans as Receptors and Organizers of the Extracellular Matrix. Cell Tissue Res. 2010, 339, 31–46. [Google Scholar] [CrossRef]
- De Rossi, G.; Whiteford, J.R. Syndecans in Angiogenesis and Endothelial Cell Biology. Biochem. Soc. Trans. 2014, 42, 1643–1646. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, A.N.; Multhaupt, H.A.B.; Couchman, J.R. Syndecans in Wound Healing, Inflammation and Vascular Biology. Int. J. Biochem. Cell Biol. 2007, 39, 505–528. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S. Syndecans in Inflammation at a Glance. Front. Immunol. 2020, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.V.; Fitzgerald, M.L.; Bernfield, M. Regulated Shedding of Syndecan-1 and -4 Ectodomains by Thrombin and Growth Factor Receptor Activation. J. Biol. Chem. 1997, 272, 14713–14720. [Google Scholar] [CrossRef] [PubMed]
- Manon-Jensen, T.; Multhaupt, H.A.B.; Couchman, J.R. Mapping of Matrix Metalloproteinase Cleavage Sites on Syndecan-1 and Syndecan-4 Ectodomains. FEBS J. 2013, 280, 2320–2331. [Google Scholar] [CrossRef] [PubMed]
- Echtermeyer, F.; Bertrand, J.; Dreier, R.; Meinecke, I.; Neugebauer, K.; Fuerst, M.; Lee, Y.J.; Song, Y.W.; Herzog, C.; Theilmeier, G.; et al. Syndecan-4 Regulates ADAMTS-5 Activation and Cartilage Breakdown in Osteoarthritis. Nat. Med. 2009, 15, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Barre, P.-E.; Redini, F.; Boumediene, K.; Vielpeau, C.; Pujol, J.-P. Semiquantitative Reverse Transcription-Polymerase Chain Reaction Analysis of Syndecan-1 and -4 Messages in Cartilage and Cultured Chondrocytes from Osteoarthritic Joints. Osteoarthr. Cartil. 2000, 8, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, M.; Pinno, K.; Ehnold, L.I.; Märtens, N.; Märtson, A.; Pap, T.; Stärke, C.; Lohmann, C.H.; Bertrand, J. MMP-9 Mediated Syndecan-4 Shedding Correlates with Osteoarthritis Severity. Osteoarthr. Cartil. 