Identification of Indicator Genes for Agar Accumulation in Gracilariopsis lemaneiformis (Rhodophyta)
Abstract
:1. Introduction
2. Results
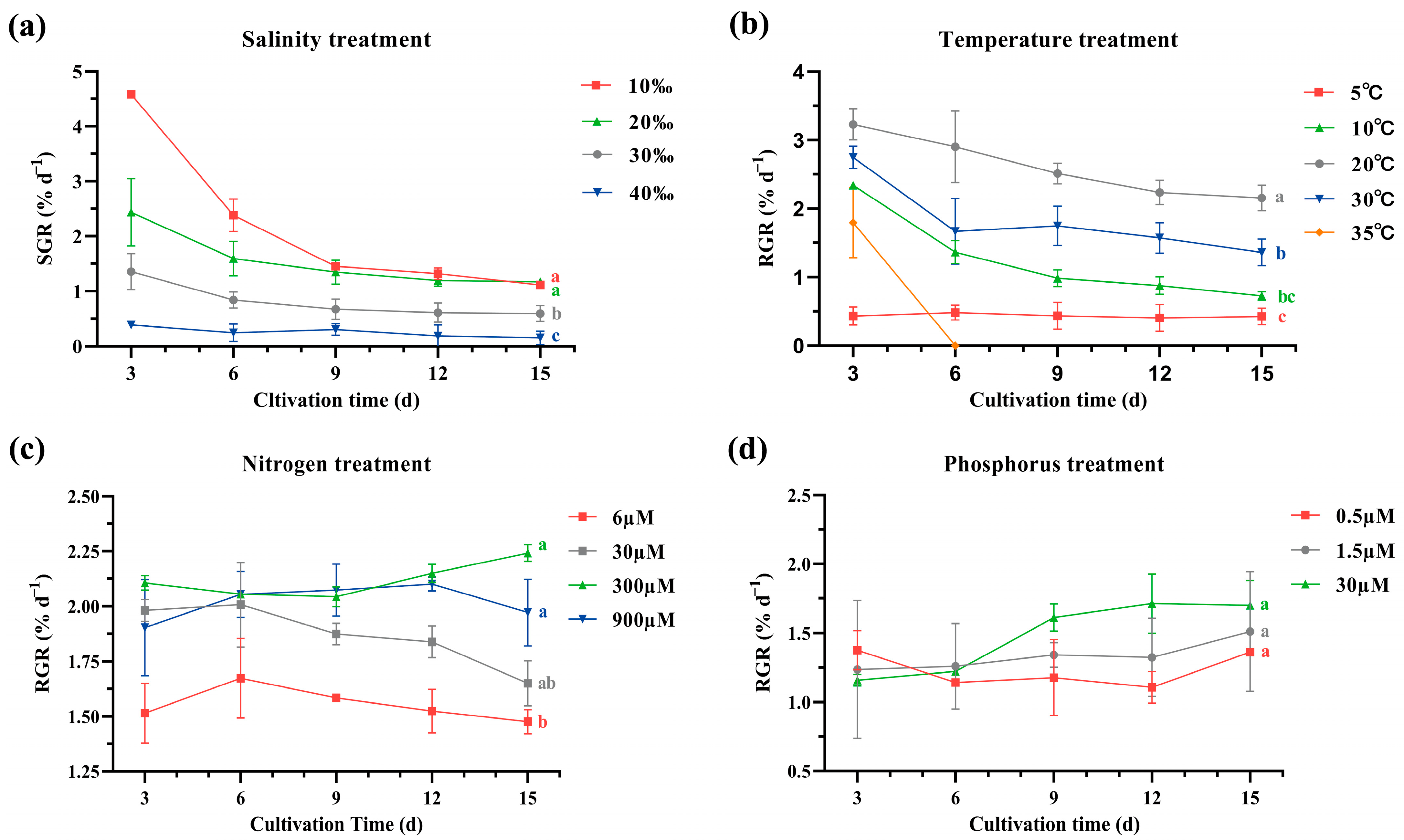
2.1. Growth Assessment
2.2. Agar Content
2.3. Soluble Polysaccharides Content
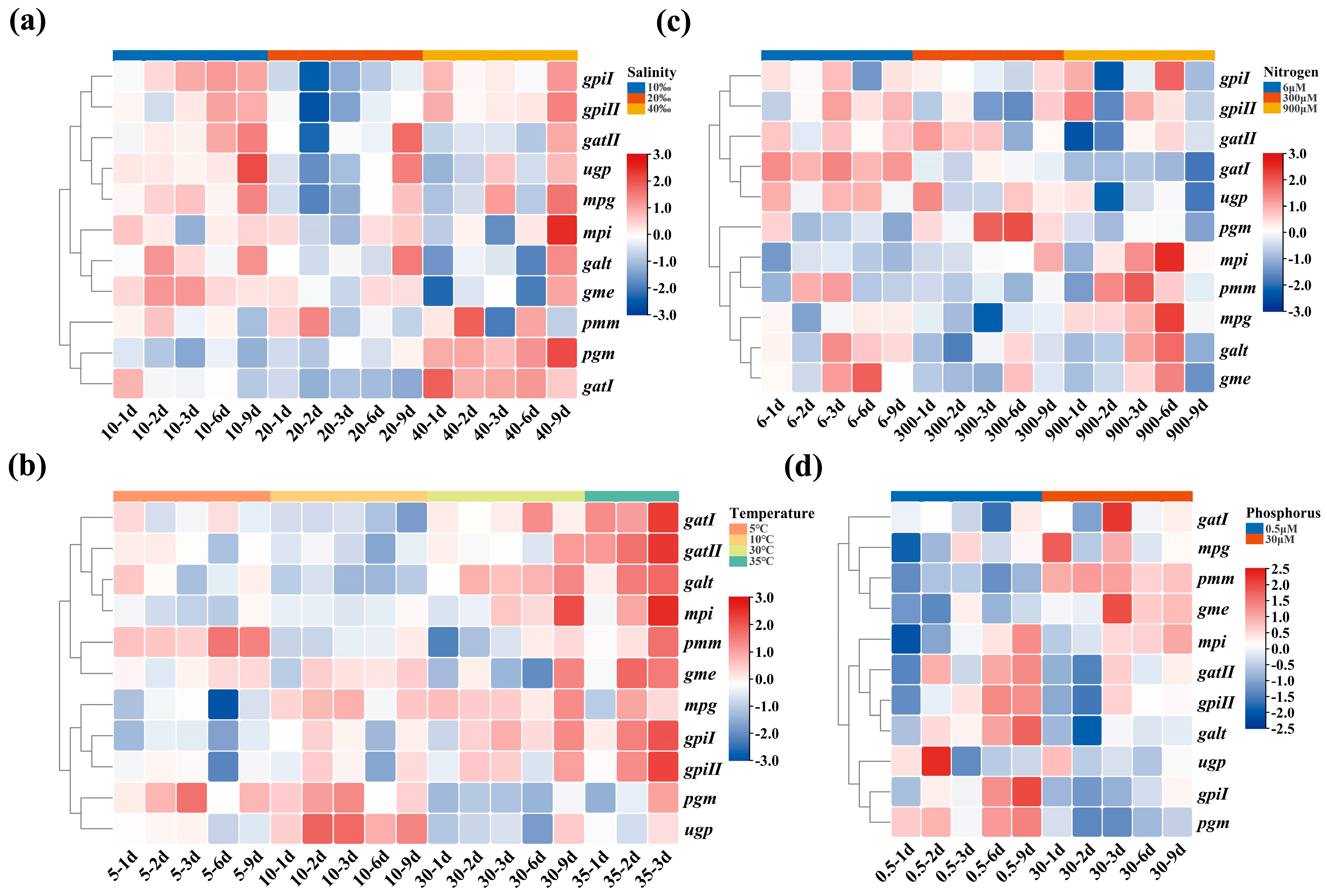
2.4. The Transcription Level of 11 Genes in G. lemaneiformis under Four Treatments
2.5. The Variation of Relationship between Agar Content and Gene Transcription Level in Different Varieties and Generations of G. lemaneiformis
2.6. The Relationship between Agar Content and Gene Transcription Level
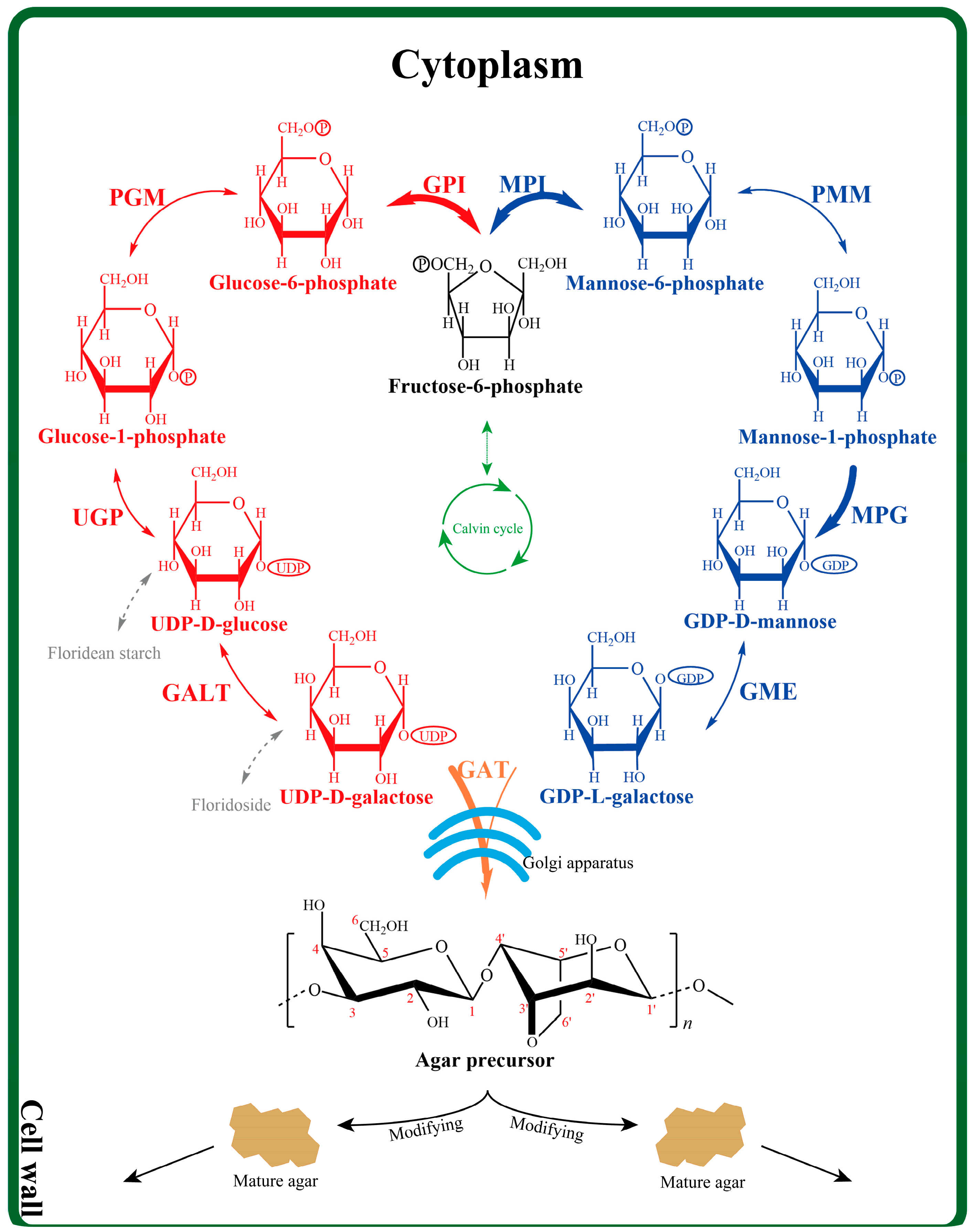
3. Discussion
4. Materials and Methods
4.1. Algae and Homogenisation Cultivation
4.2. Cultivation under Four Treatments
4.3. Determination of Relative Growth Rate
4.4. Determination of Agar Content
4.5. Measurement of Soluble Polysaccharides Content
4.6. RNA Extraction and cDNA Synthesis
4.7. Analysis of Genes Transcription Levels
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, W.A.; Knight, G.A.; Falshaw, R.; Furneaux, R.H.; Falshaw, A.; Lynds, S.M. Characterisation of the enigmatic, endemic red alga Gelidium allanii (Gelidiales) from northern New Zealand—Morphology, distribution, agar chemistry. J. Appl. Phycol. 1994, 6, 497–507. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Robledo, D.; Armisén, R.; García-Reina, G. Seasonal changes in agar characteristics of two populations of Pterocladia capillacea in Gran Canaria, Spain. J. Appl. Phycol. 1996, 8, 239–246. [Google Scholar] [CrossRef]
- Ganzon-Fortes, E.T. Photosynthetic and respiratory responses of the agarophyte Gelidiella acerosa collected from tidepool, intertidal and subtidal habitats. In Sixteenth International Seaweed Symposium, Proceedings of the Sixteenth International Seaweed Symposium held in Cebu City, Philippines, 12–17 April 1998; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Marinho-Soriano, E.; Bourret, E. Effects of season on the yield and quality of agar from Gracilaria species (Gracilariaceae, Rhodophyta). Bioresour. Technol. 2003, 90, 329–333. [Google Scholar] [CrossRef]
