Expression of Chemokines, MIP-1alpha and RANTES in Caprine Lentiviral Infection and Their Influence on Viral Replication
Abstract
:Introduction
Materials and Methods
Inoculation of goats with caprine arthritis encephalitis virus
Isolation and preparation of peripheral blood mononuclear cells (PBMC)
Goat synovial membrane cell culture & CAEV cultivation
RNA isolation & cDNA synthesis
Construction of goat MIP-1α cDNA clone
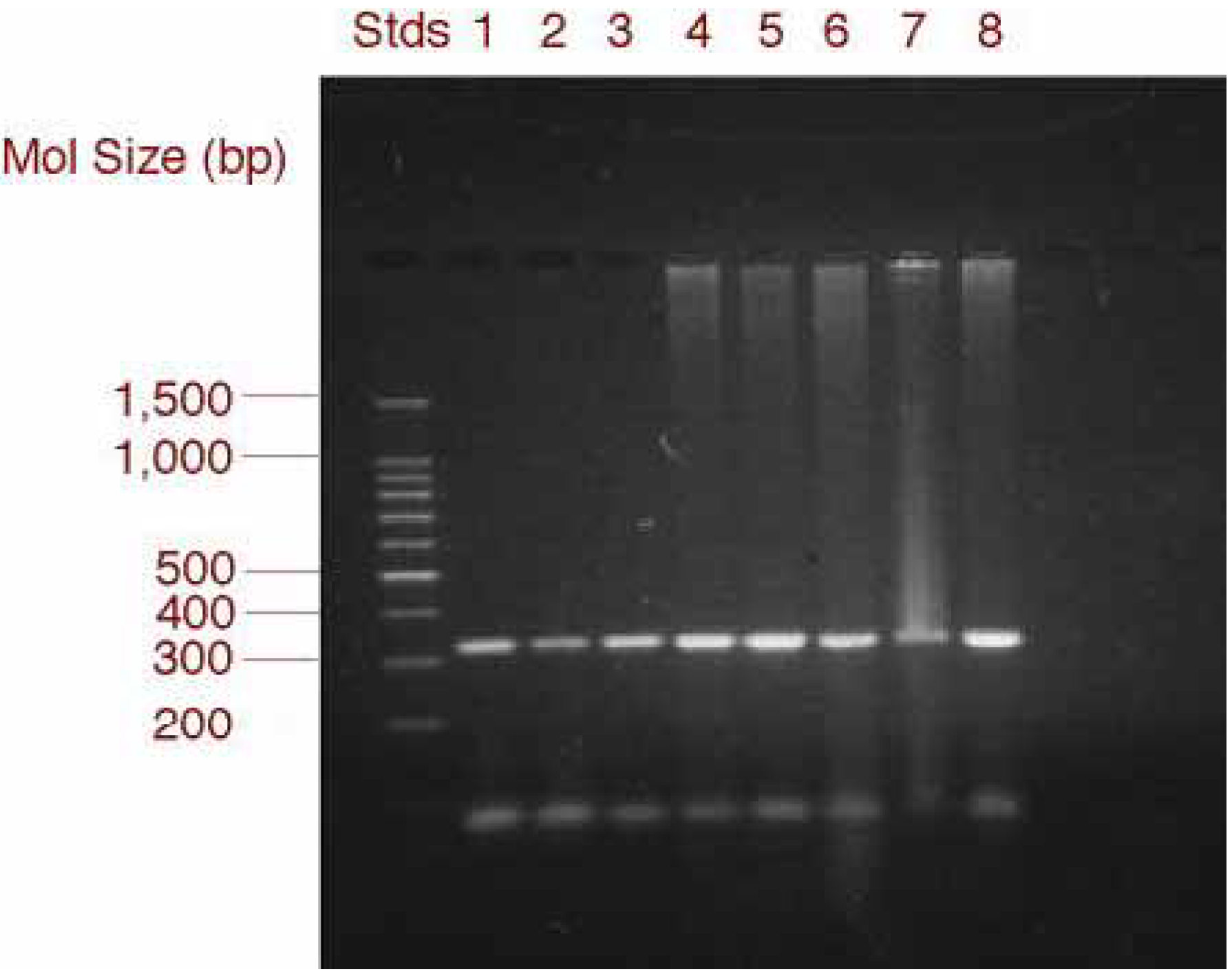
RT-PCR for MIP-1α
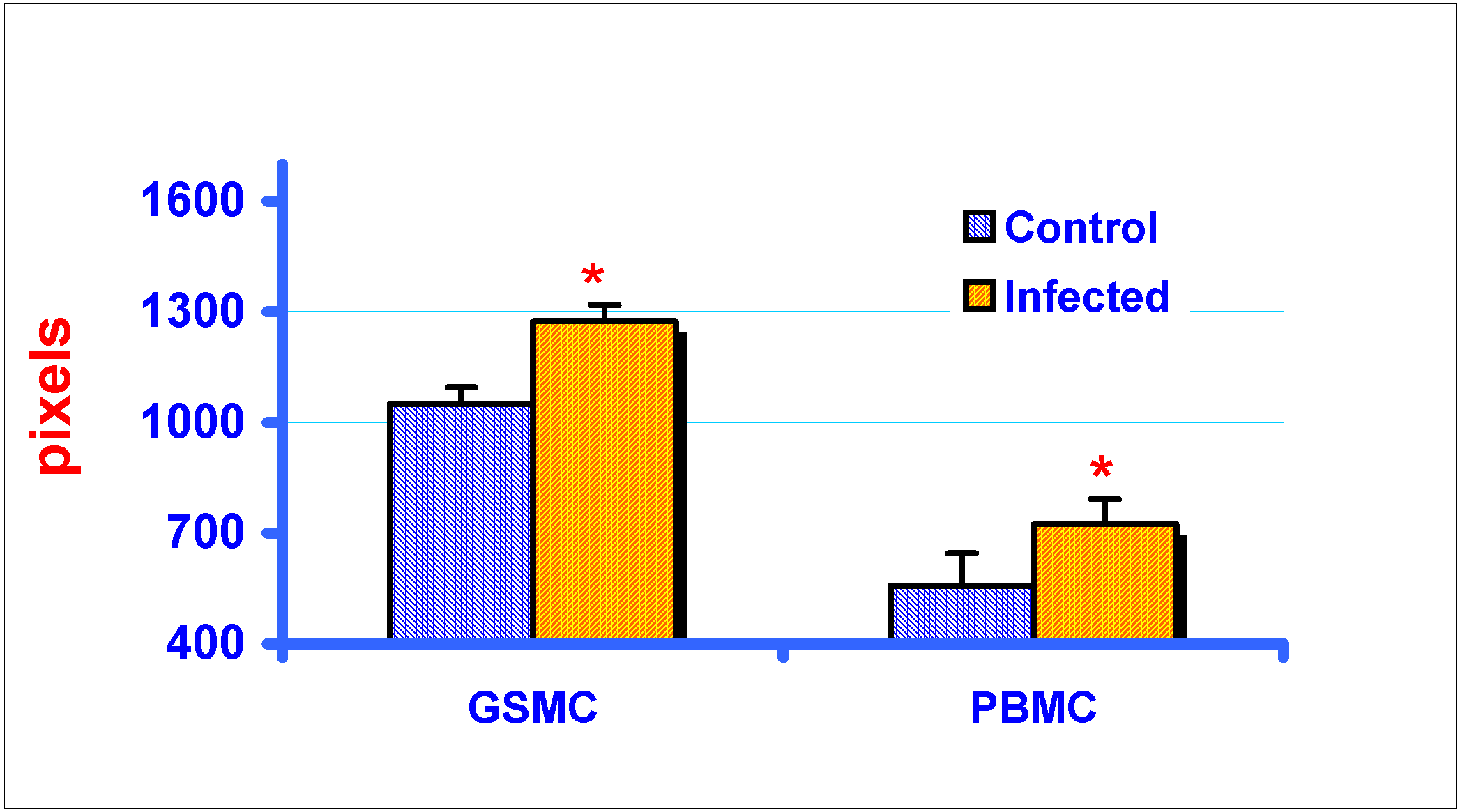
Densitometric analysis of RT-PCR products
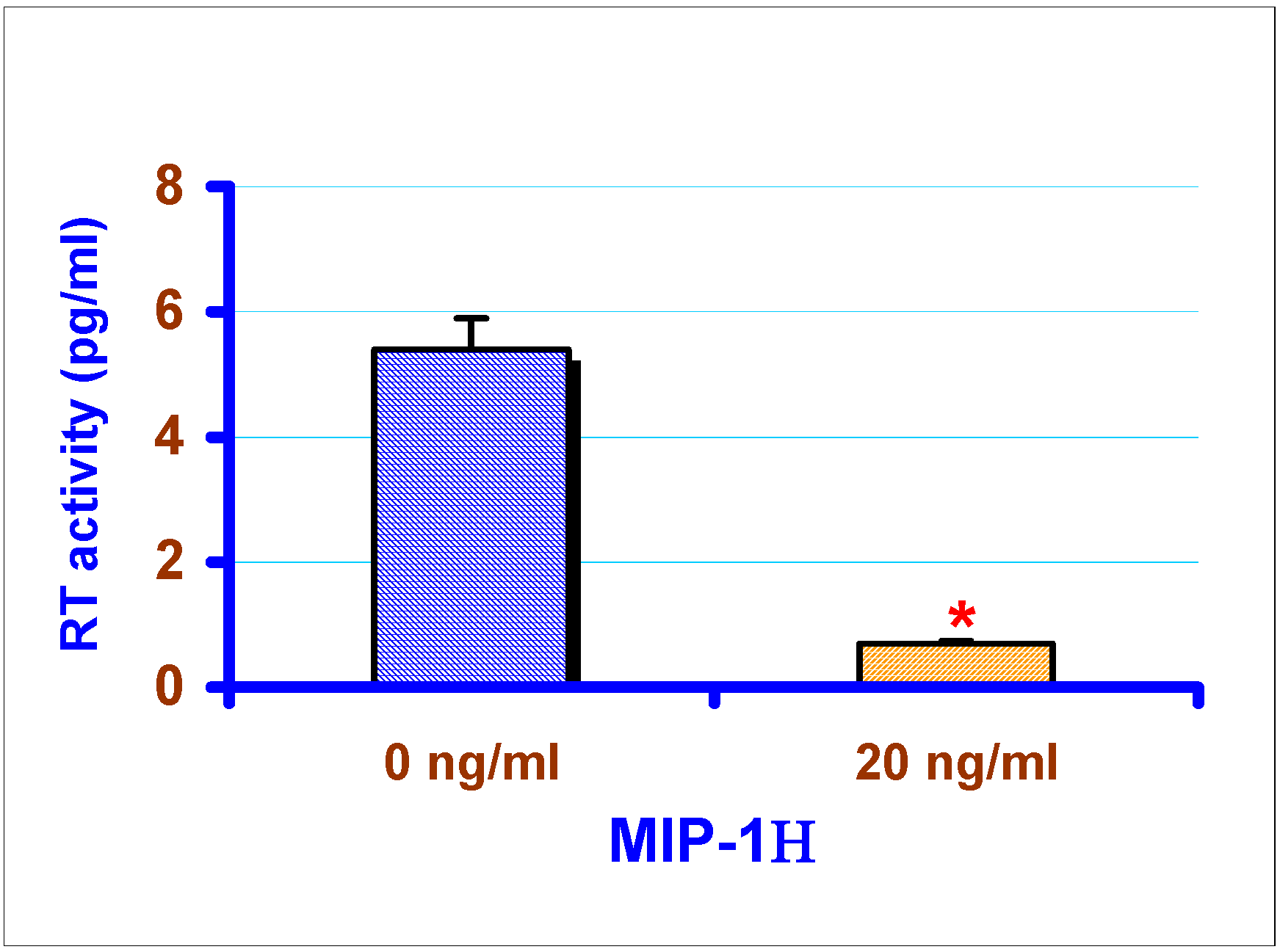
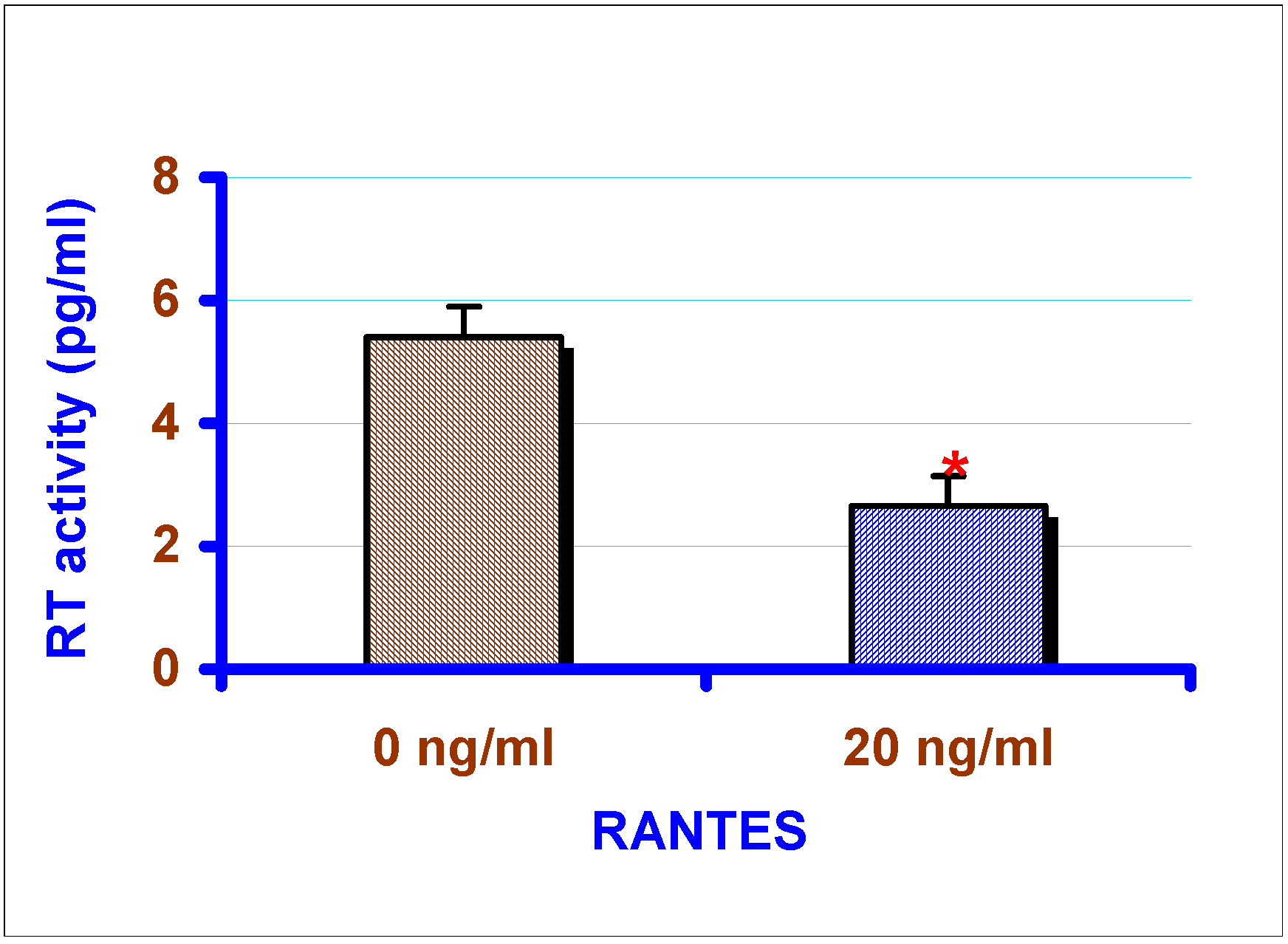
Effect of exogenous RANTES and MIP-1α on the replication of CAEV in GSMC
Determination of reverse transcriptase (RT) activity
Statistical analysis
Results
