An Acid Exchanged Montmorillonite Clay-Catalyzed Synthesis of Polyepichlorhydrin
Abstract
:Introduction
Experimental Part
Materials
“Maghnite” and “H-Maghnite” characterization
Procedure and polymer characterization
Results and Discussion
Catalyst structure
| Sample | Composition in wt% | Poly(ECH) Yield % | ||||||||||
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | SO3 | As | FL* | ||
| Raw-Maghnite | 69.39 | 14.67 | 1.16 | 0.30 | 1.07 | 0.50 | 0.79 | 0.16 | 0.91 | 0.05 | 11 | 00 |
| H-Mag0.05M | 70.75 | 14.67 | 1.05 | 0.30 | 1.01 | 0.49 | 0.78 | 0.16 | 0.75 | 0.04 | 10 | 2 |
| H-Mag0.10M | 71.00 | 14.60 | 1.00 | 0.30 | 0.98 | 0.39 | 0.78 | 0.16 | 0.55 | 0.04 | 10 | 3 |
| H-Mag0.15M | 71.58 | 14.45 | 0.95 | 0.29 | 0.91 | 0.35 | 0.77 | 0.15 | 0.42 | 0.03 | 10 | 8 |
| H-Mag0.20M | 71.65 | 14.20 | 0.80 | 0.28 | 0.85 | 0.30 | 0.77 | 0.15 | 0.39 | 0.01 | 10 | 11.2 |
