Testosterone Enhances KV Currents and Airway Smooth Muscle Relaxation Induced by ATP and UTP through P2Y4 Receptors and Adenylyl Cyclase Pathway
Abstract
:1. Introduction
2. Results
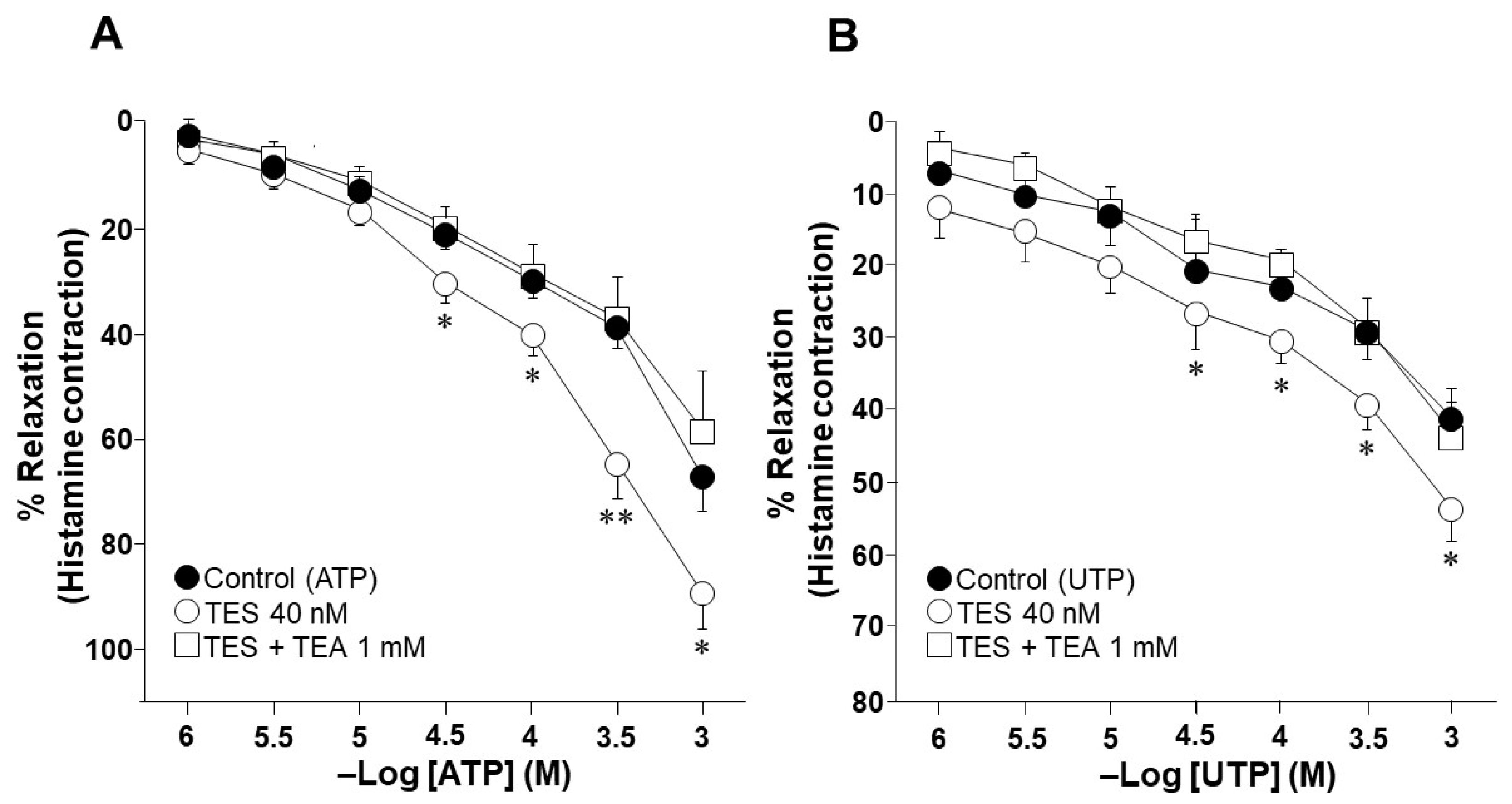
2.1. Testosterone Increases the Relaxation Response to ATP and UTP in Guinea Pig Tracheal Rings
2.2. Testosterone Increases ATP- and UTP-Induced K+ Currents in Airway Myocytes through a Genomic Pathway
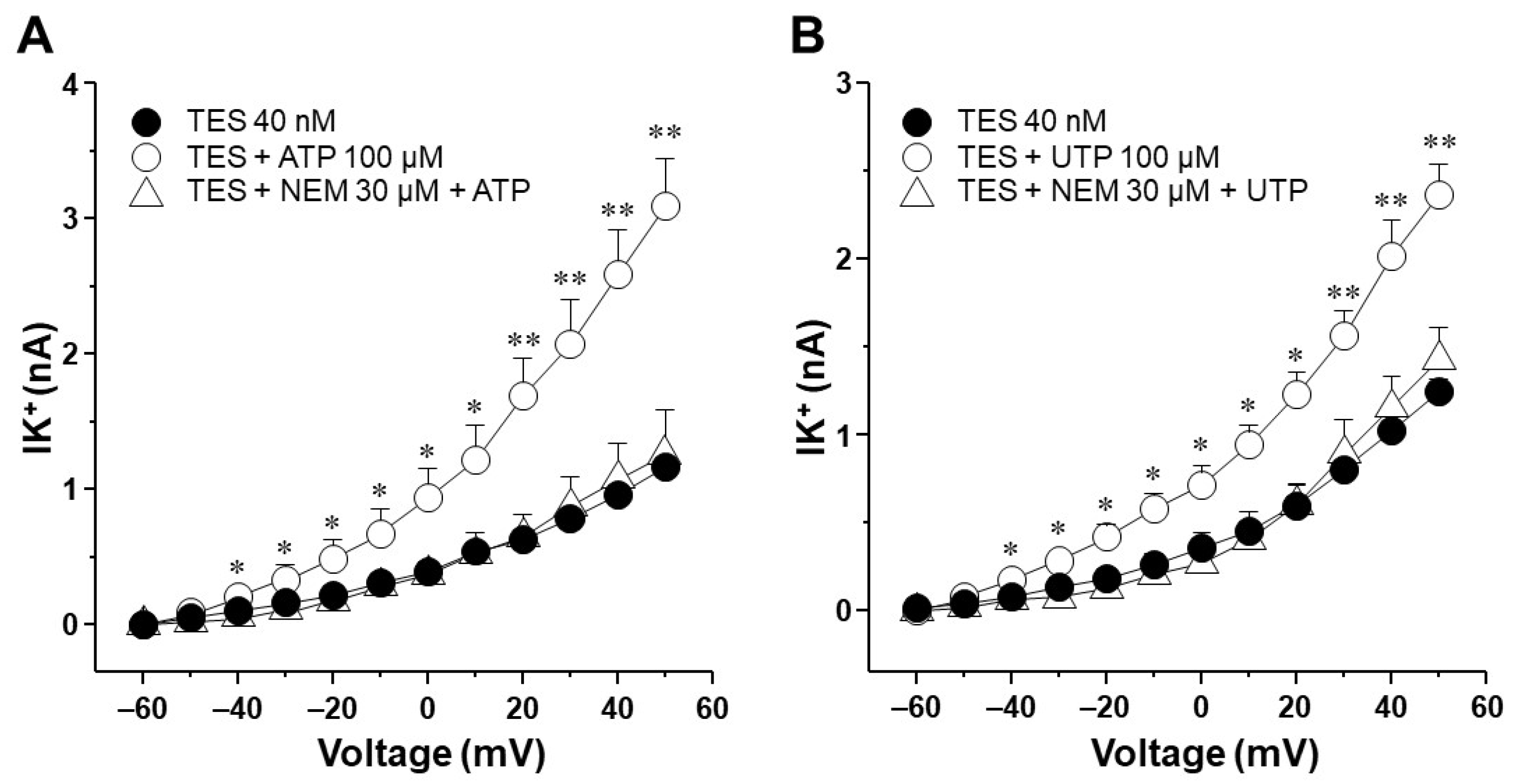
2.3. Voltage-Dependent Delayed Rectifier K+ (KV) Channels Are Responsible for Testosterone Enhancement of ATP- and UTP-Induced K+ Currents
2.4. In Tracheal Myocytes, KV Currents but Not BKCa Currents Are Upregulated by Testosterone Treatment
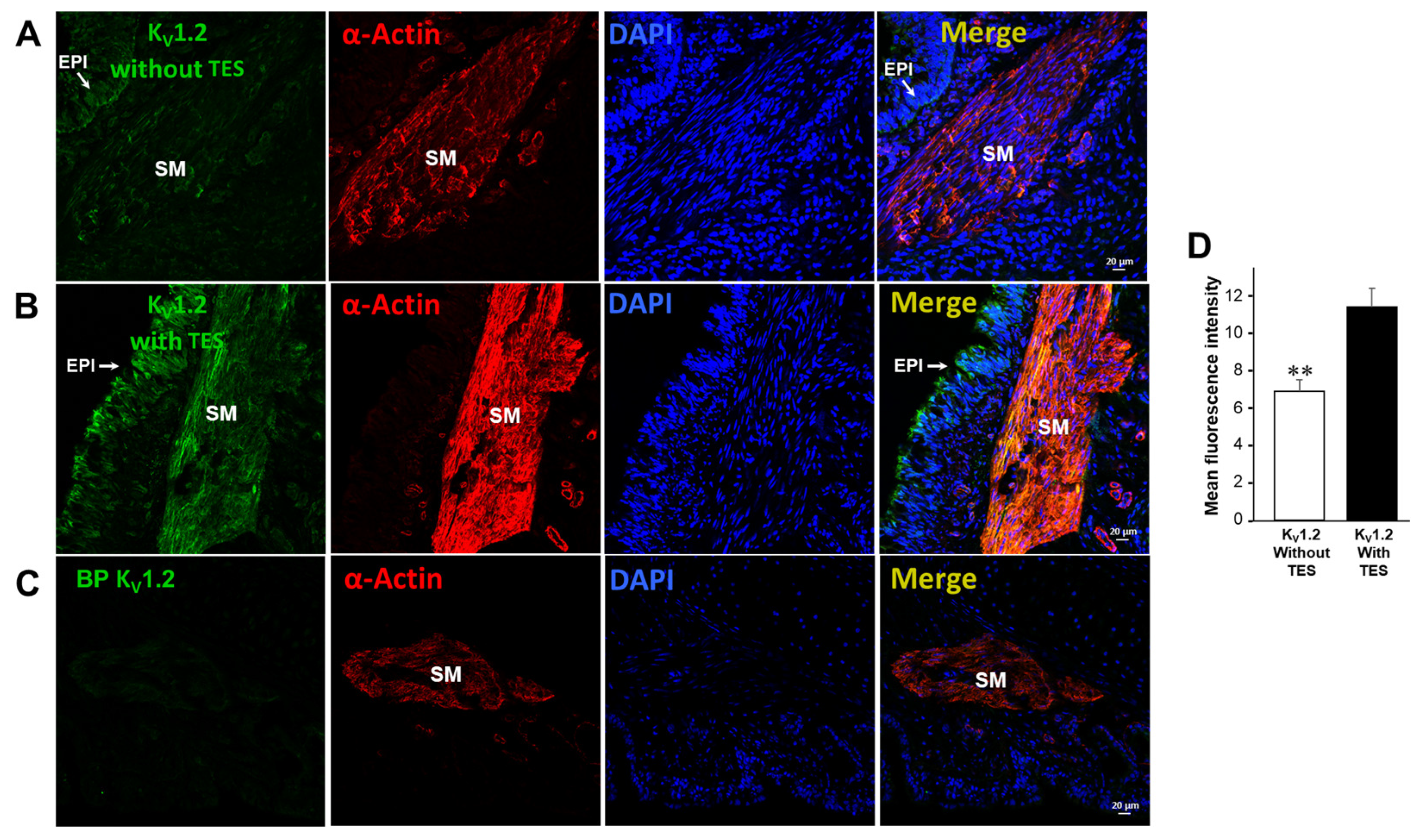
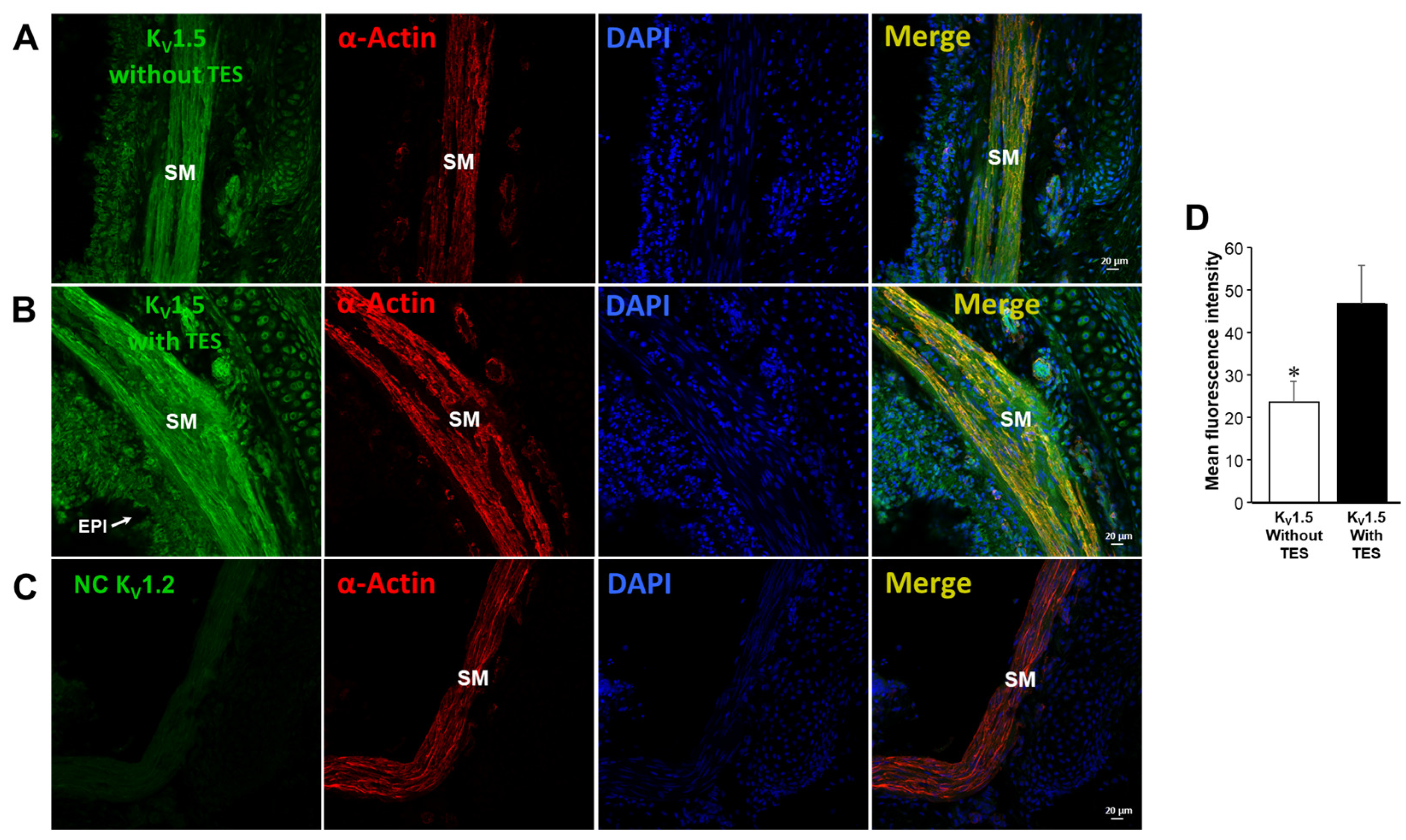
2.5. Testosterone Increases the Expression of KV1.2 and KV1.5
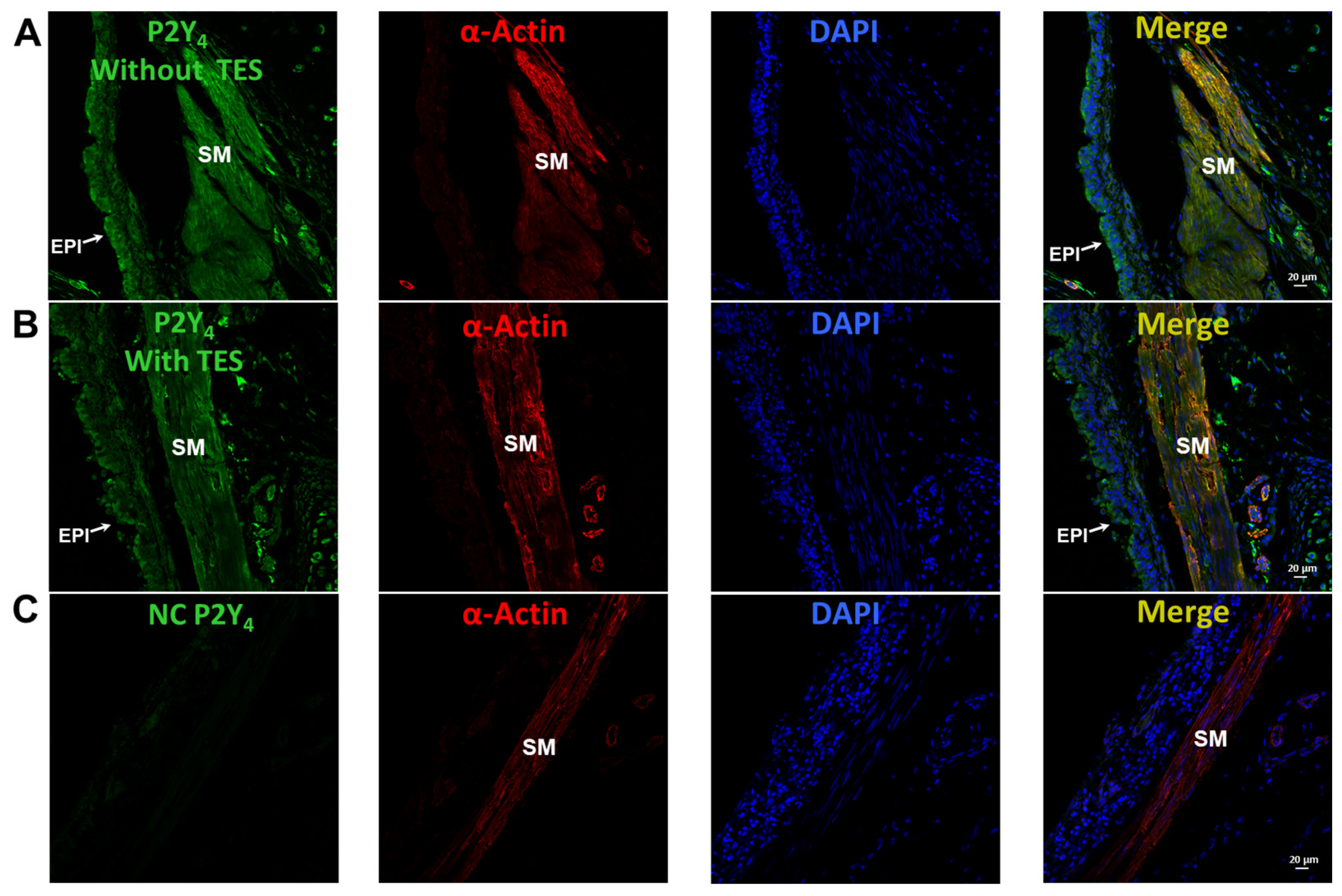
2.6. UTP Stimulates P2Y2 and P2Y4 Receptors in Airway Myocytes, and Testosterone Increases P2Y4-Mediated K+ Currents but Does Not Increase the Expression of P2Y4 Receptors
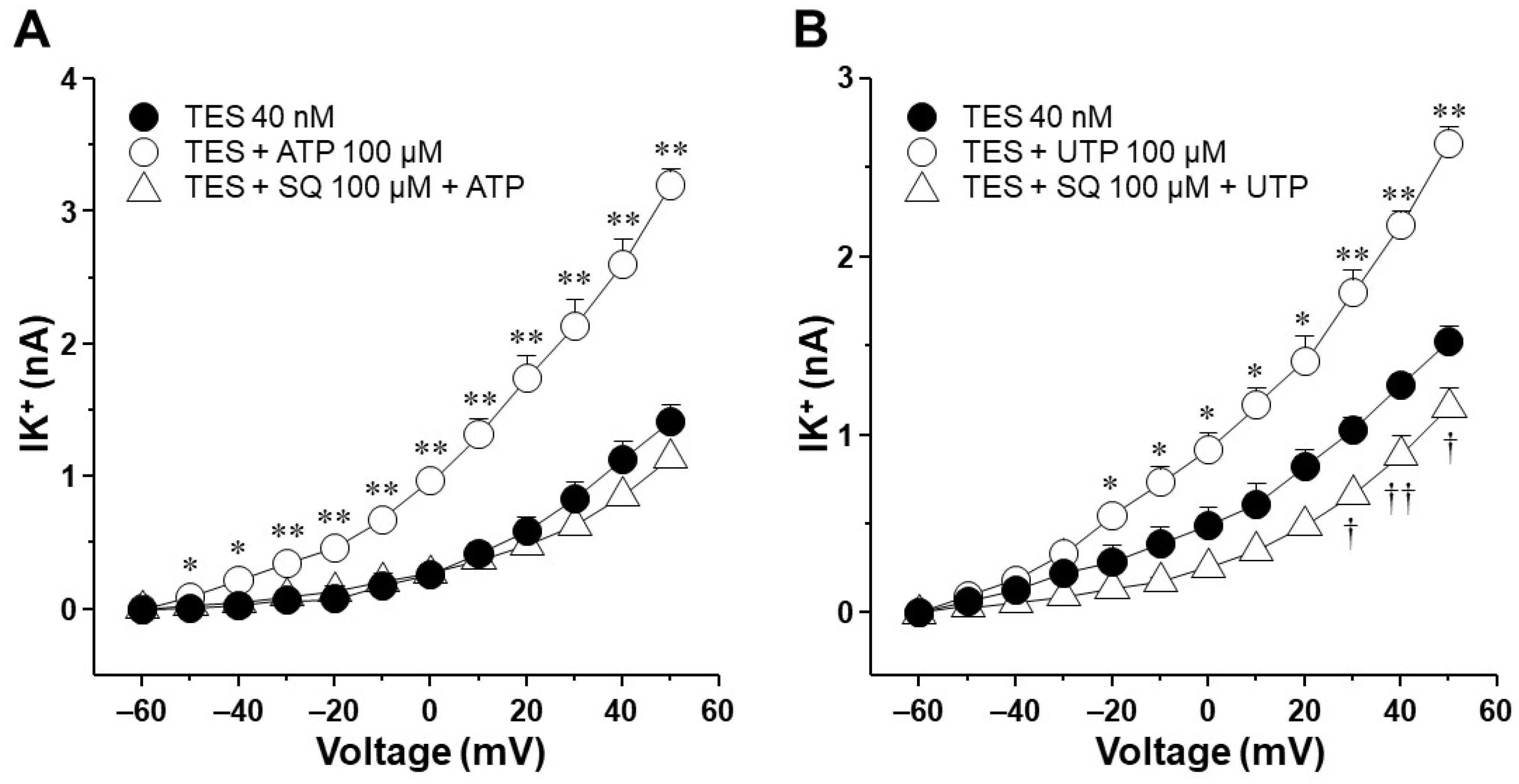
2.7. The Enhancement of ATP- and UTP-Mediated K+ Currents Depends on the GS Protein–Adenylyl Cyclase Axis
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Organ Baths
4.3. Patch-Clamp Studies
4.4. Double Immunofluorescence
4.5. Immunochemistry
4.6. Drugs and Chemicals
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arzola-Martinez, L.; Benavente, R.; Vega, G.; Rios, M.; Fonseca, W.; Rasky, A.J.; Morris, S.; Lukacs, N.W.; Villalon, M.J. Blocking ATP-releasing channels prevents high extracellular ATP levels and airway hyperreactivity in an asthmatic mouse model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L466–L476. [Google Scholar] [CrossRef] [PubMed]
- Chávez, J.; Vargas, M.H.; Martinez-Zuniga, J.; Falfan-Valencia, R.; Ambrocio-Ortiz, E.; Carbajal, V.; Sandoval-Roldan, R. Allergic sensitization increases the amount of extracellular ATP hydrolyzed by guinea pig leukocytes. Purinergic Signal. 2019, 15, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Chávez, J.; Vargas, M.H.; Rebollar-Ayala, D.C.; Diaz-Hernandez, V.; Cruz-Valderrama, J.E.; Flores-Soto, E.; Flores-Garcia, M.; Jimenez-Vargas, N.N.; Barajas-Lopez, C.; Montaño, L.M. Inhibition of extracellular nucleotides hydrolysis intensifies the allergic bronchospasm. A novel protective role of ectonucleotidases. Allergy 2013, 68, 462–471. [Google Scholar] [CrossRef]
- Liu, T.T.; Wang, Y.L.; Zhang, Z.; Jia, L.X.; Zhang, J.; Zheng, S.; Chen, Z.H.; Shen, H.H.; Piao, C.M.; Du, J. Abnormal adenosine metabolism of neutrophils inhibits airway inflammation and remodeling in asthma model induced by Aspergillus fumigatus. BMC Pulm. Med. 2023, 23, 258. [Google Scholar] [CrossRef] [PubMed]
- Pelleg, A.; Xu, F.; Zhuang, J.; Undem, B.; Burnstock, G. DT-0111: A novel drug-candidate for the treatment of COPD and chronic cough. Ther. Adv. Respir. Dis. 2019, 13, 1753466619877960. [Google Scholar] [CrossRef]
- Thompson, R.J.; Sayers, I.; Kuokkanen, K.; Hall, I.P. Purinergic Receptors in the Airways: Potential Therapeutic Targets for Asthma? Front. Allergy 2021, 2, 677677. [Google Scholar] [CrossRef] [PubMed]
- Lommatzsch, M.; Cicko, S.; Muller, T.; Lucattelli, M.; Bratke, K.; Stoll, P.; Grimm, M.; Durk, T.; Zissel, G.; Ferrari, D.; et al. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 181, 928–934. [Google Scholar] [CrossRef]