RT-PCR and gel electrophoresis analysis of MIP-1α expression in GSMC and PBMC

Effect of exogenous chemokines on CAEV replication in vitro
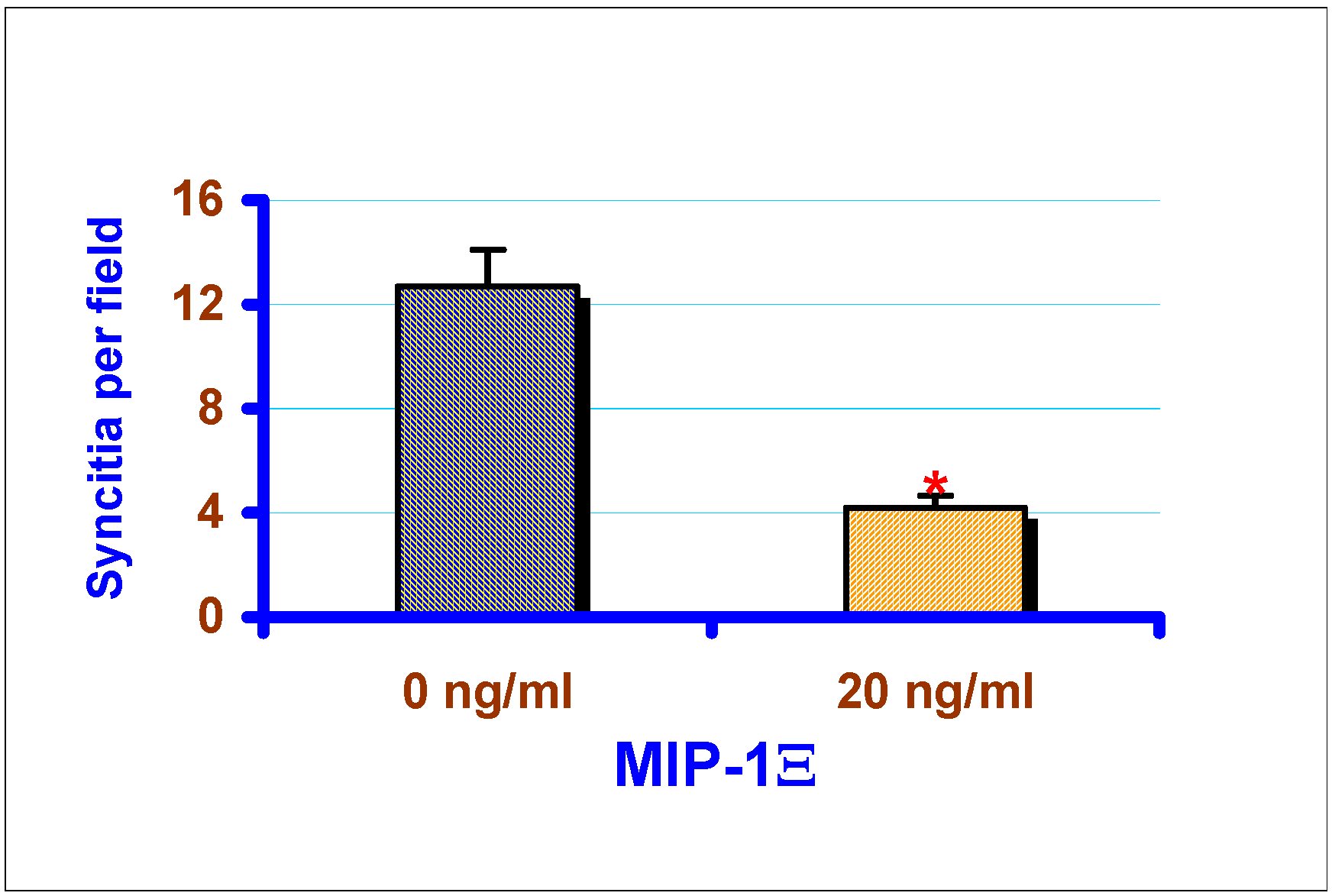
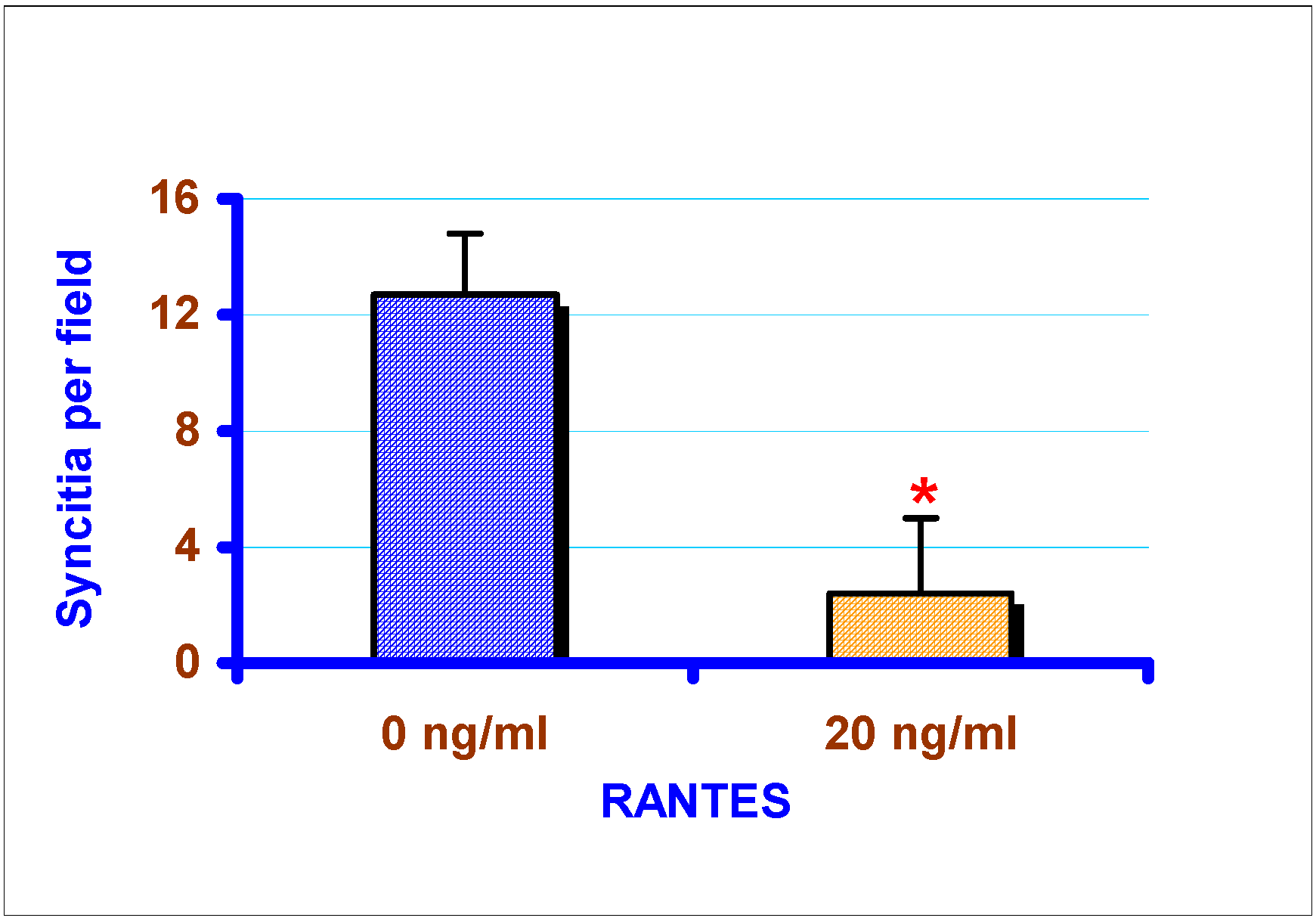
Discussion
Acknowledgements
References and Notes
- Narayan, O.; Clements, J.E. Biology and pathogenesis of lentiviruses. J. Gen. Virol. 1989, 70, 1617–1639. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Brennan, F.M.; Maini, R.N. Role of cytokines in rheumatiod arthritis. Annu. Rev. Immunol. 1996, 14, 397–440. [Google Scholar] [CrossRef] [PubMed]
- Schall, T.J.; and Bacon, K.B. Chemokines, leucocyte trafficking, and inflammation. Curr. Opin. Immunol. 1994, 6, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M.; Dewald, D.; Moser, B. Interleukin-8 and related chemotactic cytokines: CXC and CC chemokines. Adv. Immonol. 1994, 55, 97–179. [Google Scholar]