- Ficko-Blean, E.; Hervé, C.; Michel, G. Sweet and sour sugars from the sea: The biosynthesis and remodeling of sulfated cell wall polysaccharides from marine macroalgae. Perspect. Phycol. 2015, 2, 51–64. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, Q.; Zhang, G.; Ma, C.; Zhang, R. Protective effect of agar oligosaccharide on male Drosophila melanogaster suffering from oxidative stress via intestinal microflora activating the Keap1-Nrf2 signaling pathway. Carbohydr. Polym. 2023, 313, 120878. [Google Scholar] [CrossRef] [PubMed]
- Wilk, R.; Chojnacka, K. Marine Algae Extracts: Processes, Products, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2010, 23, 321–335. [Google Scholar] [CrossRef]
- Mantri, V.A.; Kambey, C.S.; Cottier-Cook, E.J.; Usandizaga, S.; Buschmann, A.H.; Chung, I.K.; Liu, T.; Sondak, C.F.; Qi, Z.; Lim, P.E.; et al. Overview of global Gracilaria production, the role of biosecurity policies and regulations in the sustainable development of this industry. Rev. Aquac. 2022, 15, 801–819. [Google Scholar] [CrossRef]
- Santos, R.; Melo, R. Global shortage of technical agars: Back to basics (resource management). J. Appl. Phycol. 2018, 30, 2463–2473. [Google Scholar] [CrossRef]
- Mantri, V.A.; Ganesan, M.; Gupta, V.; Krishnan, P.; Siddhanta, A.K. An overview on agarophyte trade in India and need for policy interventions. J. Appl. Phycol. 2019, 31, 3011–3023. [Google Scholar] [CrossRef]
- Porse, H.; Rudolph, B. The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J. Appl. Phycol. 2017, 29, 2187–2200. [Google Scholar] [CrossRef]
- Xu, J.; Sun, J.; Yin, J.; Wang, L.; Wang, X.; Liu, T.; Chi, S.; Liu, C.; Ren, L.; Wu, S.; et al. Comparative analysis of four essential Gracilariaceae species in China based on whole transcriptomic sequencing. Acta Oceanol. Sin. 2014, 33, 54–62. [Google Scholar] [CrossRef]
- Yang, Y.; Chai, Z.; Wang, Q.; Chen, W.; He, Z.; Jiang, S. Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Res. 2015, 9, 236–244. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, J.; Li, P.; Wang, J.; Mi, P.; Sui, Z. Review on recent advances of Gracilariopsis lemaneiformis (Rhodophyta). Algal Res. 2023, 79, 2211–9264. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 65, 115–217. [Google Scholar]
