The Immune Response of OAS1, IRF9, and IFI6 Genes in the Pathogenesis of COVID-19
Abstract
:1. Introduction
2. Results
2.1. Individuals Included in the Study
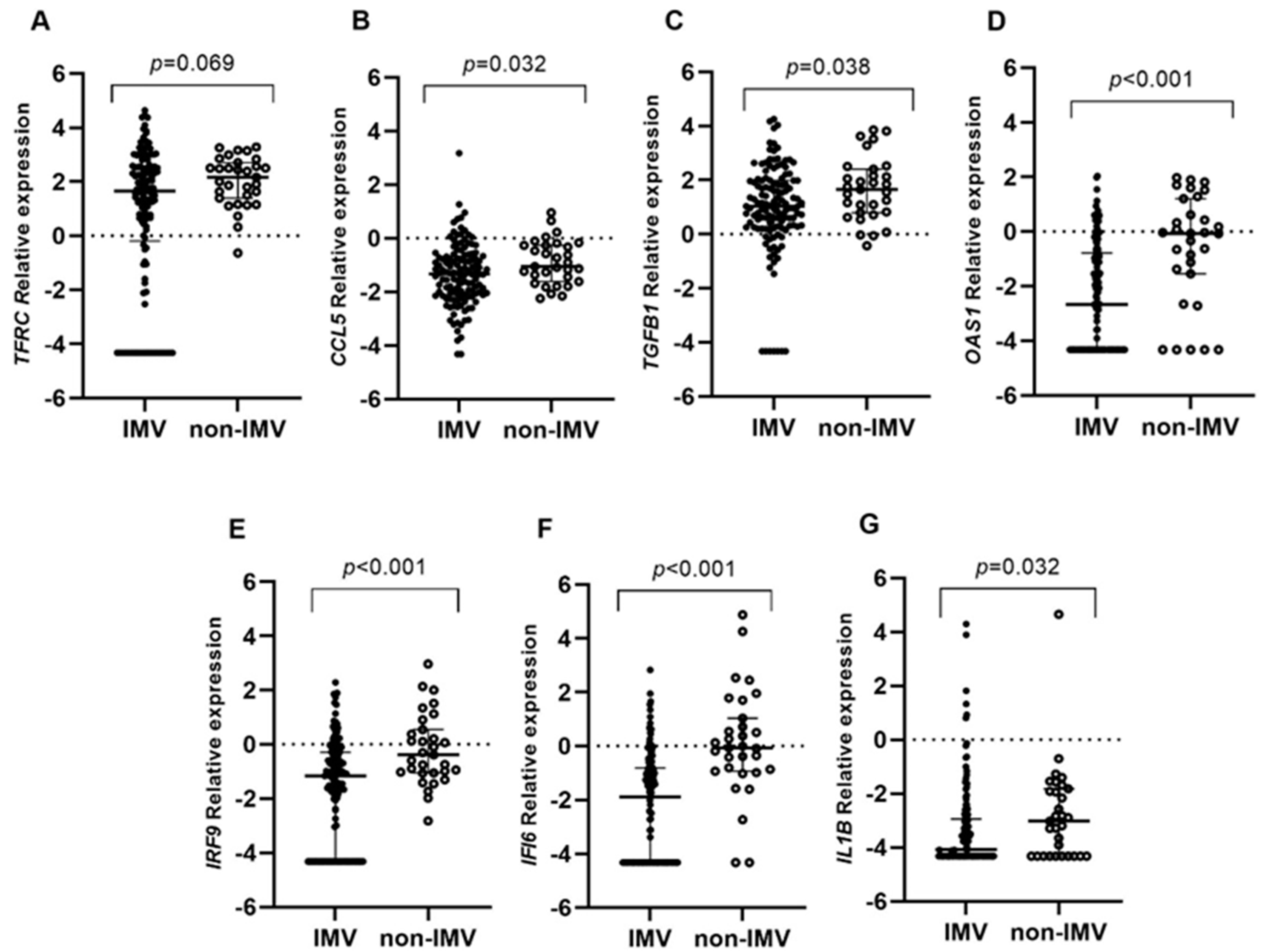
2.2. Gene Expression Analysis
3. Discussion
4. Materials and Methods
4.1. Patients and Study Samples
4.2. Gene Expression Study
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19) A Review. J. Am. Med. Assoc. 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasoon, P.; Kumari, C.; Pareek, V.; Faiq, M.A.; Narayan, R.K.; Kulandhasamy, M.; Kant, K. SARS-CoV-2-Specific Virulence Factors in COVID-19. J. Med. Virol. 2021, 93, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Rao, Z. Structural Biology of SARS-CoV-2 and Implications for Therapeutic Development. Nat. Rev. Microbiol. 2021, 19, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.H.; Lai, W.Y.; Lin, Y.Y.; Luo, Y.H.; Lin, Y.T.; Chen, H.K.; Chen, Y.M.; Lai, Y.C.; Kuo, L.C.; Chen, S.D.; et al. Clinical Manifestation and Disease Progression in COVID-19 Infection. J. Chin. Med. Assoc. 2021, 84, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Montero, M.T.V.; Rowe, K.; Kirton, R.; Kunik, F. Epidemiology, Pathogenesis, Clinical Presentations, Diagnosis and Treatment of COVID-19: A Review of Current Evidence. Expert Rev. Clin. Pharmacol. 2021, 14, 601–621. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Rubin, L.; Peng, T.; Liu, L.; Xing, X.; Lazarovici, P.; Zheng, W. Cytokine Storm in COVID-19: From Viral Infection to Immune Responses, Diagnosis and Therapy. Int. J. Biol. Sci. 2022, 18, 459–472. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 551898. [Google Scholar] [CrossRef]
- Grifoni, A.; Alonzi, T.; Alter, G.; Noonan, D.M.C.; Landay, A.L.; Albini, A.; Goletti, D. Impact of Aging on Immunity in the Context of COVID-19, HIV, and Tuberculosis. Front. Immunol. 2023, 14, 1146704. [Google Scholar] [CrossRef]
- Inde, Z.; Croker, B.A.; Yapp, C.; Joshi, G.N.; Spetz, J.; Fraser, C.; Qin, X.; Xu, L.; Deskin, B.; Ghelfi, E.; et al. Age-Dependent Regulation of SARS-CoV-2 Cell Entry Genes and Cell Death Programs Correlates with COVID-19 Severity. Sci. Adv. 2021, 7, eabf8609. [Google Scholar] [CrossRef]
- Khan, M.M.A.; Khan, M.N.; Mustagir, G.; Rana, J.; Islam, M.S.; Kabir, M.I. Effects of Underlying Morbidities on the Occurrence of Deaths in COVID-19 Patients: A Systematic Review and Meta-Analysis. J. Glob. Health 2020, 10, 020503. [Google Scholar] [CrossRef] [PubMed]