2021, 29, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.M.; Cartwright, A.; David, G.; Fitzgerald, O.; Bresnihan, B.; Ashton, B.A.; Middleton, J. Differential Expression of Syndecans and Glypicans in Chronically Inflamed Synovium. Ann. Rheum. Dis. 2007, 67, 592–601. [Google Scholar] [CrossRef]
- Busse-Wicher, M.; Wicher, K.B.; Kusche-Gullberg, M. The Extostosin Family: Proteins with Many Functions. Matrix Biol. 2014, 35, 25–33. [Google Scholar] [CrossRef]
- Jennes, I.; Pedrini, E.; Zuntini, M.; Mordenti, M.; Balkassmi, S.; Asteggiano, C.G.; Casey, B.; Bakker, B.; Sangiorgi, L.; Wuyts, W. Multiple Osteochondromas: Mutation Update and Description of the Multiple Osteochondromas Mutation Database (MOdb). Hum. Mutat. 2009, 30, 1620–1627. [Google Scholar] [CrossRef]
- Carter, N.M.; Ali, S.; Kirby, J.A. Endothelial Inflammation: The Role of Differential Expression of N-Deacetylase/N-Sulphotransferase Enzymes in Alteration of the Immunological Properties of Heparan Sulphate. J. Cell Sci. 2003, 116, 3591–3600. [Google Scholar] [CrossRef]
- Krenn, V.; Morawietz, L.; Burmester, G.; Kinne, R.W.; Mueller-Ladner, U.; Muller, B.; Haupl, T. Synovitis Score: Discrimination between Chronic Low-grade and High-grade Synovitis. Histopathology 2006, 49, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ye, X.; Zhang, Z. Syndecan-4 Is Correlated with Disease Activity and Serological Characteristic of Rheumatoid Arthritis. Adv. Rheumatol. 2022, 62, 21. [Google Scholar] [CrossRef]