- Collén, P.N.; Camitz, A.; Hancock, R.D.; Viola, R.; Pedersén, M. Effect of nutrient deprivation and resupply on metabolites and enzymes related to carbon allocation in Gracilaria tenuistipitata (Rhodophyta). J. Phycol. 2004, 40, 305–314. [Google Scholar] [CrossRef]
- Shao, Z.; Duan, D. The Cell Wall Polysaccharides Biosynthesis in Seaweeds: A Molecular Perspective. Front. Plant Sci. 2022, 13, 902823. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Du, Q.; Mi, P.; Shang, E.; Sui, Z. Gene cloning and expression regulation in the pathway of agar and floridean starch synthesis of Gracilariopsis lemaneiformis (Rhodophyta). J. Appl. Phycol. 2018, 31, 1889–1896. [Google Scholar] [CrossRef]
- Lee, W.K.; Lim, Y.Y.; Leow, A.T.C.; Namasivayam, P.; Ong Abdullah, J.; Ho, C.L. Biosynthesis of agar in red seaweeds: A review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Hu, Y.; Feng, X.; Sui, Z. Breeding of New Strains of Gracilariopsis lemaneiformis with High Agar Content by ARTP Mutagenesis and High Osmotic Pressure Screening. Mar. Biotechnol. 2022, 25, 100–108. [Google Scholar] [CrossRef]
- Chang, L.; Sui, Z.; Fu, F.; Zhou, W.; Wang, J.; Kang, K.H.; Zhang, S.; Ma, J. Relationship between gene expression of UDP-glucose pyrophosphorylase and agar yield in Gracilariopsis lemaneiformis (Rhodophyta). J. Appl. Phycol. 2014, 26, 2435–2441. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-K.; Lim, Y.-Y.; Leow, A.T.-C.; Namasivayam, P.; Abdullah, J.O.; Ho, C.-L. Factors affecting yield and gelling properties of agar. J. Appl. Phycol. 2016, 29, 1527–1540. [Google Scholar] [CrossRef]
- Bird, K.T. Agar Production and Quality from Gracilaria sp. Strain G—16: Effects of Environmental Factors. Bot. Mar. 1988, 31, 33–38. [Google Scholar] [CrossRef]