- Chenchula, S.; Vidyasagar, K.; Pathan, S.; Sharma, S.; Chavan, M.R.; Bhagavathula, A.S.; Padmavathi, R.; Manjula, M.; Chhabra, M.; Gupta, R.; et al. Global Prevalence and Effect of Comorbidities and Smoking Status on Severity and Mortality of COVID-19 in Association with Age and Gender: A Systematic Review, Meta-Analysis and Meta-Regression. Sci. Rep. 2023, 13, 6415. [Google Scholar] [CrossRef] [PubMed]
- Amati, F.; Vancheri, C.; Latini, A.; Colona, V.L.; Grelli, S.; D’Apice, M.R.; Balestrieri, E.; Passarelli, C.; Minutolo, A.; Loddo, S.; et al. Expression Profiles of the SARS-CoV-2 Host Invasion Genes in Nasopharyngeal and Oropharyngeal Swabs of COVID-19 Patients. Heliyon 2020, 6, e05143. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.-L.; Su, H.C. A Global Effort to Define the Human Genetics of Protective Immunity to SARS-CoV-2 Infection. Cell 2020, 181, 1194–1199. [Google Scholar] [CrossRef]
- Mick, E.; Kamm, J.; Pisco, A.O.; Ratnasiri, K.; Babik, J.M.; Castañeda, G.; DeRisi, J.L.; Detweiler, A.M.; Hao, S.L.; Kangelaris, K.N.; et al. Upper Airway Gene Expression Reveals Suppressed Immune Responses to SARS-CoV-2 Compared with Other Respiratory Viruses. Nat. Commun. 2020, 11, 5854. [Google Scholar] [CrossRef]
- Zaas, A.K.; Chen, M.; Varkey, J.; Veldman, T.; Hero, A.O.; Lucas, J.; Huang, Y.; Turner, R.; Gilbert, A.; Lambkin-Williams, R.; et al. Gene Expression Signatures Diagnose Influenza and Other Symptomatic Respiratory Viral Infections in Humans. Cell Host Microbe 2009, 6, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, N.A.P.; Peddu, V.; Xie, H.; Shrestha, L.; Huang, M.L.; Mears, M.C.; Cajimat, M.N.; Bente, D.A.; Shi, P.Y.; Bovier, F.; et al. In Vivo Antiviral Host Transcriptional Response to SARS-CoV-2 by Viral Load, Sex, and Age. PLoS Biol. 2020, 18, e3000849. [Google Scholar] [CrossRef] [PubMed]
- Menachery, V.D.; Eisfeld, A.J.; Schäfer, A.; Josset, L.; Sims, A.C.; Proll, S.; Fan, S.; Li, C.; Neumann, G.; Tilton, S.C.; et al. Pathogenic Influenza Viruses and Coronaviruses Utilize Similar and Contrasting Approaches to Control Interferon-Stimulated Gene Responses. MBio 2014, 5, e01174-14. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Kaur, H. In Silico Transcriptional Analysis of Asymptomatic and Severe COVID-19 Patients Reveals the Susceptibility of Severe Patients to Other Comorbidities and Non-Viral Pathological Conditions. Hum. Gene 2023, 35, 201135. [Google Scholar] [CrossRef]
