Introduction
Photophysical processes may exhibit special characteristics at the interfaces. The micelles produce nonpolar – polar interfaces where absorption and emission properties of the dye,
etc. become enhanced or quenched. Such phenomena have been exploited to account for the nature and strength of the interaction as well as understanding of the physicochemical characteristics of the surfactants. Extensive insight has been provided into the environment within micelles using steady state fluorescence quenching technique [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14]. The property of the surfactants to self-aggregate into micelles in solvents is the most fascinating aspect of these molecules and micelles remain one of the central topics of study within surface and colloid chemistry. Important characteristics of micelles are the concentration where they are formed, i.e. the CMC, their aggregation number (n), their size. The most frequently applied methods to determine aggregation number are various scattering methods and fluorescence quenching (FQ) method [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20].
An advantage with the FQ method is that n can be determined also when the micelles interact in solution [
21,
22,
23,
24,
25]. This is because the method does not rely on diffusive properties. On the other hand a drawback of this method is the necessity of adding a fluorescent probe and a quencher to the system. With FQ the effect of these molecules on the system must be kept at minimum. Another important aspect is that the partitioning of the quencher between the micelles and the surrounding solution must be known with sufficient accuracy. For concentrated micellar solutions the free concentration of the quencher can often be neglected, even if the quencher be hydrophilic [
23,
24]. However, in dilute solution a substantial amount may be in the aqueous sub phase. Recently the advantage of using quencher with properties similar to the surfactant of interest has been recognized [
25]. The quencher would be identical to the surfactant under study.
Different ionic and non-ionic surfactants have been widely studied but such studies with non-ionic surfactant Igepal (Polyoxyethylene nonyl phenol) are rare. In this paper we have determined the physicochemical characteristics of micelles of Igepal CO-210 (4-(C9H19)C6H4(OCH2CH2)nOH, n ~ 2), Igepal CO-520 (4-(C9H19) C6H4 (OCH2CH2)n OH, n~5) and Igepal CO-720 (4-(C9H19)C6H4(OCH2CH2)n OH, n ~ 12) using spectroscopic and light scattering method. Cetyl pyridinium chloride (CPCl), a cationic surfactant, has been used here as quencher. The dye Safranine T (3,7-diamino-2,8-dimethyl-5-phenyl phenazinium chloride) (ST) and 1- Anthracene sulphonate (1-AS) have been used as fluorescent probe molecule.
Experimental Details
1-AS was prepared by reducing the corresponding anthraquinone with Zn dust and 20% NH
4OH solution for 4-6 hours [
10]. The products were treated with active animal charcoal to remove traces of anthraquinone and other impurities, crystallized four times from water and obtained as sodium salt. ST (E Merck, Germany) was crystallized twice from ethanol water mixture before use. Igepal CO-210, Igepal CO-520, Igepal CO-720 and CPCl were of Aldrich products. The compounds were at least 99.9% pure. Their characterization and purity were checked by emission measurement and the impurities were found to be absent. The emission spectrum of 1-AS and ST were measured in the range 380-480 nm by exciting 1-AS molecules at 365 nm and 530-630 nm by exciting ST molecules at 520 nm in a fluorescence spectrophotometer (Fluorog FIII A Spectrofluorimeter, Spex Inc,NJ, USA) with a slit width 1.25 mm. The concentration of 1-AS and ST used in the micellar solutions was of the order of 10
-2 m.mol dm
-3. In the fluorescence quenching measurement with CPCl, the quencher concentration was varied in the range 0.1- 0.5 m.mol dm
-3. The fluorescence spectra of 1-AS and ST at different quencher concentration were recorded at a constant surfactant concentration 50 m.mol dm
-3. The quencher CPCl itself is a surfactant and at low concentration (0.1 m.mol dm
-3) much below its CMC virtually resides in the micelles of the major surfactant. The fluorescent molecule (1-AS or ST) partitioned itself between the micelles with quencher (Q) and without Q (empty micelle) [
8,
9]. The fluorescent molecule emits fluorescence when it occupies an empty micelle. The fluorescence of 1-AS or ST is quenched [
10] when it occupies a micelle containing at least one molecule of Q.
Absorption spectra were recorded using an MPS-2000 (Shimadzu) UV-Visible Spectrophotometer with a matched pair of silica cuvettes (path length 1 cm). The absorption maxima of 1-AS and ST were at 365 and 520 nm respectively. All spectral measurements were duplicated in a constant temperature water bath at 298 K accurate to within ± 0.1°C and the mean values were processed for data analysis.
The cmc of the micelles of nonionic surfactants were determined from the spectral measurements. The absorbance and fluorescence intensity of ST at different concentration of surfactant were measured and the absorbance and fluorescence intensity vs. log [surfactant] plot yielded two straight lines, the point of intersection corresponds to the cmc of the micelles. The cmc values collected in
Table 2, indicate the increasing trend with increase in oxyethylene group of Igepal.
The fluorescence lifetime of 1-AS was measured with a nanosecond single photon counting spectrofluorometer (Edinburg Instruments Model 199) with a nitrogen discharge lamp. The fluorescence from the undegassed samples was observed at right angles to the excitation beam. The lamp profile and decay curve of samples were obtained using a multichannel pulse light analyser (7100 multichannael analyzer). The data were transferred to a data analyser (Model 199M) with a plessey periferal system computer and were deconvoluted to obtain the actual lifetime. All decay curves were single exponential.