- Siow, R.-S.; Teo, S.S.; Ho, W.-Y.; Shukor, M.Y.A.; Phang, S.-M.; Ho, C.-L. Molecular cloning and biochemical characterization of galactose-1-phosphate uridylyltransferase from Gracilaria changii (rhodophyta)1. J. Phycol. 2011, 48, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M. Growth rate, epiphyte biomass and agar yield of Gracilaria conferta in an annual outdoor experiment. 1. Irradiance and nitrogen. Bioresour. Technol. 1991, 38, 203–208. [Google Scholar] [CrossRef]
- Chen, Q.; Ying, X.; Hu, C.; Liu, S.; Xu, N.; Sun, X. Genome-wide identification of the GT7 and GH16 genes and their correlations with agar metabolism in the agarophyte Gracilariopsis lemaneiformis. Front. Mar. Sci. 2023, 10, 1156490. [Google Scholar] [CrossRef]
- Macler, B.A.; West, J.A. Life history and physiology of the red alga, Gelidium coulteri, in unialgal culture. Aquaculture 1987, 61, 281–293. [Google Scholar] [CrossRef]
- Christeller, J.T.; Laing, W.A. The Effect of Environment on the Agar Yield and Gel Characteristics of Gracilaria sordida Nelson (Rhodophyta). Bot. Mar. 1989, 32, 447–456. [Google Scholar] [CrossRef]
- Liu, X.; Wen, J.; Chen, W.; Du, H. Physiological effects of nitrogen deficiency and recovery on the macroalga Gracilariopsis lemaneiformis (Rhodophyta). J. Phycol. 2019, 55, 830–839. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, X.; Liu, S.; Luo, S.; Chen, X.; Xu, N.; Sun, X. Identification, Characteristics and Function of Phosphoglucomutase (PGM) in the Agar Biosynthesis and Carbon Flux in the Agarophyte Gracilariopsis lemaneiformis (Rhodophyta). Mar. Drugs 2022, 20, 442. [Google Scholar] [CrossRef]
- Li, M.; Sui, Z.; Kang, K.-H.; Zhang, X.; Zhu, M.; Yan, B. Cloning and analysis of the galactose-1-phosphate uridylyltransferase (galt) gene of Gracilariopsis lemaneiformis (Rhodophyta) and correlation between gene expression and agar synthesis. J. Appl. Phycol. 2009, 22, 157–164. [Google Scholar] [CrossRef]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.A.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [PubMed]
- Minhas, D.; Grover, A. Transcript levels of genes encoding various glycolytic and fermentation enzymes change in response to abiotic stresses. Plant Sci. 1999, 146, 41–51. [Google Scholar] [CrossRef]