- Hunter, D.J.; Schofield, D.; Callander, E. The Individual and Socioeconomic Impact of Osteoarthritis. Nat. Rev. Rheumatol. 2014, 10, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Atukorala, I.; Kwoh, C.K.; Guermazi, A.; Roemer, F.W.; Boudreau, R.M.; Hannon, M.J.; Hunter, D.J. Synovitis in knee osteoarthritis: A precursor of disease? Ann. Rheum. Dis. 2016, 75, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Niu, J.; Neogi, T.; Goggins, J.; Nevitt, M.C.; Roemer, F.; Torner, J.; Lewis, C.E.; Guermazi, A. Synovitis and the risk of knee osteoarthritis: The MOST Study. Osteoarthr. Cartil. 2016, 24, 458–464. [Google Scholar] [CrossRef]
- Ishibashi, K.; Sasaki, E.; Ota, S.; Chiba, D.; Yamamoto, Y.; Tsuda, E.; Yoshikuni, S.; Ihara, K.; Ishibashi, Y. Detection of synovitis in early knee osteoarthritis by MRI and serum biomarkers in Japanese general population. Sci. Rep. 2020, 10, 12310. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nature reviews. Rheumatology 2015, 11, 35–44. [Google Scholar]
- Lopes de Jesus, C.C.; Dos Santos, F.C.; de Jesus, L.; Monteiro, I.; Sant’Ana, M.; Trevisani, V.F.M. Comparison between intra-articular ozone and placebo in the treatment of knee osteoarthritis: A randomized, double-blinded, placebo-controlled study. PLoS ONE 2017, 12, e0179185. [Google Scholar] [CrossRef]
- Coaccioli, S.; Sarzi-Puttini, P.; Zis, P.; Rinonapoli, G.; Varrassi, G. Osteoarthritis: New Insight on Its Pathophysiology. J. Clin. Med. 2022, 11, 6013. [Google Scholar] [CrossRef] [PubMed]
- De Roover, A.; Escribano-Nunez, A.; Monteagudo, S.; Lories, R. Fundamentals of osteoarthritis: Inflammatory mediators in osteoarthritis. Osteoarthr. Cartil. 2023, 31, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, T.; Winter, D.R.; Miller, R.J.; Miller, R.E.; Malfait, A.M. Neuroimmune Interactions and Osteoarthritis Pain: Focus on Macrophages. PR9 2021, 6, e892. [Google Scholar] [CrossRef]