| H-Mag0.25M | 71.70 | 14.03 | 0.71 | 0.28 | 0.80 | 0.21 | 0.77 | 0.15 | 0.34 | 0.01 | 11 | 56 |
| H-Mag0.30M | 73.20 | 13.85 | 0.70 | 0.27 | 0.78 | 0.20 | 0.76 | 0.13 | 0.31 | 0.02 | 9.78 | 25 |
| H-Mag0.35M | 75.31 | 13.52 | 0.71 | 0.26 | 0.78 | 0.18 | 0.75 | 0.13 | 0.32 | 0.01 | 8.03 | 20 |
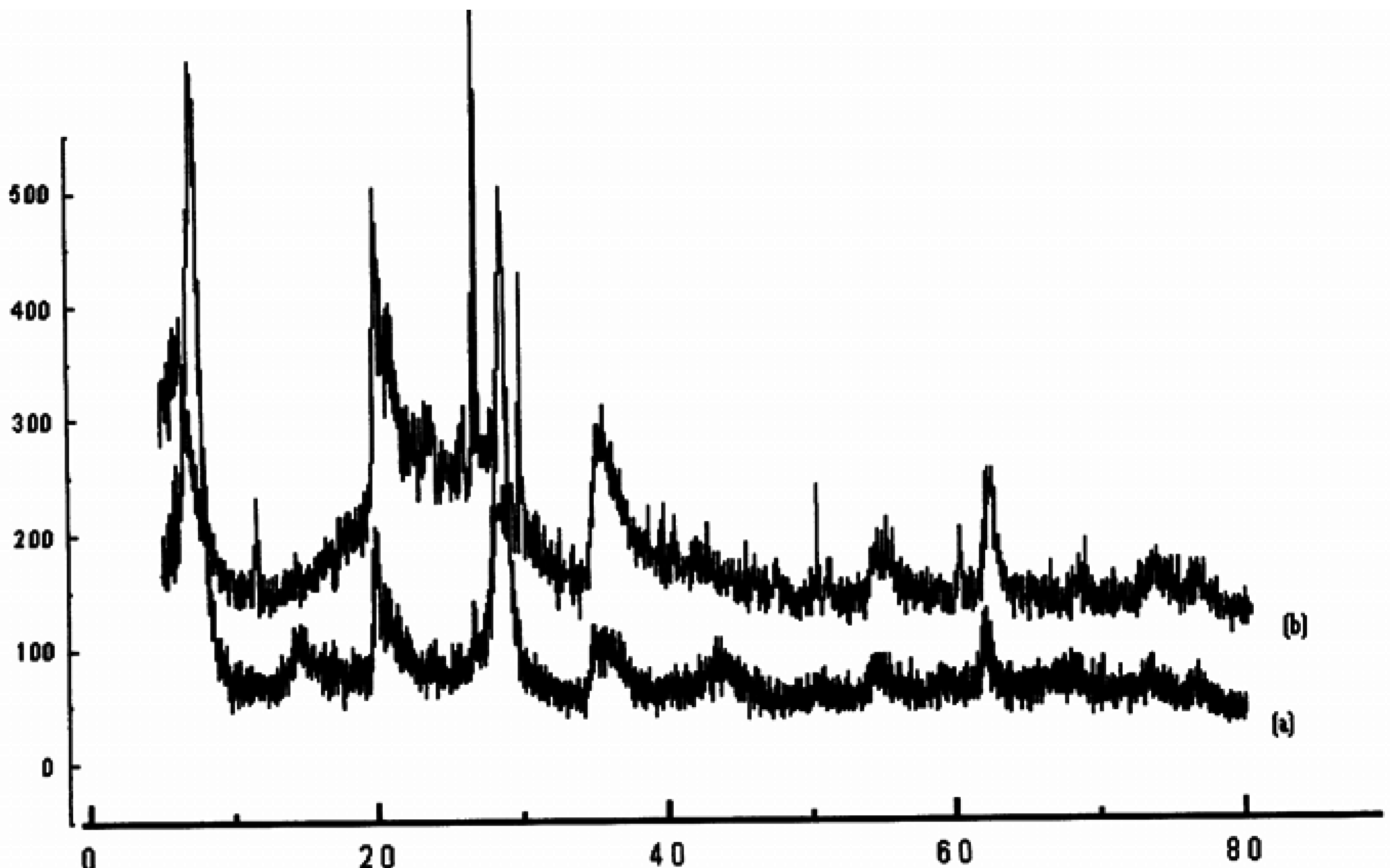
| Samples | dhkl (A°) | hkl | Nature of sample |
| Raw-Maghnite | 12.50 | 001 | Montmorillonite |
| 4.47 | 110 | Montmorillonite | |