- Malterer, M.B.; Glass, S.J.; Newman, J.P. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, H.; Zeng, T.; Chen, L.; Li, Z.; Huang, T.; Cai, Y.D. Identifying Transcriptomic Signatures and Rules for SARS-CoV-2 Infection. Front. Cell Dev. Biol. 2021, 8, 627302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Butler-Laporte, G.; Nakanishi, T.; Morrison, D.R.; Afilalo, J.; Afilalo, M.; Laurent, L.; Pietzner, M.; Kerrison, N.; Zhao, K.; et al. A Neanderthal OAS1 Isoform Protects Individuals of European Ancestry against COVID-19 Susceptibility and Severity. Nat. Med. 2021, 27, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Kwon, Y.C.; Liu, S.; Hagedorn, C.H.; Ray, R.B.; Ray, R. Interferon-α Inducible Protein 6 Impairs EGFR Activation by CD81 and Inhibits Hepatitis C Virus Infection. Sci. Rep. 2015, 5, 9012. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Halfmann, P.J.; Hill-Batorski, L.; Ozawa, M.; Lopes, T.J.S.; Neumann, G.; Schoggins, J.W.; Rice, C.M.; Kawaoka, Y. Identification of Interferon-Stimulated Genes That Attenuate Ebola Virus Infection. Nat. Commun. 2020, 11, 2953. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Agresti, N.; Lalezari, J.P.; Amodeo, P.P.; Mody, K.; Mosher, S.F.; Seethamraju, H.; Kelly, S.A.; Pourhassan, N.Z.; Sudduth, C.D.; Bovinet, C.; et al. Disruption of CCR5 Signaling to Treat COVID-19-Associated Cytokine Storm: Case Series of Four Critically Ill Patients Treated with Leronlimab. J. Transl. Autoimmun. 2021, 4, 100083. [Google Scholar] [CrossRef]
- Vaz de Paula, C.B.; Nagashima, S.; Liberalesso, V.; Collete, M.; da Silva, F.P.G.; Oricil, A.G.G.; Barbosa, G.S.; da Silva, G.V.C.; Wiedmer, D.B.; da Silva Dezidério, F.; et al. COVID-19: Immunohistochemical Analysis of TGF-β Signaling Pathways in Pulmonary Fibrosis. Int. J. Mol. Sci. 2021, 23, 168. [Google Scholar] [CrossRef]
- Gajate-Arenas, M.; García-Pérez, O.; Chao-Pellicer, J.; Domínguez-de-Barros, A.; Dorta-guerra, R.; Lorenzo-morales, J.; Córdoba-Lanús, E. Differential Expression of Antiviral and Immune-Related Genes in Individuals with COVID-19 Asymptomatic or with Mild Symptoms. Front. Cell. Infect. Microbiol. 2023, 13, 1173213. [Google Scholar] [CrossRef]
- Barek, M.A.; Aziz, M.A.; Islam, M.S. Impact of Age, Sex, Comorbidities and Clinical Symptoms on the Severity of COVID-19 Cases: A Meta-Analysis with 55 Studies and 10014 Cases. Heliyon 2020, 6, e05684. [Google Scholar] [CrossRef]
- Fricke-Galindo, I.; Falfán-Valencia, R. Genetics Insight for COVID-19 Susceptibility and Severity: A Review. Front. Immunol. 2021, 12, 622176. [Google Scholar] [CrossRef]