- Cui, L.; Xue, L.; Li, J.; Zhang, L.; Yan, H. Characterization of the glucose-6-phosphate isomerase (GPI) gene from the halotolerant alga Dunaliella salina. Mol. Biol. Rep. 2009, 37, 911–916. [Google Scholar] [CrossRef]
- Jiang, H.; Ouyang, H.; Zhou, H.; Jin, C. GDP-mannose pyrophosphorylase is essential for cell wall integrity, morphogenesis and viability of Aspergillus fumigatus. Microbiology 2008, 154, 2730–2739. [Google Scholar] [CrossRef] [PubMed]
- Yoda, K.; Kawada, T.; Kaibara, C.; Fujie, A.; Abe, M.; Hashimoto, H.; Shimizu, J.; Tomishige, N.; Noda, Y.; Yamasaki, M. Defect in Cell Wall Integrity of the Yeast Saccharomyces cerevisiae Caused by a Mutation of the GDP-mannose Pyrophosphorylase GeneVIG9. Biosci. Biotechnol. Biochem. 2000, 64, 1937–1941. [Google Scholar] [CrossRef] [PubMed]
- Gibeaut, D.M. Nucleotide sugars and glycosyltransferases for synthesis of cell wall matrix polysaccharides. Plant Physiol. Biochem. 2000, 38, 69–80. [Google Scholar] [CrossRef]
- Breton, C.; Šnajdrová, L.; Jeanneau, C.; Koča, J.; Imberty, A. Structures and mechanisms of glycosyltransferases. Glycobiology 2005, 16, 29R–37R. [Google Scholar] [CrossRef]
- Collén, J.; Porcel, B.; Carré, W.; Ball, S.G.; Chaparro, C.; Tonon, T.; Barbeyron, T.; Michel, G.; Noel, B.; Valentin, K.; et al. Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc. Natl. Acad. Sci. USA 2013, 110, 5247–5252. [Google Scholar] [CrossRef]
- Berges, J.A.; Franklin, D.J.; Harrison, P.J. Evolution of an artifcial seawater medium: Improvements in enriched seawater, artifcial water over the last two decades. J. Phycol. 2001, 37, 1138–1145. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms: I. cyclotella nana hustedt, and detonula confervacea (cleve) gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Abreu, M.H.; Varela, D.A.; Henríquez, L.; Villarroel, A.; Yarish, C.; Sousa-Pinto, I.; Buschmann, A.H. Traditional vs. Integrated Multi-Trophic Aquaculture of Gracilaria chilensis C. J. Bird, J. McLachlan & E. C. Oliveira: Productivity and physiological performance. Aquaculture 2009, 293, 211–220. [Google Scholar]
- Yang, X.; Liu, M.; Qi, B.; Deng, J.; Hao, S.; Cen, J.; Zhou, W. Comparison of methods in determination of polysaccharide in Gracilaria lemaneiformis. Sci. Technol. Food Ind. 2013, 34, 4. [Google Scholar]