| 4.16 | ,, | Quartz | |
| 3.35 | ,, | Quartz | |
| 3.21 | ,, | Feldspath | |
| 3.03 | ,, | Calcite | |
| 2.55 | 200 | Montmorillonite | |
| 1.68 | 009 | Montmorillonite | |
| 1.49 | 060 | Montmorillonite | |
| H-Maghnite0.25M | 15.02 | 001 | Montmorillonite |
| 4.47 | 110 | Montmorillonite | |
| 4.16 | ,, | Quartz | |
| 3.35 | ,, | Quartz | |
| 3.21 | ,, | Feldspath | |
| 3.03 | ,, | Calcite | |
| 2.55 | 200 | Montmorillonite | |
| 1.68 | 009 | Montmorillonite | |
| 1.49 | 060 | Montmorillonite |

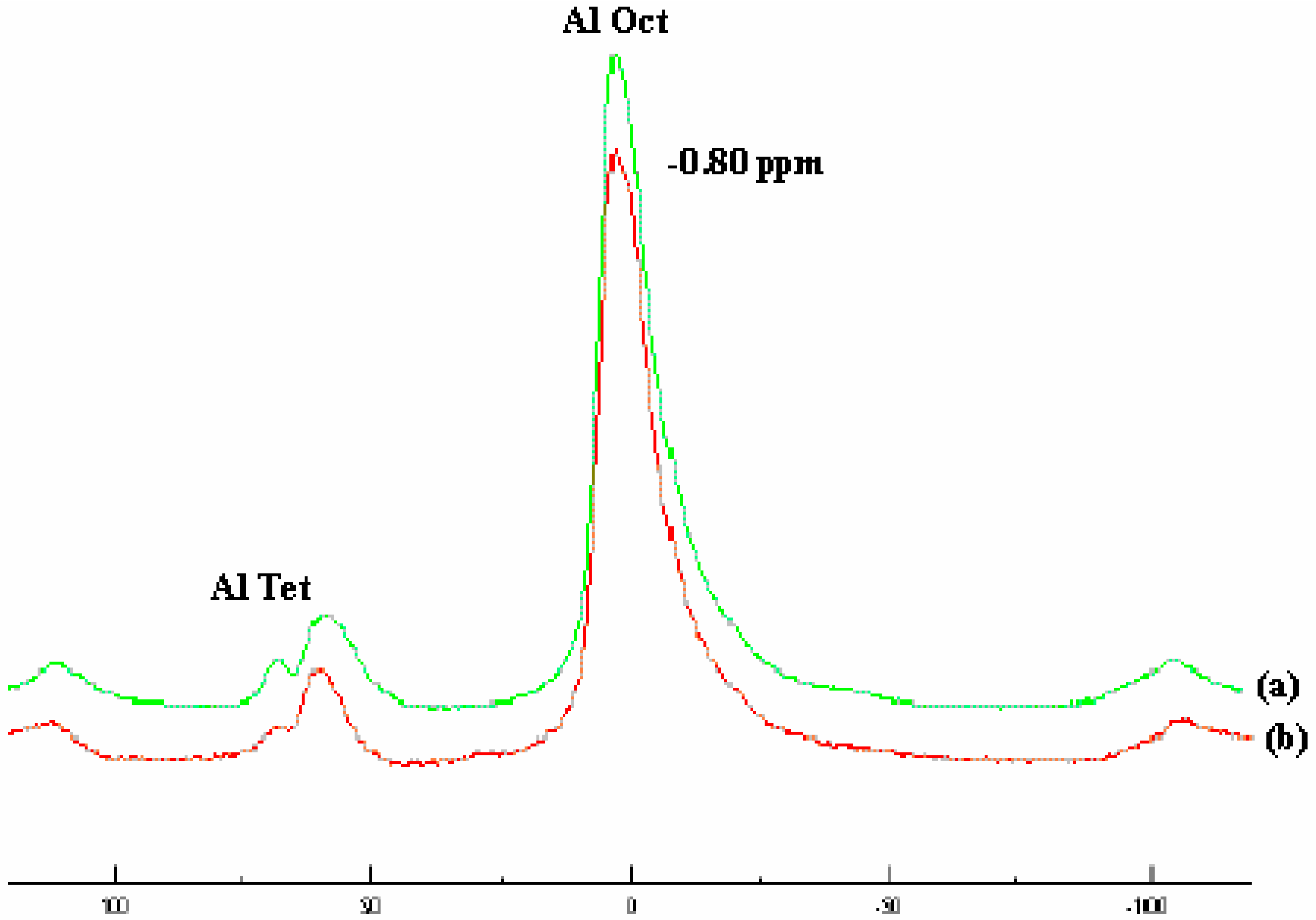
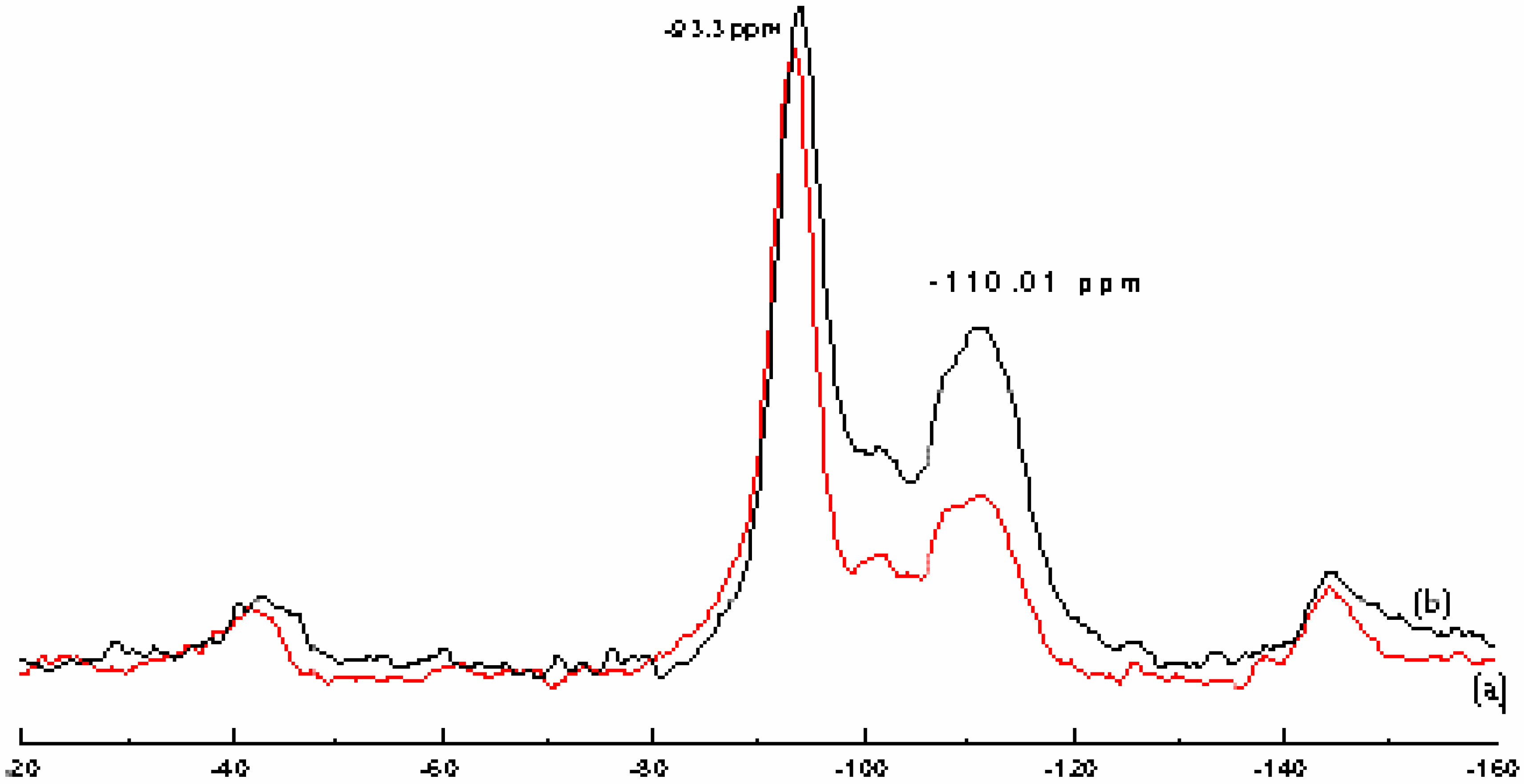
Polymerization and products characterization
| Experiment | ECH (g) | “H-Maghnite0.25M”(g) | Yield % | Mv | Mn | Mw |
| 1 | 10 | 1 | 56 | 4012 | 2435 | 4385 |
| 2 | 10 | 0.5 | 12 | 4245 | 3458 | 5390 |
Effect of “H-Maghnite0.25M” proportion
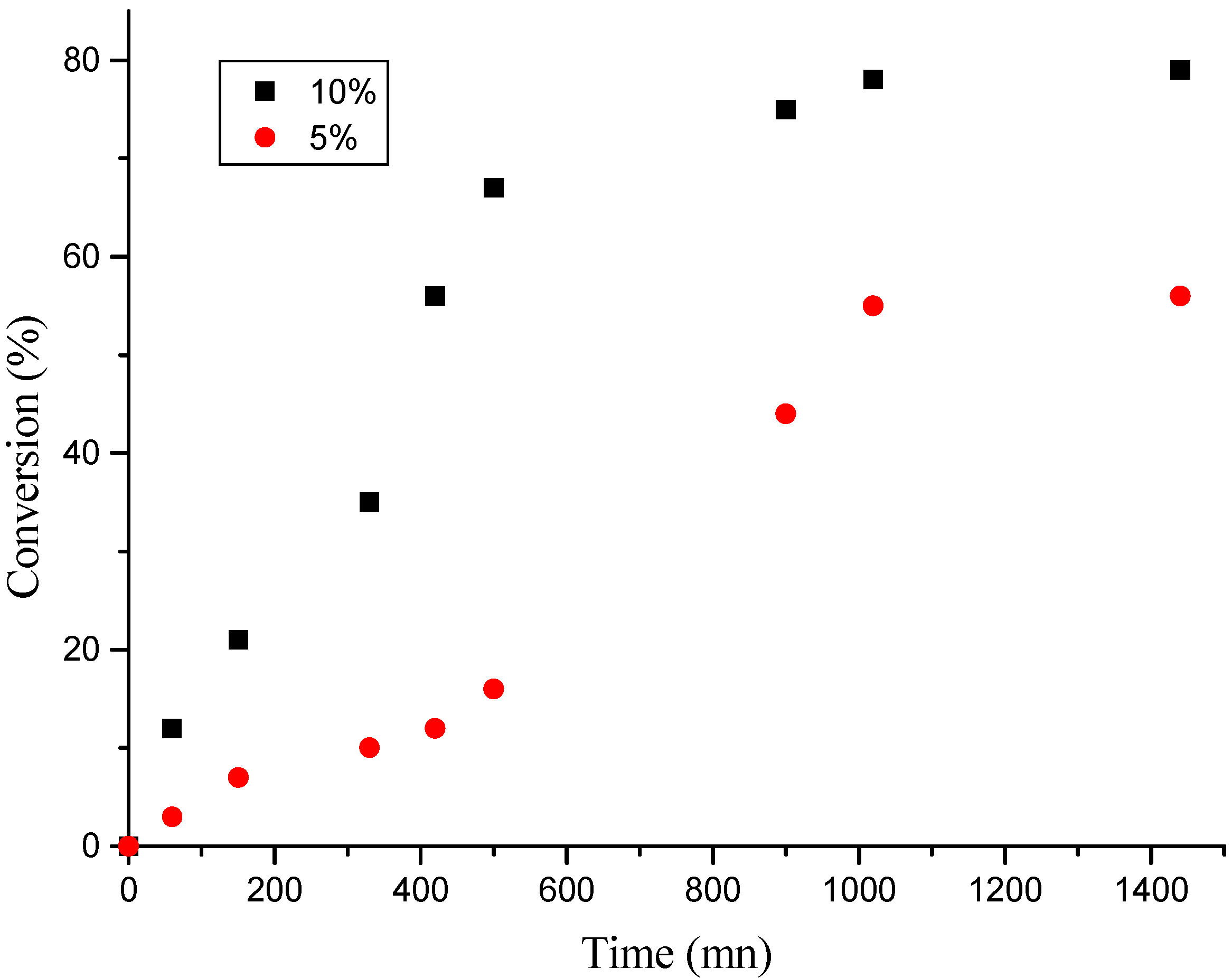
| Time(mn) | 60 | 150 | 330 | 420 | 500 | 900 | 1020 | 1440 |