- Bischoff, S.; Krieger, M.; Brunner, T.; Rot, A.; von Tscharner, V.; baggioini, M.; Dahinden, C. RANTES and related chemokines activate human basophil granulocytes through different G protein-coupled receptors. Eur. J. Immunol. 1993, 23, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Rathanaswami, P.; Hachicha, M.; Sadick, M.; Schall, T.J.; McColl, S.R. Expression of the cytokine RANTES in human rheumatiod synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J. Biol. Chem. 1993, 268, 5834–5839. [Google Scholar] [PubMed]
- Lechner, F.; Machado, J.; Bertoni, G.; Seow, H.F.; Dobbalaere, D.A.E.; Peterhans, E. Caprine arthritis encephalitis virus dysregulates the expression of cytokines in macrophages. J. Virol. 1997, 71, 7488–7497. [Google Scholar]
- Cocchi, F.; Devico, A.L.; Garzino-Demo, A.; Arya, S.K.; Gallo, R.C.; Lusso, P. Identification of RANTES, MIP-1alpha, MIP-1beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995, 270, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Coffey, M.J.; Woffendin, C.; Phare, S.M.; Strieter, R.M.; Markovitz, D.M. RANTES inhibits HIV-1 replication in human peripheral blood monocytes and alveolar macrophages. Am. J. Physiol. 1997, 272, L1025–L1029. [Google Scholar] [PubMed]
- Crawford, T.B.; Adams, D.S.; Cheevers, W.P.; Cork, L.C. Chronic arthritis in goats caused by retrovirus. Science 1980, 207, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.G.; Sapp, W.J.; Heneine, W. Detection of caprine arthritis encephalitis virus by polymerase chain reaction. J. Clin. Microbiol. 1993, 31, 3042–3043. [Google Scholar] [PubMed]