- Kristiansen, H.; Gad, H.H.; Eskildsen-Larsen, S.; Despres, P.; Hartmann, R. The Oligoadenylate Synthetase Family: An Ancient Protein Family with Multiple Antiviral Activities. J. Interf. Cytokine Res. 2011, 31, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wickenhagen, A.; Sugrue, E.; Lytras, S.; Kuchi, S.; Noerenberg, M.; Turnbull, M.L.; Loney, C.; Herder, V.; Allan, J.; Jarmson, I.; et al. A Prenylated DsRNA Sensor Protects against Severe COVID-19. Science 2021, 374, eabj3624. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.R.; Stanifer, M.L.; Florez-Vargas, O.; Onabajo, O.O.; Papenberg, B.W.; Zahoor, M.A.; Mirabello, L.; Ring, T.J.; Lee, C.H.; Albert, P.S.; et al. Genetic Regulation of OAS1 Nonsense-Mediated Decay Underlies Association with COVID-19 Hospitalization in Patients of European and African Ancestries. Nat. Genet. 2022, 54, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Cheriyath, V.; Kaur, J.; Davenport, A.; Khalel, A.; Chowdhury, N.; Gaddipati, L. G1P3 (IFI6), a Mitochondrial Localised Antiapoptotic Protein, Promotes Metastatic Potential of Breast Cancer Cells through MtROS. Br. J. Cancer 2018, 119, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Park, G.-H.; Kim, K.-Y.; Cho, S.W.; Cheong, J.Y.; Yu, G.I.; Shin, D.H.; Kwack, K.B. Association between Interferon-Inducible Protein 6 (IFI6) Polymorphisms and Hepatitis B Virus Clearance. Genomics Inform. 2013, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- García-Morato, M.B.; Calvo Apalategi, A.; Bravo-Gallego, L.Y.; Blázquez Moreno, A.; Simón-Fuentes, M.; Garmendia, J.V.; Méndez Echevarría, A.; del Rosal Rabes, T.; Domínguez-Soto, Á.; López-Granados, E.; et al. Impaired Control of Multiple Viral Infections in a Family with Complete IRF9 Deficiency. J. Allergy Clin. Immunol. 2019, 144, 309–312.e10. [Google Scholar] [CrossRef]
- Gothe, F.; Stremenova Spegarova, J.; Hatton, C.F.; Griffin, H.; Sargent, T.; Cowley, S.A.; James, W.; Roppelt, A.; Shcherbina, A.; Hauck, F.; et al. Aberrant Inflammatory Responses to Type I Interferon in STAT2 or IRF9 Deficiency. J. Allergy Clin. Immunol. 2022, 150, 955–964.e16. [Google Scholar] [CrossRef] [PubMed]
- Assou, S.; Ahmed, E.; Morichon, L.; Nasri, A.; Foisset, F.; Bourdais, C.; Gros, N.; Tieo, S.; Petit, A.; Vachier, I.; et al. The Transcriptome Landscape of the In Vitro Human Airway Epithelium Response to SARS-CoV-2. Int. J. Mol. Sci. 2023, 24, 12017. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Xie, Z.; Suleman, M.; Shah, A.; Khan, S.; Luo, S. Roles and Functions of SARS-CoV-2 Proteins in Host Immune Evasion. Front. Immunol. 2022, 13, 940756. [Google Scholar] [CrossRef]
- Liu, Q.; Chi, S.; Dmytruk, K.; Dmytruk, O.; Tan, S. Coronaviral Infection and Interferon Response: The Virus-Host Arms Race and COVID-19. Viruses 2022, 14, 1349. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.L.; Flavell, R.A. Transforming Growth Factor- β Regulation of Immune Responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef] [PubMed]