- Qin, F.; Zang, X.; Shui, G.; Wang, Z. Transcriptome analysis of Gracilariopsis lemaneiformis at low temperature. J. Appl. Phycol. 2021, 33, 4035–4050. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, H.; Zhang, R.; Yang, Q.; Liu, Y.; Zang, X.; Zhang, X. Selection of reference gene from Gracilaria lemaneiformis under temperature stress. J. Appl. Phycol. 2014, 27, 1365–1372. [Google Scholar] [CrossRef]
- Sun, X.; Wu, J.; Wang, G.; Kang, Y.; Ooi, H.S.; Shen, T.; Wang, F.; Yang, R.; Xu, N.; Zhao, X. Genomic analyses of unique carbohydrate and phytohormone metabolism in the macroalga Gracilariopsis lemaneiformis (Rhodophyta). BMC Plant Biol. 2018, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Hou, L.; Dong, M.; Shi, J.; Huang, X.; Ding, Y.; Cong, X.; Zhang, F.; Zhang, X.; Zang, X. Transcriptome Analysis in Haematococcus pluvialis: Astaxanthin Induction by High Light with Acetate and Fe2+. Int. J. Mol. Sci. 2018, 19, 175. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Gao, G.; Beardall, J.; Inaba, K.; Hall-Spencer, J.M.; Xu, D.; Zhang, X.; Han, W.; McMinn, A.; et al. Decreased motility of flagellated microalgae long-term acclimated to CO2-induced acidified waters. Nat. Clim. Chang. 2020, 10, 561–567. [Google Scholar] [CrossRef]




| Genes | gpiI | gpiII | pgm | ugp | galt | mpi | pmm | mpg | gme | gatI | gatII |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCC | 0.503 | 0.925 * | −0.278 | −0.023 | 0.742 | 0.776 * | −0.532 | 0.901 * | −0.636 | 0.209 | 0.856 * |
| Sample Collection Time | Treatments | Groups (Levels) | ||||
|---|---|---|---|---|---|---|
| 13 August 2022 | Salinity (‰) | 10 | 20 | 30 (control) | 40 | - |
| 3 September 2022 | Temperature (°C) | 5 | 10 | 20 (control) | 30 | 35 |
| 12 October 2022 | N (µM) | 6 | 30 (control) | 300 | 900 | - |
| 11 November 2022 | P (µM) | 0.5 | 1.5 (control) | 30 | - | - |
| Component | Concentration (g/L) |
|---|---|
| NaCl | 24.470 |
| Na2SO4 | 3.9170 |
| KCl | 0.6640 |
| KBr | 0.0960 |
| SrCl2·6H2O | 0.0402 |
| MgCl·6H2O | 4.9810 |