- Chen, Y.; Corriden, R.; Inoue, Y.; Yip, L.; Hashiguchi, N.; Zinkernagel, A.; Nizet, V.; Insel, P.A.; Junger, W.G. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 2006, 314, 1792–1795. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Braber, S.; Nazary, M.; Givi, M.E.; Nijkamp, F.P.; Folkerts, G. ATP in the pathogenesis of lung emphysema. Eur. J. Pharmacol. 2009, 619, 92–96. [Google Scholar] [CrossRef]
- Nocker, R.E.; Schoonbrood, D.F.; van de Graaf, E.A.; Hack, C.E.; Lutter, R.; Jansen, H.M.; Out, T.A. Interleukin-8 in airway inflammation in patients with asthma and chronic obstructive pulmonary disease. Int. Arch. Allergy Immunol. 1996, 109, 183–191. [Google Scholar] [CrossRef]
- Warringa, R.A.; Mengelers, H.J.; Raaijmakers, J.A.; Bruijnzeel, P.L.; Koenderman, L. Upregulation of formyl-peptide and interleukin-8-induced eosinophil chemotaxis in patients with allergic asthma. J. Allergy Clin. Immunol. 1993, 91, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Vultaggio-Poma, V.; Falzoni, S.; Salvi, G.; Giuliani, A.L.; Di Virgilio, F. Signalling by extracellular nucleotides in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119237. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Hammad, H.; van Nimwegen, M.; Kool, M.; Willart, M.A.; Muskens, F.; Hoogsteden, H.C.; Luttmann, W.; Ferrari, D.; Di Virgilio, F.; et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat. Med. 2007, 13, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Flores-Soto, E.; Carbajal, V.; Reyes-García, J.; Garcia-Hernandez, L.M.; Figueroa, A.; Checa, M.; Barajas-Lopez, C.; Montaño, L.M. In airways ATP refills sarcoplasmic reticulum via P2X smooth muscle receptors and induces contraction through P2Y epithelial receptors. Pflug. Arch. 2011, 461, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.Y.; Kim, K.C.; Lee, W.J.; Jo, M.J.; Park, K.H.; Dalby, R.; Ko, K.H. Inhaled ATP causes mucin release from goblet cells of intact rats. Exp. Lung Res. 2000, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Su, X.; Huang, G.; Xin, X.F.; Cao, E.H.; Shi, Y.; Song, Y. Adenosine Triphosphate Promotes Allergen-Induced Airway Inflammation and Th17 Cell Polarization in Neutrophilic Asthma. J. Immunol. Res. 2017, 2017, 5358647. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Dichmann, S.; Ferrari, D.; Di Virgilio, F.; la Sala, A.; Girolomoni, G.; Panther, E.; Norgauer, J. Nucleotides induce chemotaxis and actin polymerization in immature but not mature human dendritic cells via activation of pertussis toxin-sensitive P2Y receptors. Blood 2002, 100, 925–932. [Google Scholar] [CrossRef] [PubMed]
- McFadden, E.R., Jr.; Lyons, H.A. Arterial-blood gas tension in asthma. N. Engl. J. Med. 1968, 278, 1027–1032. [Google Scholar] [CrossRef]
- Tai, E.; Read, J. Blood-gas tensions in bronchial asthma. Lancet 1967, 1, 644–646. [Google Scholar] [CrossRef]
- Eckert, D.J.; Catcheside, P.G.; Smith, J.H.; Frith, P.A.; McEvoy, R.D. Hypoxia suppresses symptom perception in asthma. Am. J. Respir. Crit. Care Med. 2004, 169, 1224–1230. [Google Scholar] [CrossRef]
- Okada, S.F.; Ribeiro, C.M.; Sesma, J.I.; Seminario-Vidal, L.; Abdullah, L.H.; van Heusden, C.; Lazarowski, E.R.; Boucher, R.C. Inflammation promotes airway epithelial ATP release via calcium-dependent vesicular pathways. Am. J. Respir. Cell Mol. Biol. 2013, 49, 814–820. [Google Scholar] [CrossRef] [PubMed]