- Florindo, H.F.; Kleiner, R.; Vaskovich-Koubi, D.; Acúrcio, R.C.; Carreira, B.; Yeini, E.; Tiram, G.; Liubomirski, Y.; Satchi-Fainaro, R. Immune-Mediated Approaches against COVID-19. Nat. Nanotechnol. 2020, 15, 630–645. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Yoo, J.R.; Park, Y.; Kim, S.H.; Heo, S.T.; Park, S.H.; Kim, M.; Kim, T.J.; Oh, S.; Lee, M.S.; et al. Fatal Outcome of Severe Fever with Thrombocytopenia Syndrome (SFTS) and Severe and Critical COVID-19 Is Associated with the Hyperproduction of IL-10 and IL-6 and the Low Production of TGF-β. J. Med. Virol. 2023, 95, e28894. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.B.; Huh, C.G.; Becker, D.; Geiser, A.; Lyght, M.; Flanders, K.C.; Roberts, A.B.; Sporn, M.B.; Ward, J.M.; Karlsson, S. Transforming Growth Factor Β1 Null Mutation in Mice Causes Excessive Inflammatory Response and Early Death. Proc. Natl. Acad. Sci. USA 1993, 90, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.E.; Guabiraba, R.; Russo, R.C.; Teixeira, M.M. Targeting CCL5 in Inflammation. Expert Opin. Ther. Targets 2013, 17, 1439–1460. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.K.; Seethamraju, H.; Dhody, K.; Corley, M.J.; Kazempour, K.; Lalezari, J.P.; Pang, A.P.; Sugai, C.; Francisco, E.B.; Pise, A.; et al. Disruption of the CCL5/RANTES-CCR5 Pathway Restores Immune Homeostasis and Reduces Plasma Viral Load in Critical COVID-19. medRxiv 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Pita-Martínez, C.; Pérez-García, F.; Virseda Berdices, A.; Martin-Vicente, M.; Castilla-García, L.; Hervás Fernández, I.; González Ventosa, V.; Muñoz-Gómez, M.J.; Cuadros-González, J.; Bermejo-Martin, J.F.; et al. A Deficient Immune Response to SARS-CoV-2 in the Nasopharynx Is Associated with Severe COVID-19 Pneumonia. Int. J. Infect. Dis. 2023, 134, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, F.; Martin-Vicente, M.; Rojas-Garcia, R.L.; Castilla-Garcia, L.; Munoz-Gomez, M.J.; Hervas Fernandez, I.; Gonzalez Ventosa, V.; Vidal-Alcantara, E.J.; Cuadros-Gonzalez, J.; Bermejo-Martin, J.F.; et al. High SARS-CoV-2 Viral Load and Low CCL5 Expression Levels in the Upper Respiratory Tract Are Associated with COVID-19 Severity. J. Infect. Dis. 2022, 225, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, B.; Mao, J. The Pathogenesis and Treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1β, IL-6, and TNF Cytokine Triad Is Associated with Post-Acute Sequelae of COVID-19. Cell Reports Med. 2022, 3, 100663. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.; Guo, Y.; Gan, D.; Zhang, C.; Wang, R.; Hua, L.; Zhu, L.; Ma, P.; Shi, J.; et al. Role of TFRC as a Novel Prognostic Biomarker and in Immunotherapy for Pancreatic Carcinoma. Front. Mol. Biosci. 2022, 9, 756895. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, J.S.; ElGhazali, G.; Shafarin, J.; Mohammad, M.G.; Abu-Qiyas, A.; Hamad, M. SARS-CoV-2-Induced Hypomethylation of the Ferritin Heavy Chain (FTH1) Gene Underlies Serum Hyperferritinemia in Severe COVID-19 Patients. Biochem. Biophys. Res. Commun. 2022, 631, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wicik, Z.; Eyileten, C.; Jakubik, D.; Simões, S.N.; Martins, D.C.; Pavão, R.; Siller-Matula, J.M.; Postula, M. ACE2 Interaction Networks in COVID-19: A Physiological Framework for Prediction of Outcome in Patients with Cardiovascular Risk Factors. J. Clin. Med. 2020, 9, 3743. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, D.T.; Boldrini, V.O.; Bonfante, I.L.P.; Duft, R.G.; Mateus, K.; Costa, L.; Chacon-Mikahil, M.P.T.; Teixeira, A.M.; Farias, A.S.; Cavaglieri, C.R. Obesity Increases Gene Expression of Markers Associated With Immunosenescence in Obese Middle-Aged Individuals. Front. Immunol. 2022, 12, 806400. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.J.; van der Made, C.I.; Keur, N.; Setiabudiawan, T.; Röring, R.J.; Damoraki, G.; Dijkstra, H.; Lemmers, H.; Ioannou, S.; Poulakou, G.; et al. Dexamethasone Attenuates Interferon-Related Cytokine Hyperresponsiveness in COVID-19 Patients. Front. Immunol. 2023, 14, 1233318. [Google Scholar] [CrossRef] [PubMed]
- Pinski, A.N.; Steffen, T.L.; Zulu, M.Z.; George, S.L.; Dickson, A.; Tifrea, D.; Maroney, K.J.; Tedeschi, N.; Zhang, Y.; Scheuermann, R.H.; et al. Corticosteroid Treatment in COVID-19 Modulates Host Inflammatory Responses and Transcriptional Signatures of Immune Dysregulation. J. Leukoc. Biol. 2021, 110, 1225–1239. [Google Scholar] [CrossRef]
- Gutiérrez-Pérez, I.A.; Buendía-Roldán, I.; Pérez-Rubio, G.; Chávez-Galán, L.; Hernández-Zenteno, R.d.J.; Aguilar-Duran, H.; Fricke-Galindo, I.; Zaragoza-García, O.; Falfán-Valencia, R.; Guzmán-Guzmán, I.P. Outcome Predictors in COVID-19: An Analysis of Emergent Systemic Inflammation Indices in Mexican Population. Front. Med. 2022, 9, 1000147. [Google Scholar] [CrossRef]

| Characteristics | Moderate (n = 63) | Severe (n = 97) | p-Value |
|---|---|---|---|
| Age, years (mean ± SD) | 53.9 ± 13.5 | 60.9 ± 15.1 | 0.003 |
| Age category | 0.003 | ||
| 50 (79.4%) | 55 (56.7%) | |
| 13 (20.6%) | 42 (43.3%) | |
| Sex | 0.278 | ||
| 43 (68.3%) | 58 (59.8%) | |
| 20 (31.7%) | 39 (40.2%) | |