- Adams, D.H.; Shaw, S. Luecocyte-endothlial interactions and regulation of leucocyte migration. The Lancet 1994, 343, 831–836. [Google Scholar] [CrossRef]
- Furie, M.B.; Randolph, G.J. Chemokines and tissue injury. Am. J. Pathol. 1995, 146, 1287–1301. [Google Scholar] [PubMed]
- Pulsatelli, L.; Meliconi, R.; Boiardi, L.; Macchioni, P.; Salvarani, C.; Faccini, A. Elevated serum concentrations of chemokine RANTES in patients with polymyalgia rheumatica. Clin. Exp. Rheumatol. 1998, 16, 263–268. [Google Scholar] [PubMed]
- Kunkel, S.L.; Lukacs, N.; Kasama, T.; Strieter, R.M. The role of chemokines in inflmmatory joint disease. J. Leukoc. Biol. 1996, 59, 6–12. [Google Scholar] [PubMed]
- Peterhans, E.; Pohl, B.; Zanoni, R.; Lazary, S. Caprine arthritis encephalitis. In Rheumatoid arthritis; Smolen, J.S., Kalden, J.R., Maini, R.N., Eds.; Springer: Heidelberg, Germany, 1992; pp. 216–230. [Google Scholar]
- Walker, C.M.; Levy, J.A. A diffusible lymphokine produced by CD8+ T lymphocytes suppresses HIV replication. Immunol. 1989, 66, 628–630. [Google Scholar]
- Baier, M.; Werner, A.; Bannert, N.; Metzner, K.; Kurth, R. HIV suppression by interleukin-16. Nature 1995, 378, 561. [Google Scholar] [CrossRef] [PubMed]
- Zink, M.C.; Narayan, O. Lentivirus-induced interferon inhibits maturation and proliferation of monocytes and restricts the replication of caprine arthritis-encephalitis virus. J. Virol. 1989, 63, 2578–2584. [Google Scholar] [PubMed]
- Bembaruch, A.; Michiel, D.F.; Oppenheim, J.J. Signals and receptors involved in recruitment of inflammatory cells. J. Biol. Chem. 1995, 270, 11703–11706. [Google Scholar] [CrossRef] [PubMed]