- Dainese, P.; Mahieu, H.; De Mits, S.; Wittoek, R.; Stautemas, J.; Calders, P. Associations between markers of inflammation and altered pain perception mechanisms in people with knee osteoarthritis: A systematic review. RMD Open 2023, 9, e002945. [Google Scholar] [CrossRef]
- Wood, M.J.; Miller, R.E.; Malfait, A.M. The Genesis of Pain in Osteoarthritis: Inflammation as a Mediator of Osteoarthritis Pain. Clin. Geriatr. Med. 2022, 38, 221–238. [Google Scholar] [CrossRef]
- Knights, A.J.; Farrell, E.C.; Ellis, O.M.; Lammlin, L.; Junginger, L.M.; Rzeczycki, P.M.; Bergman, R.F.; Pervez, R.; Cruz, M.; Knight, E.; et al. Synovial fibroblasts assume distinct functional identities and secrete R-spondin 2 in osteoarthritis. Ann. Rheum. Dis. 2023, 82, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Presto, J.; Thuveson, M.; Carlsson, P.; Busse, M.; Wilen, M.; Eriksson, I.; Kusche-Gullberg, M.; Kjellen, L. Heparan sulfate biosynthesis enzymes EXT1 and EXT2 affect NDST1 expression and heparan sulfate sulfation. Proc. Natl. Acad. Sci. USA 2008, 105, 4751–4756. [Google Scholar] [CrossRef]
- Deligny, A.; Dierker, T.; Dagalv, A.; Lundequist, A.; Eriksson, I.; Nairn, A.V.; Moremen, K.W.; Merry, C.L.R.; Kjellen, L. NDST2 (N-Deacetylase/N-Sulfotransferase-2) Enzyme Regulates Heparan Sulfate Chain Length. J. Biol. Chem. 2016, 291, 18600–18607. [Google Scholar] [CrossRef]
- Najm, A.; le Goff, B.; Venet, G.; Garraud, T.; Amiaud, J.; Biha, N.; Charrier, C.; Touchais, S.; Crenn, V.; Blanchard, F.; et al. IMSYC immunologic synovitis score: A new score for synovial membrane characterization in inflammatory and non-inflammatory arthritis. Jt. Bone Spine 2019, 86, 77–81. [Google Scholar] [CrossRef]