- De Ita, M.; Vargas, M.H.; Carbajal, V.; Ortiz-Quintero, B.; Lopez-Lopez, C.; Miranda-Morales, M.; Barajas-Lopez, C.; Montaño, L.M. ATP releases ATP or other nucleotides from human peripheral blood leukocytes through purinergic P2 receptors. Life Sci. 2016, 145, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xia, Q.; Feng, X.; Cao, F.; Yu, H.; Song, Y.; Ni, X. Effect of P2X4R on airway inflammation and airway remodeling in allergic airway challenge in mice. Mol. Med. Rep. 2016, 13, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Oguma, T.; Ito, S.; Kondo, M.; Makino, Y.; Shimokata, K.; Honjo, H.; Kamiya, K.; Kume, H. Roles of P2X receptors and Ca2+ sensitization in extracellular adenosine triphosphate-induced hyperresponsiveness in airway smooth muscle. Clin. Exp. Allergy 2007, 37, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Bergner, A.; Sanderson, M.J. ATP stimulates Ca2+ oscillations and contraction in airway smooth muscle cells of mouse lung slices. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L1271–L1279. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, V.; Martin, J.G.; Maghni, K.; Ferraro, P.; Michoud, M.C. The effects of extracellular purines and pyrimidines on human airway smooth muscle cells. J. Pharmacol. Exp. Ther. 2005, 315, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Montaño, L.M.; Cruz-Valderrama, J.E.; Figueroa, A.; Flores-Soto, E.; García-Hernández, L.M.; Carbajal, V.; Segura, P.; Mendez, C.; Díaz, V.; Barajas-Lopez, C. Characterization of P2Y receptors mediating ATP induced relaxation in guinea pig airway smooth muscle: Involvement of prostaglandins and K+ channels. Pflug. Arch. 2011, 462, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Bonvini, S.J.; Birrell, M.A.; Dubuis, E.; Adcock, J.J.; Wortley, M.A.; Flajolet, P.; Bradding, P.; Belvisi, M.G. Novel airway smooth muscle-mast cell interactions and a role for the TRPV4-ATP axis in non-atopic asthma. Eur. Respir. J. 2020, 56, 1901458. [Google Scholar] [CrossRef]
- Ai, Y.; Wang, H.; Liu, L.; Qi, Y.; Tang, S.; Tang, J.; Chen, N. Purine and purinergic receptors in health and disease. MedComm 2023, 4, e359. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, N.; Illes, P.; Di Virgilio, F.; Ulrich, H.; Semyanov, A.; Verkhratsky, A.; Sperlagh, B.; Yu, S.G.; Huang, C.; et al. From purines to purinergic signalling: Molecular functions and human diseases. Signal Transduct. Target. Ther. 2021, 6, 162. [Google Scholar] [CrossRef]
- Mounkaila, B.; Marthan, R.; Roux, E. Biphasic effect of extracellular ATP on human and rat airways is due to multiple P2 purinoceptor activation. Respir. Res. 2005, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. The journey to establish purinergic signalling in the gut. Neurogastroenterol. Motil. 2008, 20 (Suppl. 1), 8–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhong, H.; Vollmer, C.; Nurse, C.A. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J. Physiol. 2000, 525 Pt 1, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Bennett, W.C.; Jania, C.M.; Chason, K.D.; German, Z.; Adouli, J.; Budney, S.D.; Oby, B.T.; van Heusden, C.; Lazarowski, E.R.; et al. Identification of an ATP/P2X7/mast cell pathway mediating ozone-induced bronchial hyperresponsiveness. JCI Insight 2021, 6, e140207. [Google Scholar] [CrossRef] [PubMed]
- Schulman, E.S.; Glaum, M.C.; Post, T.; Wang, Y.; Raible, D.G.; Mohanty, J.; Butterfield, J.H.; Pelleg, A. ATP modulates anti-IgE-induced release of histamine from human lung mast cells. Am. J. Respir. Cell Mol. Biol. 1999, 20, 530–537. [Google Scholar] [CrossRef]