- Neote, K.; DiGregorio, D.; Mark, J.Y.; Horuk, R.; Schall, T.J. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell 1993, 72, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Liu, R.; Ellmeier, W.; Choe, S.; Untmaz, D.; Burkhart, M.; DiMarzio, P.; Marmon, S.; Sutton, R.E.; Hill, C.M.; Davis, C.B.; Peiper, S.C.; Schall, T.J.; Littman, D.R.; Landau, N.R. Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996, 381, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Mackewicz, C.E.; Blackbourn, D.J.; Levy, J.A. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. J. Immunol. 1995, 163, 3653–3661. [Google Scholar]
© 2002 by MDPI (http://www.mdpi.org).
Share and Cite
Punna, S.; Jones, A.K.; Reddy, P.G. Expression of Chemokines, MIP-1alpha and RANTES in Caprine Lentiviral Infection and Their Influence on Viral Replication. Int. J. Mol. Sci. 2002, 3, 1177-1187. https://doi.org/10.3390/i3111177
Punna S, Jones AK, Reddy PG. Expression of Chemokines, MIP-1alpha and RANTES in Caprine Lentiviral Infection and Their Influence on Viral Replication. International Journal of Molecular Sciences. 2002; 3(11):1177-1187. https://doi.org/10.3390/i3111177
Chicago/Turabian StylePunna, S., A. K. Jones, and P. G. Reddy. 2002. "Expression of Chemokines, MIP-1alpha and RANTES in Caprine Lentiviral Infection and Their Influence on Viral Replication" International Journal of Molecular Sciences 3, no. 11: 1177-1187. https://doi.org/10.3390/i3111177
APA StylePunna, S., Jones, A. K., & Reddy, P. G. (2002). Expression of Chemokines, MIP-1alpha and RANTES in Caprine Lentiviral Infection and Their Influence on Viral Replication. International Journal of Molecular Sciences, 3(11), 1177-1187. https://doi.org/10.3390/i3111177