- Schmidt, T.; Najm, A.; Mussawy, H.; Burghardt, R.; Oehler, N.; Krenn, V.; Ruther, W.; Niemeier, A. General synovitis score and immunologic synovitis score reflect clinical disease activity in patients with advanced stage rheumatoid arthritis. Sci. Rep. 2019, 9, 8448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ling, X.; Zhou, H.; Xie, J. Syndecan-4 is the key proteoglycan involved in mediating sepsis-associated lung injury. Heliyon 2023, 9, e18600. [Google Scholar] [CrossRef] [PubMed]
- Angsana, J.; Chen, J.; Smith, S.; Xiao, J.; Wen, J.; Liu, L.; Haller, C.A.; Chaikof, E.L. Syndecan-1 modulates the motility and resolution responses of macrophages. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.H.; Aquino, R.S.; Park, P.W. Molecular functions of syndecan-1 in disease. Matrix Biol. J. Int. Soc. Matrix Biol. 2012, 31, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Sebestyen, A.; Totth, A.; Mihalik, R.; Szakacs, O.; Paku, S.; Kopper, L. Syndecan-1-dependent homotypic cell adhesion in HT58 lymphoma cells. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2000, 21, 349–357. [Google Scholar]
- Duplancic, R.; Roguljic, M.; Puhar, I.; Vecek, N.; Dragun, R.; Vukojevic, K.; Saraga-Babic, M.; Kero, D. Syndecans and Enzymes for Heparan Sulfate Biosynthesis and Modification Differentially Correlate With Presence of Inflammatory Infiltrate in Periodontitis. Front. Physiol. 2019, 10, 1248. [Google Scholar] [CrossRef] [PubMed]
- Chanalaris, A.; Clarke, H.; Guimond, S.E.; Vincent, T.L.; Turnbull, J.E.; Troeberg, L. Heparan Sulfate Proteoglycan Synthesis Is Dysregulated in Human Osteoarthritic Cartilage. Am. J. Pathol. 2019, 189, 632–647. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Hasegawa, M.; Iino, T.; Imanaka-Yoshida, K.; Sudo, A. Role of Syndecan-4 in the Inhibition of Articular Cartilage Degeneration in Osteoarthritis. Biomedicines 2023, 11, 2257. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, G.; Ji, B.; Jin, H.; Naranmandakh, S.; Li, Y. Research Trends and Foci in Osteoarthritis Pain from 2012 to 2022: Bibliometric and Visualization Study. J. Pain Res. 2023, 16, 2567–2585. [Google Scholar] [CrossRef]
- Chou, C.H.; Jain, V.; Gibson, J.; Attarian, D.E.; Haraden, C.A.; Yohn, C.B.; Laberge, R.M.; Gregory, S.; Kraus, V.B. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci. Rep. 2020, 10, 10868. [Google Scholar] [CrossRef]
- Mussawy, H.; Zustin, J.; Luebke, A.M.; Strahl, A.; Krenn, V.; Ruther, W.; Rolvien, T. The histopathological synovitis score is influenced by biopsy location in patients with knee osteoarthritis. Arch. Orthop. Trauma Surg. 2022, 142, 2991–2997. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.; Bollmann, M. Soluble syndecans: Biomarkers for diseases and therapeutic options. Br. J. Pharmacol. 2019, 176, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Caric, D.; Zekic Tomas, S.; Filipovic, N.; Soljic, V.; Benzon, B.; Glumac, S.; Rakovac, I.; Vukojevic, K. Expression Pattern of iNOS, BCL-2 and MMP-9 in the Hip Synovium Tissue of Patients with Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1489. [Google Scholar] [CrossRef] [PubMed]
- Pavic, B.; Ogorevc, M.; Boric, K.; Vukovic, D.; Saraga-Babic, M.; Mardesic, S. Connexin 37, 40, 43 and Pannexin 1 Expression in the Gastric Mucosa of Patients with Systemic Sclerosis. Biomedicines 2023, 11, 2487. [Google Scholar] [CrossRef] [PubMed]