| CaCl2 | 0.9480 |
| NaHCO3 | 0.1920 |
| NaF | 0.0039 |
| NaH2PO4·2H2O | X |
| H3BO3 | 0.0260 |
| NaNO3 | Y |
| Na2SiO3·9H2O | 0.0230 |
| Treatments | X, g/L (µM) | Y, g/L (µM) |
|---|---|---|
| Various N Concentrations | 4.68 × 10−3 (30) | 5.10 × 10−5 (6) |
| 2.55 × 10−3 (30) | ||
| 2.55 × 10−2 (300) | ||
| 7.65 × 10−2 (900) | ||
| Various P Concentrations | 7.80 × 10−5 (0.5) | 2.55 × 10−2 (300) |
| 2.35 × 10−4 (1.5) | ||
| 4.68 × 10−3 (30) |
| Genes Name | Forward Primers (5′–3′) | Reverse Primer (5′–3′) | PCR Products (bp) |
|---|---|---|---|
| gpiI | TGCTCCAACTTGCTGCCGA | GCTCGCAGGGCGGTATGAA | 193 |
| gpiII | ACCACCGCCGAAACCATGC | CCAGTCCCAGAATCCAAACACG | 165 |
| pgm | TCCTTCTGATTCTGTCGCTGTC | ATTTCCAACCTGTAGGCACTTC | 167 |
| ugp | GACCTTATCGTCCAGCAAATCG | CCTTGACAATCCTCGGGTAGCG | 181 |
| galt | CGTTCCTTTGCTTCCATTTCG | TCTGGGCATCTGAGTTCGTTC | 127 |
| mpi | CTCCGCCGACTCCGTTCAAGA | TACGCCGCAAAGCAGCCCACAT | 138 |
| pmm | CGTTCTGGGATTACGGTCTC | GCATAGTTTCTTCCGGGTTC | 219 |
| mpg | CCTTGATTCTCGTCGGTGGTTA | GCATCTTCTCGGGCTGGTAGTT | 174 |
| gme | TGGGTAAGATGGTGCTCGGATTT | TCAAAAGTCCTCCTGAGCCCGT | 163 |
| gatI | GCTGGATTTGTAATGTTGATGTTGC | TGGATAAGCCTCGTGCGTTC | 211 |
| gatII | CCTTGAGCTGGTTGTGCAATGT | CATGAGCATCCAGTATCCCG | 233 |
| act | CTACTCGTTTACCACTTCTGCTGA | TTCCATTCCGACCAACTCTG | 220 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Tu, M.; Qin, F.; Shui, G.; Xu, D.; Zang, X. Identification of Indicator Genes for Agar Accumulation in Gracilariopsis lemaneiformis (Rhodophyta). Int. J. Mol. Sci. 2024, 25, 4606. https://doi.org/10.3390/ijms25094606
Li Z, Tu M, Qin F, Shui G, Xu D, Zang X. Identification of Indicator Genes for Agar Accumulation in Gracilariopsis lemaneiformis (Rhodophyta). International Journal of Molecular Sciences. 2024; 25(9):4606. https://doi.org/10.3390/ijms25094606
Chicago/Turabian StyleLi, Zheng, Mengge Tu, Feng Qin, Guangqiang Shui, Di Xu, and Xiaonan Zang. 2024. "Identification of Indicator Genes for Agar Accumulation in Gracilariopsis lemaneiformis (Rhodophyta)" International Journal of Molecular Sciences 25, no. 9: 4606. https://doi.org/10.3390/ijms25094606