| Tobacco smoking (%) | 23 (36.5%) | 28 (28.9%) | 0.311 |
| BMI (median (P25–P75)) | 27.9 (25.5–32.4) | 28.5 (25.3–33.4) | 0.407 |
| BMI category | 0.647 | ||
| 13 (21.0%) | 20 (21.3%) | |
| 23 (37.1%) | 33 (35.1%) | |
| 25 (40.3%) | 41 (43.6%) | |
| Hospitalization days (median (P25–P75)) | 16.5 (11–35.5) | 36 (23.7–58) | <0.001 |
| PaO2/FiO2 (mean ± SD) | 289.9 ± 55.5 | 72.1 ± 17.2 | <0.001 |
| IMV (%) | 32 (50.8%) | 97 (100%) | <0.001 |
| IMV days (median (P25–P75)) | 1.5 (0–21.7) | 28.5 (17.7–41) | <0.001 |
| Outcome | 0.055 | ||
| 43 (74.1%) | 51 (58.6%) | |
| 15 (25.9%) | 36 (41.4%) | |
| Type 2 diabetes (%) | 20 (31.7%) | 33 (34.0%) | 0.765 |
| Hypertension (%) | 20 (31.7%) | 39 (40.2%) | 0.278 |
| Chronic respiratory disease (%) | 10 (15.9%) | 7 (7.2%) | 0.083 |
| Steroid treatment (%) | 44 (69.8%) | 89 (92.7%) | <0.001 |
| Gene | OR | CI (95%) | p-Value |
|---|---|---|---|
| Survival | |||
| TFRC | - | - | ns |
| CCL5 | 0.574 | 0.396–0.832 | 0.003 |
| TGFB1 | 0.646 | 0.500–0.835 | 0.001 |
| OAS1 | - | - | ns |
| IRF9 | 0.800 | 0.653–0.979 | 0.030 |
| IFI6 | 0.827 | 0.690–0.991 | 0.039 |
| IL1B | - | - | ns |
| IMV | |||
| TFRC | 0.787 | 0.620–0.999 | 0.049 |
| CCL5 | - | - | ns |
| TGFB1 | - | - | ns |
| OAS1 | 0.642 | 0.516–0.798 | 0.001 |
| IRF9 | 0.581 | 0.427–0.790 | 0.001 |
| IFI6 | 0.544 | 0.391–0.688 | <0.001 |
| IL1B | - | - | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajate-Arenas, M.; Fricke-Galindo, I.; García-Pérez, O.; Domínguez-de-Barros, A.; Pérez-Rubio, G.; Dorta-Guerra, R.; Buendía-Roldán, I.; Chávez-Galán, L.; Lorenzo-Morales, J.; Falfán-Valencia, R.; et al. The Immune Response of OAS1, IRF9, and IFI6 Genes in the Pathogenesis of COVID-19. Int. J. Mol. Sci. 2024, 25, 4632. https://doi.org/10.3390/ijms25094632
Gajate-Arenas M, Fricke-Galindo I, García-Pérez O, Domínguez-de-Barros A, Pérez-Rubio G, Dorta-Guerra R, Buendía-Roldán I, Chávez-Galán L, Lorenzo-Morales J, Falfán-Valencia R, et al. The Immune Response of OAS1, IRF9, and IFI6 Genes in the Pathogenesis of COVID-19. International Journal of Molecular Sciences. 2024; 25(9):4632. https://doi.org/10.3390/ijms25094632
Chicago/Turabian StyleGajate-Arenas, Malena, Ingrid Fricke-Galindo, Omar García-Pérez, Angélica Domínguez-de-Barros, Gloria Pérez-Rubio, Roberto Dorta-Guerra, Ivette Buendía-Roldán, Leslie Chávez-Galán, Jacob Lorenzo-Morales, Ramcés Falfán-Valencia, and et al. 2024. "The Immune Response of OAS1, IRF9, and IFI6 Genes in the Pathogenesis of COVID-19" International Journal of Molecular Sciences 25, no. 9: 4632. https://doi.org/10.3390/ijms25094632