- Aksoy, M.O.; Kelsen, S.G. Relaxation of rabbit tracheal smooth muscle by adenine nucleotides: Mediation by P2-purinoceptors. Am. J. Respir. Cell Mol. Biol. 1994, 10, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Fortner, C.N.; Breyer, R.M.; Paul, R.J. EP2 receptors mediate airway relaxation to substance P, ATP, and PGE2. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L469–L474. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Kotlikoff, M.I. Activation of KCa channels in airway smooth muscle cells by endogenous protein kinase A. Am. J. Physiol. 1996, 271, L100–L105. [Google Scholar] [CrossRef] [PubMed]
- Campos-Bedolla, P.; Vargas, M.H.; Segura, P.; Carbajal, V.; Calixto, E.; Figueroa, A.; Flores-Soto, E.; Barajas-López, C.; Mendoza-Patiño, N.; Montaño, L.M. Airway smooth muscle relaxation induced by 5-HT2A receptors: Role of Na+/K+-ATPase pump and Ca2+-activated K+ channels. Life Sci. 2008, 83, 438–446. [Google Scholar] [CrossRef]
- Carbajal-García, A.; Reyes-García, J.; Casas-Hernández, M.F.; Flores-Soto, E.; Díaz-Hernández, V.; Solis-Chagoyán, H.; Sommer, B.; Montaño, L.M. Testosterone augments β2 adrenergic receptor genomic transcription increasing salbutamol relaxation in airway smooth muscle. Mol. Cell. Endocrinol. 2020, 510, 110801. [Google Scholar] [CrossRef]
- Mason, H.S.; Latten, M.J.; Godoy, L.D.; Horowitz, B.; Kenyon, J.L. Modulation of Kv1.5 currents by protein kinase A, tyrosine kinase, and protein tyrosine phosphatase requires an intact cytoskeleton. Mol. Pharmacol. 2002, 61, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Laffont, S.; Blanquart, E.; Guery, J.C. Sex Differences in Asthma: A Key Role of Androgen-Signaling in Group 2 Innate Lymphoid Cells. Front. Immunol. 2017, 8, 1069. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, J.G.; Guo, W.; Andersen, K.J.; Baker, S.E.; Wiggins, C.C.; Shepherd, J.R.A.; Carter, R.E.; Welch, B.T.; Joyner, M.J.; Dominelli, P.B. Sex differences in paediatric airway anatomy. Exp. Physiol. 2020, 105, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Sathish, V.; Martin, Y.N.; Prakash, Y.S. Sex steroid signaling: Implications for lung diseases. Pharmacol. Ther. 2015, 150, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Montaño, L.M.; Flores-Soto, E.; Sommer, B.; Solis-Chagoyan, H.; Perusquia, M. Androgens are effective bronchodilators with anti-inflammatory properties: A potential alternative for asthma therapy. Steroids 2020, 153, 108509. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Al-Azzawi, F. Mechanism of androgen receptor action. Maturitas 2009, 63, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Herald, A.K.; Alves-Lopes, R.; Montezano, A.C.; Ahmed, S.F.; Touyz, R.M. Genomic and non-genomic effects of androgens in the cardiovascular system: Clinical implications. Clin. Sci. 2017, 131, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Kouloumenta, V.; Hatziefthimiou, A.; Paraskeva, E.; Gourgoulianis, K.; Molyvdas, P.A. Non-genomic effect of testosterone on airway smooth muscle. Br. J. Pharmacol. 2006, 149, 1083–1091. [Google Scholar] [CrossRef]
- Bordallo, J.; de Boto, M.J.; Meana, C.; Velasco, L.; Bordallo, C.; Suarez, L.; Cantabrana, B.; Sanchez, M. Modulatory role of endogenous androgens on airway smooth muscle tone in isolated guinea-pig and bovine trachea; involvement of β2 adrenoceptors, the polyamine system and external calcium. Eur. J. Pharmacol. 2008, 601, 154–162. [Google Scholar] [CrossRef]
- Foster, P.S.; Goldie, R.G.; Paterson, J.W. Effect of steroids on β-adrenoceptor-mediated relaxation of pig bronchus. Br. J. Pharmacol. 1983, 78, 441–445. [Google Scholar] [CrossRef]