- Vrouwe, J.P.M.; Burggraaf, J.; Kloppenburg, M.; Stuurman, F.E. Challenges and opportunities of pharmacological interventions for osteoarthritis: A review of current clinical trials and developments. Osteoarthr. Cartil. Open 2021, 3, 100212. [Google Scholar] [CrossRef] [PubMed]
- Terkawi, M.A.; Matsumae, G.; Shimizu, T.; Takahashi, D.; Kadoya, K.; Iwasaki, N. Interplay between Inflammation and Pathological Bone Resorption: Insights into Recent Mechanisms and Pathways in Related Diseases for Future Perspectives. Int. J. Mol. Sci. 2022, 23, 1786. [Google Scholar] [CrossRef]
- Agere, S.A.; Kim, E.Y.; Akhtar, N.; Ahmed, S. Syndecans in chronic inflammatory and autoimmune diseases: Pathological insights and therapeutic opportunities. J. Cell Physiol. 2018, 233, 6346–6358. [Google Scholar] [CrossRef]
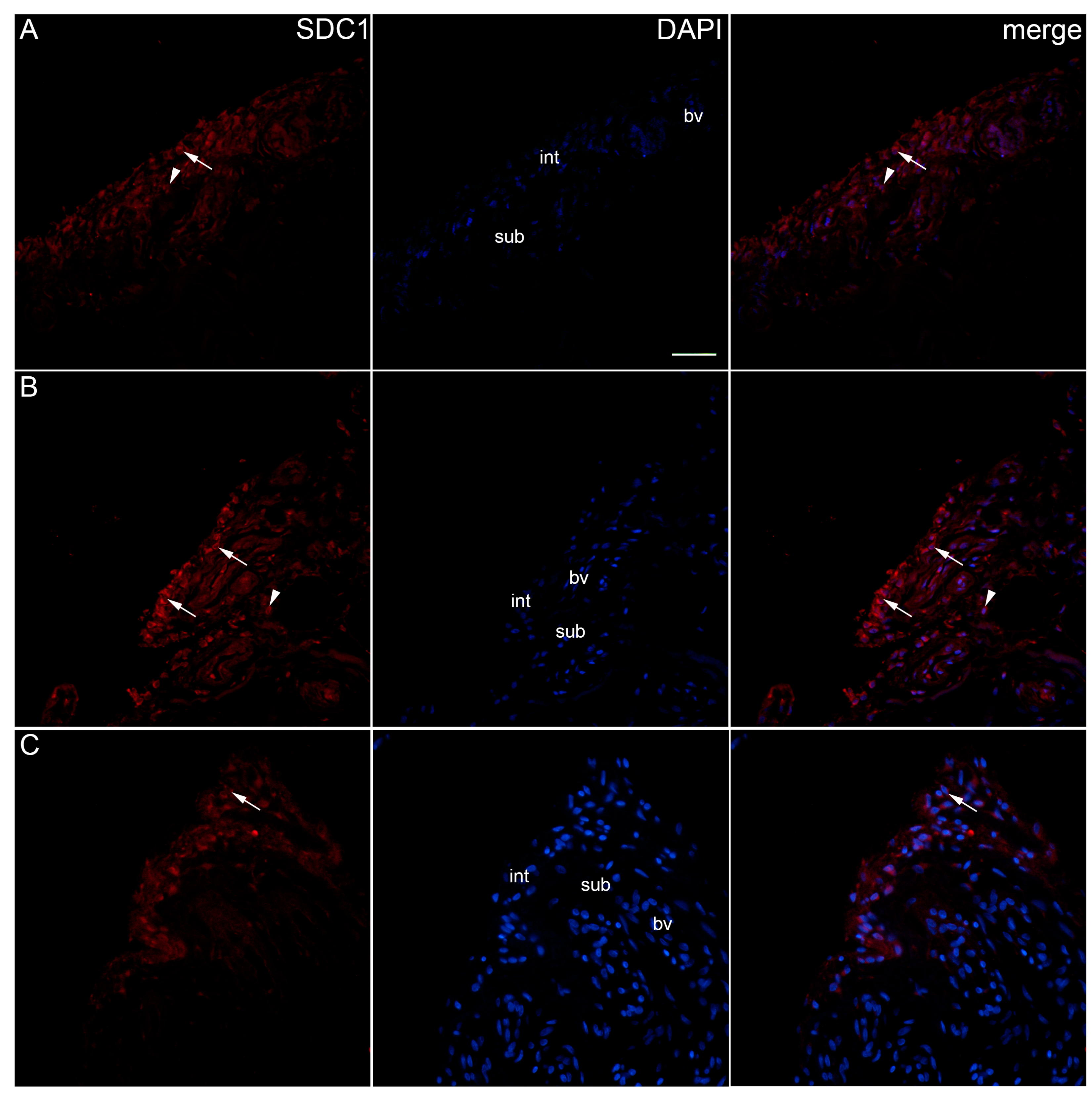


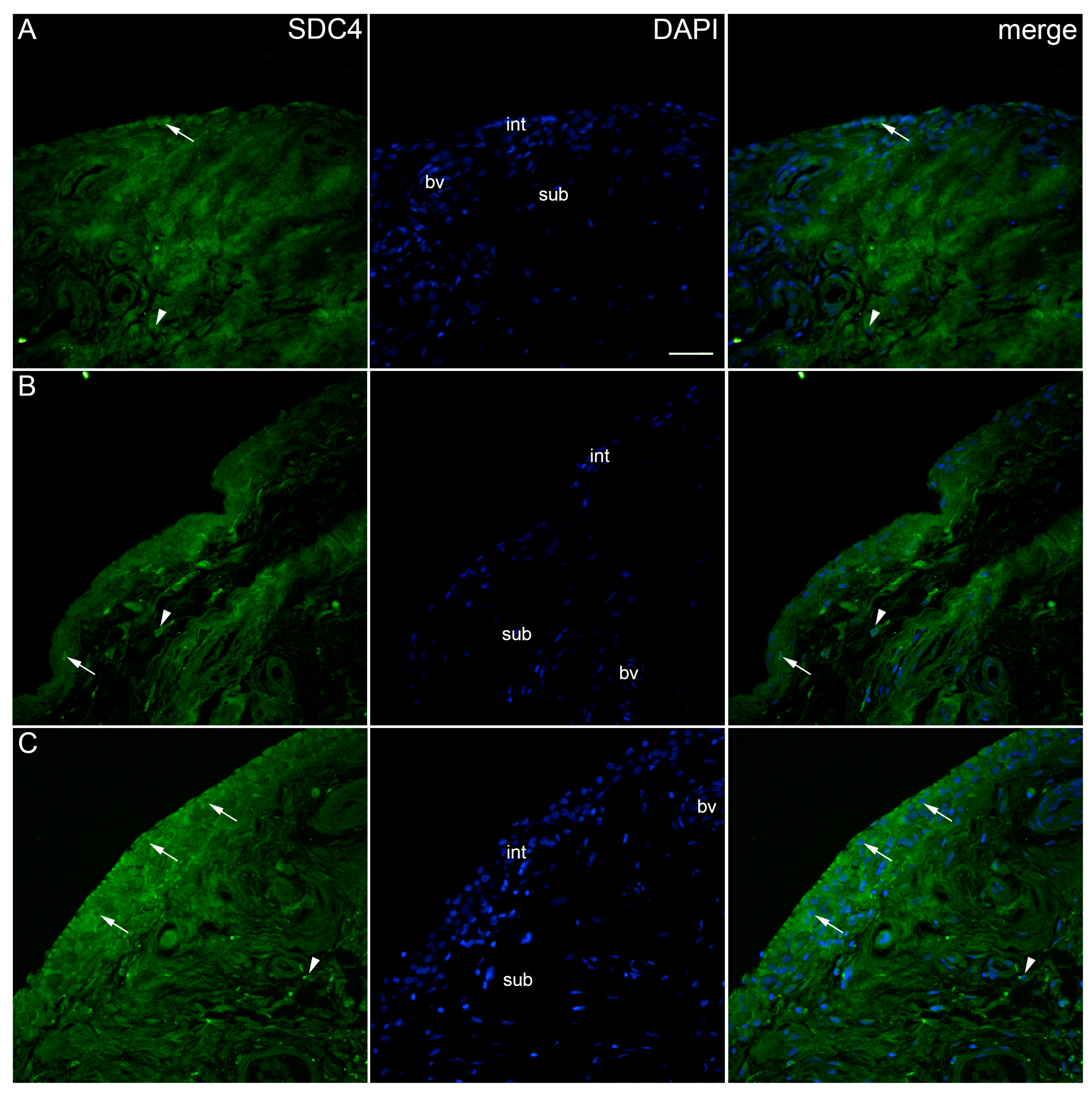
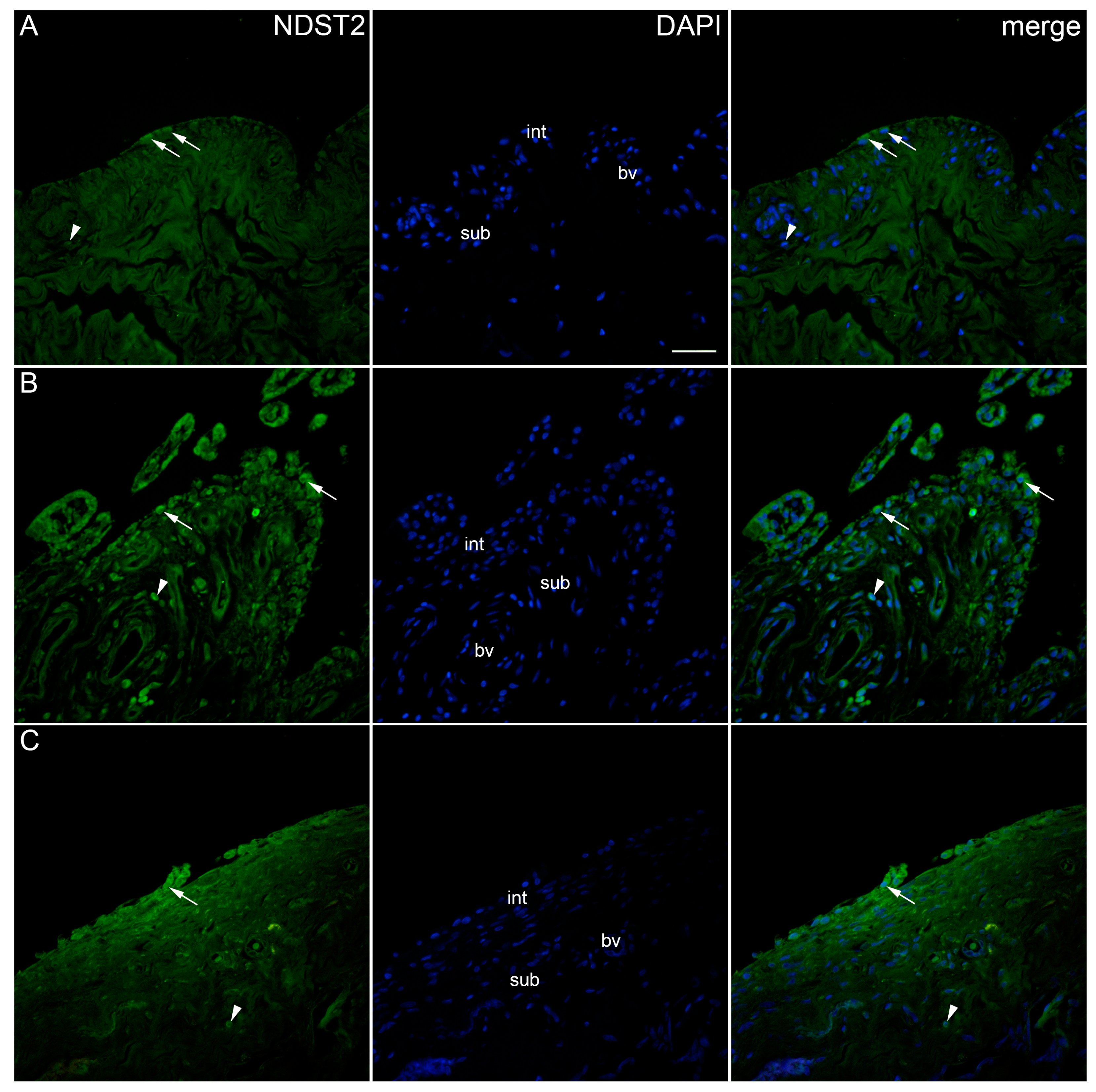

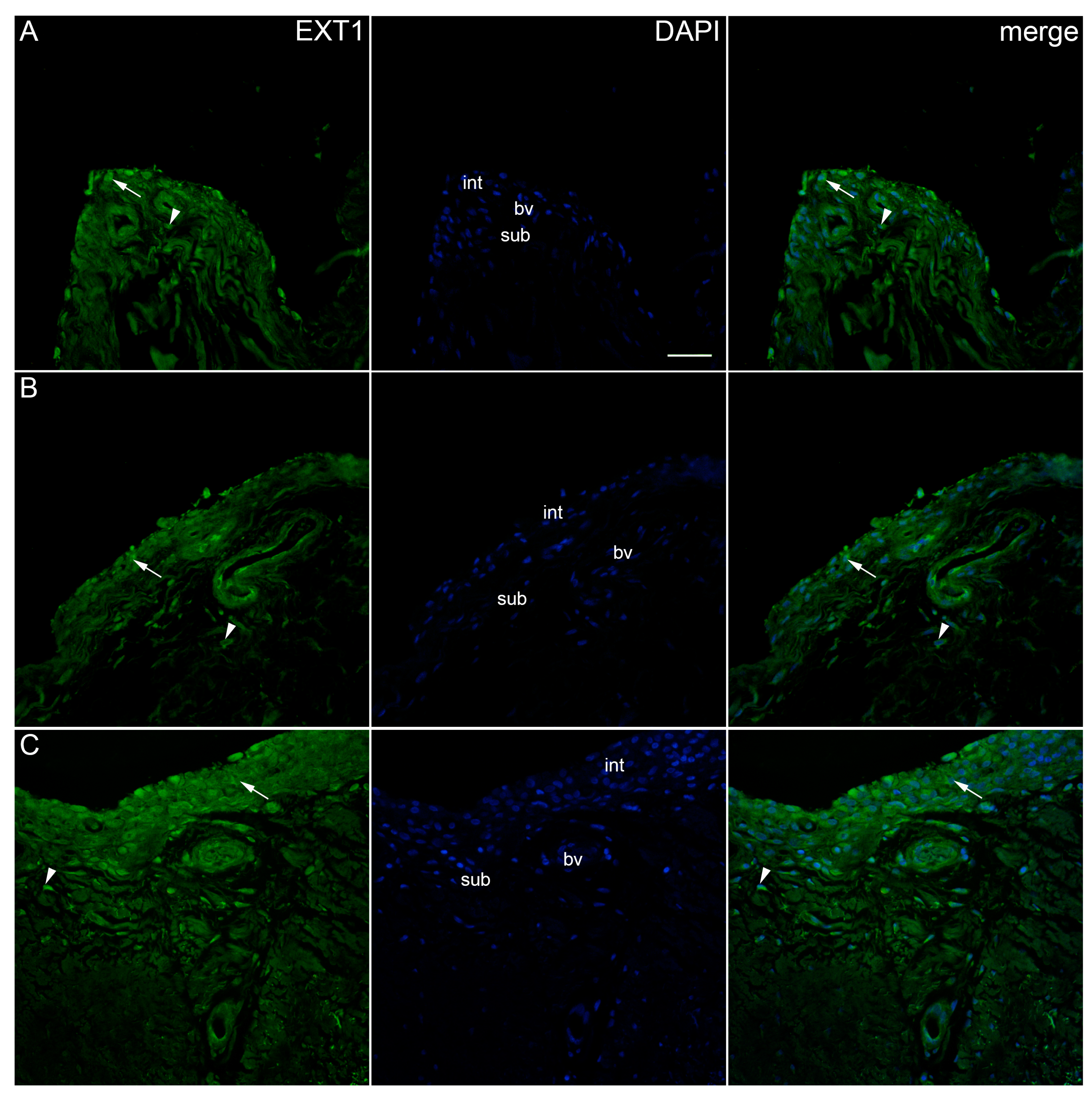
| Controls | OA Krenn Synovitis Score 0–2 | OA Krenn Synovitis Score ≥ 3 | * p Value | |
|---|---|---|---|---|
| Age (median ± IQR, years) | 74 (73.55–76.05) | 73 (63.7–75.9) | 73 (66–78) | 0.854 |
| Sex (male/female) | (6/4) | (7/5) | (6/6) | 0.732 |
| BMI (median ± IQR, kg/m2) | 25.87 (23.97–26.6) | 24.7 (23.25–25.82) | 26.7 (25.5–29.43) | 0.054 |
| K-L grade (median ± IQR) | 0.5 (0–1) | 2 (2–2) | 4 (3–4) | <0.0001 |
| Krenn score (median ± IQR) | 0 (0–0) | 6.4 (5.6–9) | 9 (7–9) | <0.0001 |
| HHS (median ± IQR) | - | 48.7 (43.58–56.8) | 41 (33.48–49.6) | 0.272 |
| VAS (median ± IQR) | - | 6 (4.6–6.8) | 6 (5–7) | 0.784 |
| WOMAC (median ± IQR) | - | 46.2 (40.2–56.4) | 47.3 (36.1–55.3) | 0.918 |
| Antibodies | Host | Code No. | Dilution | Source | |
|---|---|---|---|---|---|
| Primary | anti-Sdc1 [B-A38] | Mouse | ab34164 | 1:100 | Abcam, Cambridge, UK |