| Yield(%)(a) | 13 | 21 | 35 | 56 | 67 | 75 | 78 | 79 |
| Yield(%)(b) | 3 | 7 | 10 | 12 | 16 | 44 | 55 | 56 |
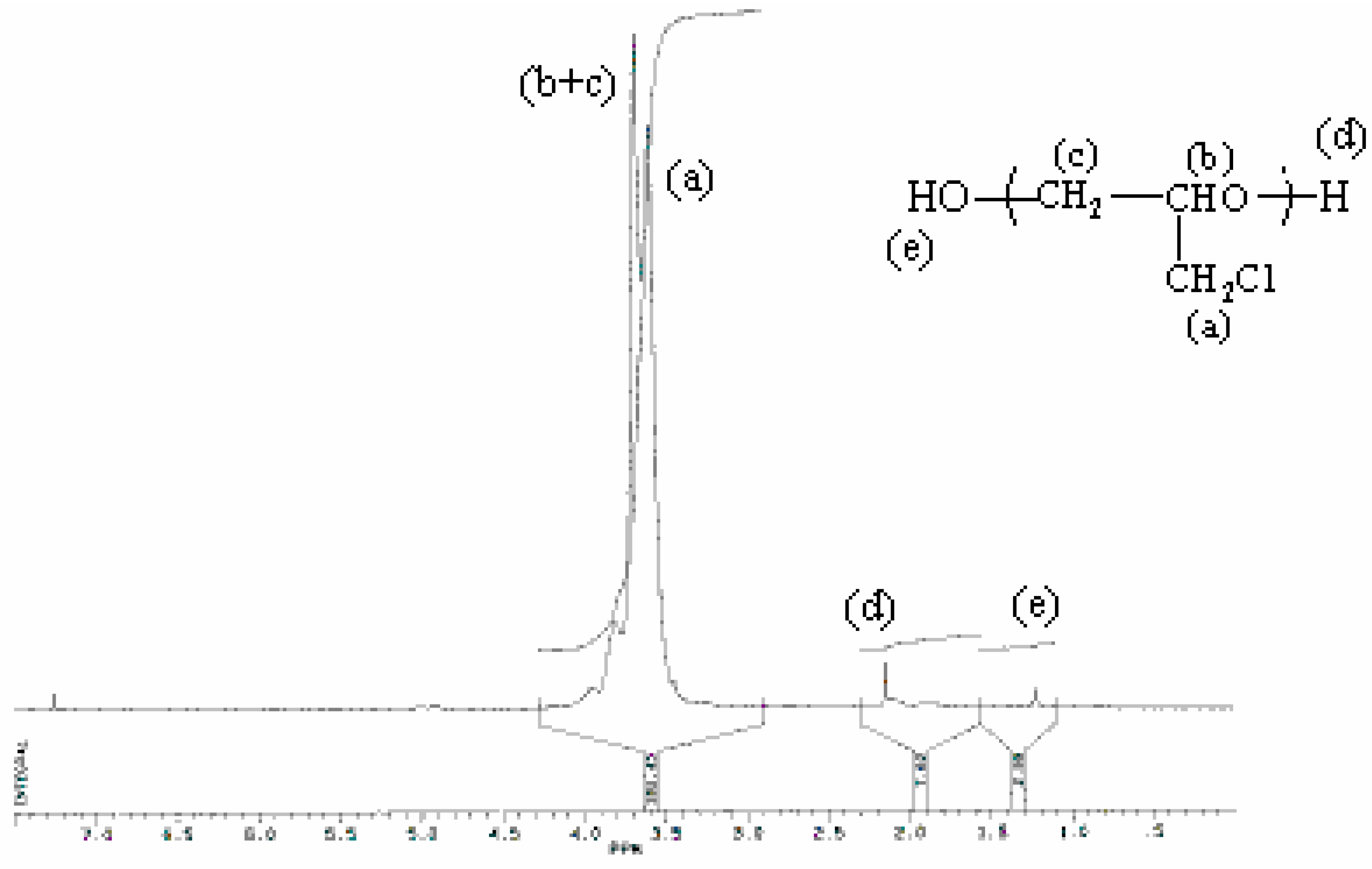
Characterisation of products

| Proton type | (a) | (b) | (c) | (d) | (e) |
| δ(ppm) | 3.60 | 3.70 | 3.70 | 2.14 | 1.22 |
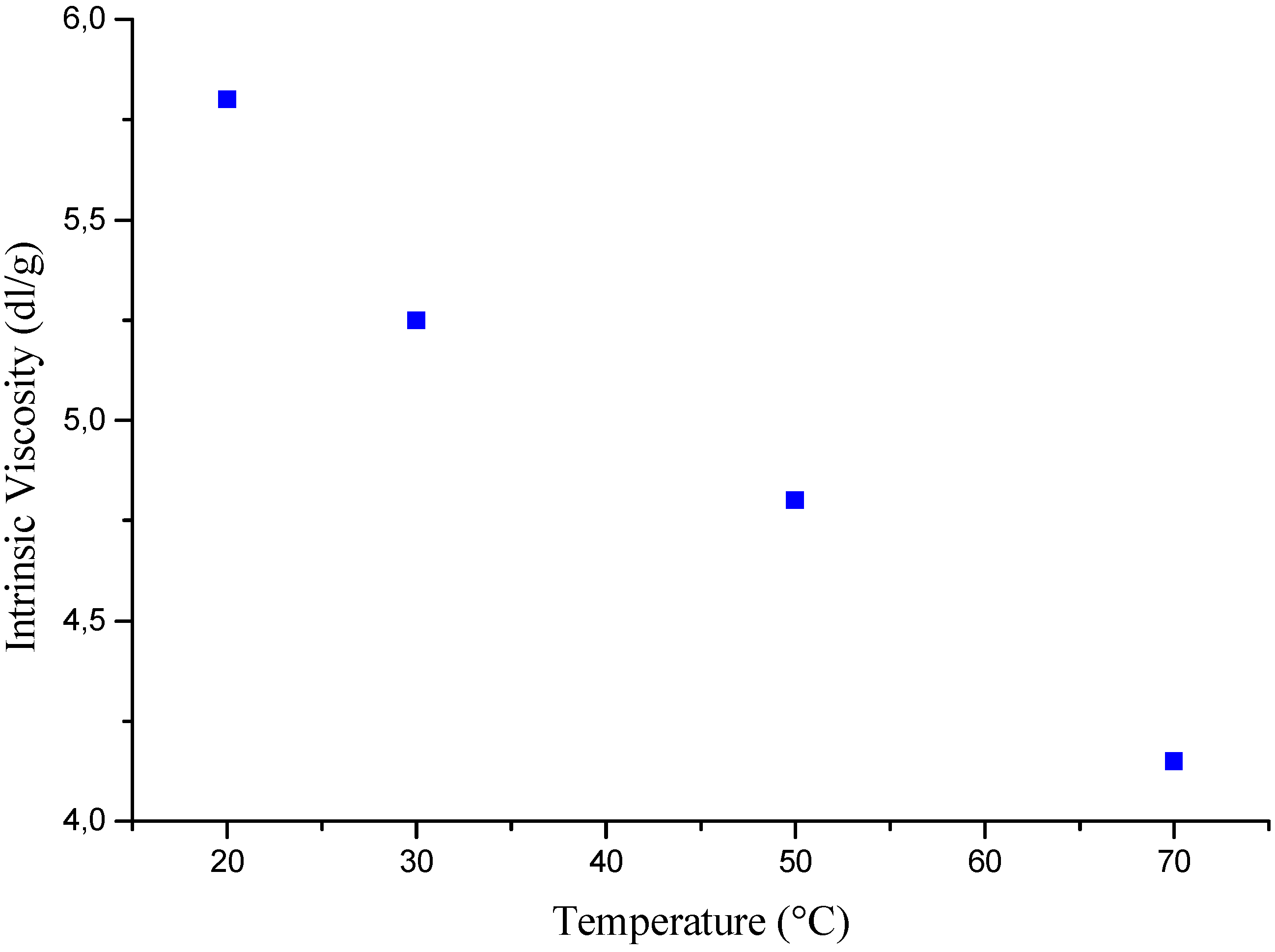
Temperature effects


Polymerization mechanism







Conclusion
Acknowledgements
References
- Aldridge, L. P.; McLaughlin, J. R.; Pope, C. G. J. Catal. 1973, 30, 409. [CrossRef]
- Forni, L. Catal. Rev. 1973, 8, 65.
- Fripiat, J. J.; Gastuche, M. C.; Richard, R. B. J. Phys. Chem. 1962, 66, 806.
- Theng, B. K. G. Dev. Sedimentol. 1982, 35, 197.
- Kowalska, M.; Cock, D.L. Chemosphere 1998, 36, 547–552.
- Evangetou, V.P.; Marsi, M.; Vandiviere, M.M. Plant and Sol. 1999, 213, 63–74.
- Kwon, O.Y.; Park, K.W.; Jeong, S.Y. Bull. Korean Chem. Soc. 2001, 22, 678–684.