- Montaño, L.M.; Espinoza, J.; Flores-Soto, E.; Chavez, J.; Perusquia, M. Androgens are bronchoactive drugs that act by relaxing airway smooth muscle and preventing bronchospasm. J. Endocrinol. 2014, 222, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Perusquia, M.; Flores-Soto, E.; Sommer, B.; Campuzano-Gonzalez, E.; Martinez-Villa, I.; Martinez-Banderas, A.I.; Montaño, L.M. Testosterone-induced relaxation involves L-type and store-operated Ca2+ channels blockade, and PGE2 in guinea pig airway smooth muscle. Pflug. Arch. 2015, 467, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sanderson, M.J. Airway smooth muscle relaxation results from a reduction in the frequency of Ca2+ oscillations induced by a cAMP-mediated inhibition of the IP3 receptor. Respir. Res. 2006, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Garcia, J.; Flores-Soto, E.; Carbajal-Garcia, A.; Sommer, B.; Montano, L.M. Maintenance of intracellular Ca2+ basal concentration in airway smooth muscle (Review). Int. J. Mol. Med. 2018, 42, 2998–3008. [Google Scholar] [CrossRef] [PubMed]
- Flores-Soto, E.; Reyes-García, J.; Carbajal-García, A.; Campuzano-Gonzalez, E.; Perusquia, M.; Sommer, B.; Montaño, L.M. Sex steroids effects on guinea pig airway smooth muscle tone and intracellular Ca2+ basal levels. Mol. Cell. Endocrinol. 2017, 439, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Adda, S.; Fleischmann, B.K.; Freedman, B.D.; Yu, M.; Hay, D.W.; Kotlikoff, M.I. Expression and function of voltage-dependent potassium channel genes in human airway smooth muscle. J. Biol. Chem. 1996, 271, 13239–13243. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yin, H.; Dong, Y.; Wang, X.; Liu, Y.; Wang, K. A Novel Role of Uricosuric Agent Benzbromarone in BK Channel Activation and Reduction of Airway Smooth Muscle Contraction. Mol. Pharmacol. 2023, 103, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Reyes-García, J.; Díaz-Hernández, V.; Carbajal-García, A.; Casas-Hernández, M.F.; Sommer, B.; Montaño, L.M. Theophylline-induced relaxation is enhanced after testosterone treatment via increased KV1.2 and KV1.5 protein expression in guinea pig tracheal smooth muscle. Int. J. Mol. Sci. 2023, 24, 5884. [Google Scholar] [CrossRef] [PubMed]
- Van Crombruggen, K.; Van Nassauw, L.; Timmermans, J.P.; Lefebvre, R.A. Inhibitory purinergic P2 receptor characterisation in rat distal colon. Neuropharmacology 2007, 53, 257–271. [Google Scholar] [CrossRef]
- Wildman, S.S.; Unwin, R.J.; King, B.F. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br. J. Pharmacol. 2003, 140, 1177–1186. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Costanzi, S.; Joshi, B.V.; Besada, P.; Shin, D.H.; Ko, H.; Ivanov, A.A.; Mamedova, L. Agonists and antagonists for P2 receptors. Novartis Found. Symp. 2006, 276, 58–68; discussion 68–72, 107–112, 275–281. [Google Scholar] [CrossRef] [PubMed]
- von Kugelgen, I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol. Ther. 2006, 110, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Attah, I.; Al-Hroub, H.; Namasivayam, V.; Muller, C.E. Discovery of P2Y2 Receptor Antagonist Scaffolds through Virtual High-Throughput Screening. J. Chem. Inf. Model. 2022, 62, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Kamikawa, Y.; Shimo, Y. Mediation of prostaglandin E2 in the biphasic response to ATP of the isolated tracheal muscle of guinea-pigs. J. Pharm. Pharmacol. 1976, 28, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Metzger, W.J.; Olanrewaju, H.A.; Mustafa, S.J. Adenosine receptor-mediated relaxation of rabbit airway smooth muscle: A role for nitric oxide. Am. J. Physiol. 1997, 273, L581–L587. [Google Scholar] [CrossRef] [PubMed]