| anti-Sdc2 | Rabbit | ab191062 | 1:200 | Abcam, Cambridge, UK | |
| anti-Sdc4 | Rabbit | ab24511 | 1:100 | Abcam, Cambridge, UK | |
| anti-EXT1 | Rabbit | ab126305 | 1:100 | Abcam, Cambridge, UK | |
| Anti-EXT2 | Rabbit | ab102843 | 1:50 | Abcam, Cambridge, UK | |
| Anti-NDST1 | Rabbit | ab151141 | 1:50 | Abcam, Cambridge, UK | |
| Anti-NDST2 | Rabbit | ab1511141 | 1:100 | Abcam, Cambridge, UK | |
| Secondary | Rhodamine Red™-X Anti-Mouse IgG | Donkey | 715-295-151 | 1:400 | Jackson Immuno Research Laboratories, Inc., Baltimore, PA, USA |
| Alexa Fluor®488 Anti-Rabbit lgG | Donkey | 711-545-152 | 1:400 | Jackson Immuno Research Laboratories, Inc., Baltimore, PA, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rošin, M.; Kelam, N.; Jurić, I.; Racetin, A.; Ogorevc, M.; Corre, B.; Čarić, D.; Filipović, N.; Vukojević, K. Syndecans, Exostosins and Sulfotransferases as Potential Synovial Inflammation Moderators in Patients with Hip Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 4557. https://doi.org/10.3390/ijms25084557
Rošin M, Kelam N, Jurić I, Racetin A, Ogorevc M, Corre B, Čarić D, Filipović N, Vukojević K. Syndecans, Exostosins and Sulfotransferases as Potential Synovial Inflammation Moderators in Patients with Hip Osteoarthritis. International Journal of Molecular Sciences. 2024; 25(8):4557. https://doi.org/10.3390/ijms25084557
Chicago/Turabian StyleRošin, Matko, Nela Kelam, Ivana Jurić, Anita Racetin, Marin Ogorevc, Brieuc Corre, Davor Čarić, Natalija Filipović, and Katarina Vukojević. 2024. "Syndecans, Exostosins and Sulfotransferases as Potential Synovial Inflammation Moderators in Patients with Hip Osteoarthritis" International Journal of Molecular Sciences 25, no. 8: 4557. https://doi.org/10.3390/ijms25084557