- Süd-Chemie AG. Munich: West Germany.
- Ballantine, J. A.; Davies, M.; O’Neil, R. M.; Patel, I.; Purnell, J. H.; Williams, K.J.; Thomas, J. M. J. Mol. Catal. 1984, 26, 57. [CrossRef]
- Lee, D. G.; Nomeldin, N. Tetrahedron Lett. 1981, 22, 4889.
- Chiang, C. S.; McKillop, A.; Taylor, E. C.; White., J. F. J. Am. Chem. Soc. 1976, 98, 6750. [CrossRef]
- Coenélis, A.; Laszlo, P. Synthesis 1985, 909.
- Ballantine, J. A.; Puenell, J. H.; Thomas, J. M. J. Mol. Catal. 1984, 26, 157. [CrossRef]
- Hojabri, F. J. Appl. Chem. Biotechnol. 1971, 21, 87. [CrossRef]
- Odian, G. La Polymerisation: Principes et Applications; Ed.Technica: New York, 1994; pp. 222–226. [Google Scholar]
- Breen, C.; Madejovà, J.; Komadel, P. J. Mater.Chem. 1995, 5(3), 496–474.
- Farmer, V. C. Infrared Spectra of Minerals; Farmer, V. C., Ed.; Mineralogical Society: London, 1974; p. 331. [Google Scholar]
- Moeke, H. H. W. Infrared Spectra of Minerals; Farmer, V. C., Ed.; Mineralogical Society: London, 1974; p. 365. [Google Scholar]
- Madejovà, J.; Bednànikovà, E.; Komadel, P.; Cicel, B. Proc. 11th Conf. Chem. Miner. Petrol. Ceske Budéjovica 1990; Konta, J., Ed.; Charles University: Prague, 1993; p. 267. [Google Scholar]
- Komadel, P. Clay Minerals 2003, 38, 127.
- Benharrats, N.; Belbachir, M.; Legran, A. P.; D’espinose de le Caillerie, J. B. Clays Miner. 2003, 38, 49–61.
- Samajovà, E.; Kraus, I.; Lajcàkovà, A. Geol. Carpath. Ser. Clays. 1992, 42, 21.
- Thompson, J. G. Clay Miner. 1984, 19, 169.
- Tkàc, I.; Komadel, P.; Müle, D. Clay Miner. 1994, 29, 11.
- Lagarde, F.; Reibel, L.; Franta, E. Macromol. Chem. 1992, 193, 1087–1097.
- Schacht, E.; Bailey, D.; Vogl, O. Polymer Sci., Polym. Chem. Ed. 1978, 16, 2343–2351. [CrossRef]
- Inoue, S.; Aida, T. Ring–opening Polymérization; Vol. 1, Ivin, K. J., Sagusa, T., Eds.; Applied Science Publishers: New York, NY, 1984. [Google Scholar]
- Goethals, A. Pure Appl. Chem. 1976, 48, 335.
- Miyazaka, T.; Tanaka, S. Polymer J. 1984, 16, 365–369.
© 2003 by MDPI (http://www.mdpi.org). Reproduction for noncommercial purposes permitted.
Share and Cite
Yahiaoui, A.; Belbachir, M.; Hachemaoui, A. An Acid Exchanged Montmorillonite Clay-Catalyzed Synthesis of Polyepichlorhydrin. Int. J. Mol. Sci. 2003, 4, 548-561. https://doi.org/10.3390/i4100548
Yahiaoui A, Belbachir M, Hachemaoui A. An Acid Exchanged Montmorillonite Clay-Catalyzed Synthesis of Polyepichlorhydrin. International Journal of Molecular Sciences. 2003; 4(10):548-561. https://doi.org/10.3390/i4100548
Chicago/Turabian StyleYahiaoui, Ahmed, Mohammed Belbachir, and Aïcha Hachemaoui. 2003. "An Acid Exchanged Montmorillonite Clay-Catalyzed Synthesis of Polyepichlorhydrin" International Journal of Molecular Sciences 4, no. 10: 548-561. https://doi.org/10.3390/i4100548