- Breschi, M.C.; Blandizzi, C.; Fogli, S.; Martinelli, C.; Adinolfi, B.; Calderone, V.; Camici, M.; Martinotti, E.; Nieri, P. In vivo adenosine A2B receptor desensitization in guinea-pig airway smooth muscle: Implications for asthma. Eur. J. Pharmacol. 2007, 575, 149–157. [Google Scholar] [CrossRef]
- Piper, A.S.; Hollingsworth, M. ATP and β, γ-methylene ATP produce relaxation of guinea-pig isolated trachealis muscle via actions at P1 purinoceptors. Eur. J. Pharmacol. 1996, 307, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-García, A.; Reyes-García, J.; Montaño, L.M. Androgen Effects on the Adrenergic System of the Vascular, Airway, and Cardiac Myocytes and Their Relevance in Pathological Processes. Int. J. Endocrinol. 2020, 2020, 8849641. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.P.; Tomasic, M.; Kotlikoff, M.I. Delayed rectifier potassium channels in canine and porcine airway smooth muscle cells. J. Physiol. 1992, 447, 329–350. [Google Scholar] [CrossRef]
- Masuda, K.; Takanari, H.; Morishima, M.; Ma, F.; Wang, Y.; Takahashi, N.; Ono, K. Testosterone-mediated upregulation of delayed rectifier potassium channel in cardiomyocytes causes abbreviation of QT intervals in rats. J. Physiol. Sci. 2018, 68, 759–767. [Google Scholar] [CrossRef]
- Cairrao, E.; Alvarez, E.; Santos-Silva, A.J.; Verde, I. Potassium channels are involved in testosterone-induced vasorelaxation of human umbilical artery. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 376, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Deenadayalu, V.P.; White, R.E.; Stallone, J.N.; Gao, X.; Garcia, A.J. Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1720–H1727. [Google Scholar] [CrossRef]
- Burnstock, G. Purine and purinergic receptors. Brain Neurosci. Adv. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Communi, D.; Motte, S.; Boeynaems, J.M.; Pirotton, S. Pharmacological characterization of the human P2Y4 receptor. Eur. J. Pharmacol. 1996, 317, 383–389. [Google Scholar] [CrossRef]
- Nicholas, R.A.; Watt, W.C.; Lazarowski, E.R.; Li, Q.; Harden, K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: Identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol. Pharmacol. 1996, 50, 224–229. [Google Scholar]
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Knight, G.E.; Fumagalli, M.; Gachet, C.; Jacobson, K.A.; et al. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006, 58, 281–341. [Google Scholar] [CrossRef] [PubMed]
- Filippov, A.K.; Simon, J.; Barnard, E.A.; Brown, D.A. Coupling of the nucleotide P2Y4 receptor to neuronal ion channels. Br. J. Pharmacol. 2003, 138, 400–406. [Google Scholar] [CrossRef]
- Henriquez, M.; Fonseca, M.; Perez-Zoghbi, J.F. Purinergic receptor stimulation induces calcium oscillations and smooth muscle contraction in small pulmonary veins. J. Physiol. 2018, 596, 2491–2506. [Google Scholar] [CrossRef]
- Tanaka, H.; Watanabe, K.; Tamaru, N.; Yoshida, M. Arachidonic acid metabolites and glucocorticoid regulatory mechanism in cultured porcine tracheal smooth muscle cells. Lung 1995, 173, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Erb, L.; Weisman, G.A. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 789–803. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future. Bioessays 2012, 34, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Historical review: ATP as a neurotransmitter. Trends Pharmacol. Sci. 2006, 27, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, K.R.; Stokes, L.; Prince, L.R.; Marriott, H.M.; Meis, S.; Kassack, M.U.; Bingle, C.D.; Sabroe, I.; Surprenant, A.; Whyte, M.K. Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor. J. Immunol. 2007, 179, 8544–8553. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A.; Musset, B.; Limberg, S.H.; Renigunta, V.; Sus, R.; Dalpke, A.H.; Heeg, K.M.; Robaye, B.; Hanley, P.J. “Host tissue damage” signal ATP promotes non-directional migration and negatively regulates toll-like receptor signaling in human monocytes. J. Biol. Chem. 2005, 280, 32459–32467. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Meichle, S.; Kim, U.S.; Wong, T.; Moody, M.W. P2Y11, a purinergic receptor acting via cAMP, mediates secretion by pancreatic duct epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G795–G804. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Morielli, A.D.; Peralta, E.G. Molecular basis of cardiac potassium channel stimulation by protein kinase A. Proc. Natl. Acad. Sci. USA 1994, 91, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Jonas, E.A.; Kaczmarek, L.K. Regulation of potassium channels by protein kinases. Curr. Opin. Neurobiol. 1996, 6, 318–323. [Google Scholar] [CrossRef]
- Chiba, Y.; Sakai, H.; Misawa, M. Possible involvement of Gi3 protein in augmented contraction of bronchial smooth muscle from antigen-induced airway hyperresponsive rats. Biochem. Pharmacol. 2001, 61, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Abebe, W.; Agrawal, D.K. Identification of guanine nucleotide binding regulatory proteins in bovine tracheal smooth muscle. Mol. Cell. Biochem. 1996, 154, 179–184. [Google Scholar] [CrossRef]
- Barnes, P.J. Pharmacology of airway smooth muscle. Am. J. Respir. Crit. Care Med. 1998, 158, S123–S132. [Google Scholar] [CrossRef]
- Thomas, J.M.; Hoffman, B.B. Isoform-specific sensitization of adenylyl cyclase activity by prior activation of inhibitory receptors: Role of beta gamma subunits in transducing enhanced activity of the type VI isoform. Mol. Pharmacol. 1996, 49, 907–914. [Google Scholar]
- Gordon, J.L. Extracellular ATP: Effects, sources and fate. Biochem. J. 1986, 233, 309–319. [Google Scholar] [CrossRef]
- Kowal, K.; Pampuch, A.; Kowal-Bielecka, O.; DuBuske, L.M.; Bodzenta-Lukaszyk, A. Platelet activation in allergic asthma patients during allergen challenge with Dermatophagoides pteronyssinus. Clin. Exp. Allergy 2006, 36, 426–432. [Google Scholar] [CrossRef]
- Yoshida, A.; Ohba, M.; Wu, X.; Sasano, T.; Nakamura, M.; Endo, Y. Accumulation of platelets in the lung and liver and their degranulation following antigen-challenge in sensitized mice. Br. J. Pharmacol. 2002, 137, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Bours, M.J.; Swennen, E.L.; Di Virgilio, F.; Cronstein, B.N.; Dagnelie, P.C. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 2006, 112, 358–404. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Footitt, J.; Marczynski, M.; Radicioni, G.; Cross, M.T.; Finney, L.J.; Trujillo-Torralbo, M.B.; Calderazzo, M.; Zhu, J.; Aniscenko, J.; et al. Airway mucins promote immunopathology in virus-exacerbated chronic obstructive pulmonary disease. J. Clin. Investig. 2022, 132, 20220305364. [Google Scholar] [CrossRef] [PubMed]
- Gosens, R.; Zaagsma, J.; Meurs, H.; Halayko, A.J. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir. Res. 2006, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.J. The Action of Histamine on the Respiratory Tract in Normal and Asthmatic Subjects. J. Clin. Investig. 1946, 25, 785–791. [Google Scholar] [CrossRef]
- Gelfand, E.W. Role of histamine in the pathophysiology of asthma: Immunomodulatory and anti-inflammatory activities of H1-receptor antagonists. Am. J. Med. 2002, 113 (Suppl. 9A), 2S–7S. [Google Scholar] [CrossRef]
- Hallstrand, T.S.; Henderson, W.R., Jr. An update on the role of leukotrienes in asthma. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 60–66. [Google Scholar] [CrossRef]
- Chen, L.C.; Tseng, H.M.; Kuo, M.L.; Chiu, C.Y.; Liao, S.L.; Su, K.W.; Tsai, M.H.; Hua, M.C.; Lai, S.H.; Yao, T.C.; et al. Levels of 15-HETE and TXB2 in exhaled breath condensates as markers for diagnosis of childhood asthma and its therapeutic outcome. Pediatr. Allergy Immunol. 2021, 32, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, G.; Gallelli, L.; Vatrella, A.; Grembiale, R.D.; Maselli, R.; De Sarro, G.B.; Marsico, S.A. Potential role of potassium channel openers in the treatment of asthma and chronic obstructive pulmonary disease. Life Sci. 2002, 70, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Radaeli, A.; Mancuso, S.; Polosa, R. The potential therapeutic role of potassium channel modulators in asthma and chronic obstructive pulmonary disease. J. Biol. Regul. Homeost. Agents 2010, 24, 123–130. [Google Scholar] [PubMed]
- Nielsen-Kudsk, J.E. Potassium channel modulation: A new drug principle for regulation of smooth muscle contractility. Studies on isolated airways and arteries. Dan. Med. Bull. 1996, 43, 429–447. [Google Scholar]
- Small, R.C.; Berry, J.L.; Burka, J.F.; Cook, S.J.; Foster, R.W.; Green, K.A.; Murray, M.A. Potassium channel activators and bronchial asthma. Clin. Exp. Allergy 1992, 22, 11–18. [Google Scholar] [CrossRef]
- Small, R.C.; Berry, J.L.; Foster, R.W. Potassium channel opening drugs and the airways. Braz. J. Med. Biol. Res. 1992, 25, 983–998. [Google Scholar] [PubMed]
- Snetkov, V.A.; Ward, J.P. Ion currents in smooth muscle cells from human small bronchioles: Presence of an inward rectifier K+ current and three types of large conductance K+ channel. Exp. Physiol. 1999, 84, 835–846. [Google Scholar] [CrossRef]
- Morley, J. K+ channel openers and suppression of airway hyperreactivity. Trends Pharmacol. Sci. 1994, 15, 463–468. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbajal-García, A.; Reyes-García, J.; Díaz-Hernández, V.; Casas-Hernández, M.F.; Flores-Murrieta, F.J.; Montaño, L.M. Testosterone Enhances KV Currents and Airway Smooth Muscle Relaxation Induced by ATP and UTP through P2Y4 Receptors and Adenylyl Cyclase Pathway. Int. J. Mol. Sci. 2024, 25, 4652. https://doi.org/10.3390/ijms25094652
Carbajal-García A, Reyes-García J, Díaz-Hernández V, Casas-Hernández MF, Flores-Murrieta FJ, Montaño LM. Testosterone Enhances KV Currents and Airway Smooth Muscle Relaxation Induced by ATP and UTP through P2Y4 Receptors and Adenylyl Cyclase Pathway. International Journal of Molecular Sciences. 2024; 25(9):4652. https://doi.org/10.3390/ijms25094652
Chicago/Turabian StyleCarbajal-García, Abril, Jorge Reyes-García, Verónica Díaz-Hernández, María F. Casas-Hernández, Francisco Javier Flores-Murrieta, and Luis M. Montaño. 2024. "Testosterone Enhances KV Currents and Airway Smooth Muscle Relaxation Induced by ATP and UTP through P2Y4 Receptors and Adenylyl Cyclase Pathway" International Journal of Molecular Sciences 25, no. 9: 4652. https://doi.org/10.3390/ijms25094652