DLS Measurements
The particle size (hydrodynamic diameter) of the micelles was determined using a dynamic light scattering instrument of Otsuka Electronics, Japan.The light source used was 632 nm Neon laser which acted on the micellar solution (filtered a couple of times using 0.45 μm millipore microfilter and taken in a cylinderical cell). Measurements were taken at 90ºangle and the intensity data were processed using a computer. Essentially the instrument measures the diffusion coefficient (D) of the dispersed droplets (taken to be spherical) and evaluates the hydrodynamic diameter (dh) in terms of the Stokes- Einstein equation, dh = where η, k, T are the viscosity coefficient of the medium, the Boltzmann constant and the absolute temperature, respectively.
Results
DLS results
The hydrodynamic diameter (d
h), polydispersity indices (PDI) and diffusion coefficients (D) were determined by DLS method. The results are presented in
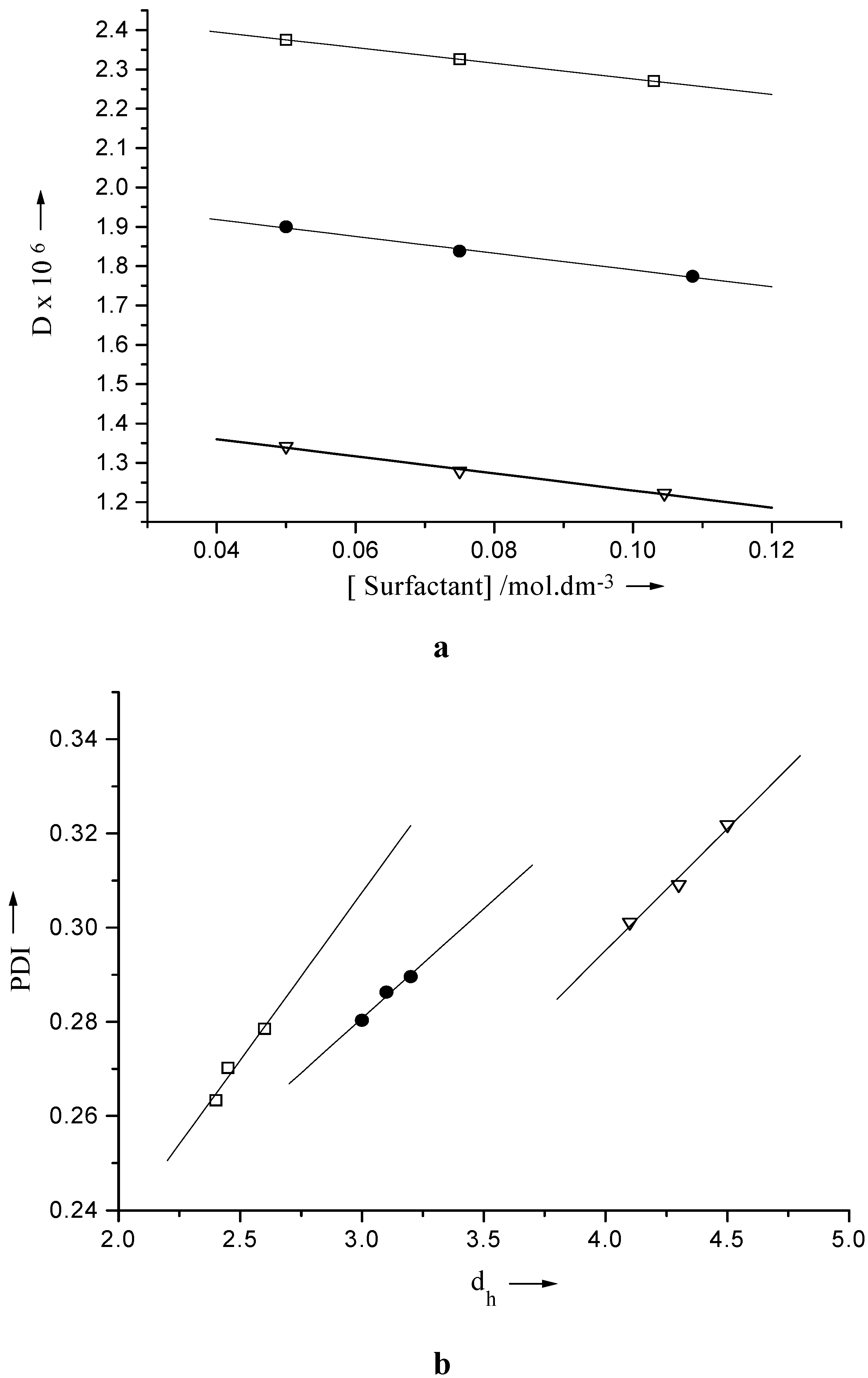
Table 1. The hydrodynamic diameter of the micelle follow the trend Igepal CO-720 > CO-520 > CO- 210. The polydispersity indices of the studied micellar solution are fairly large.
The polydispersity indices (PDI) represents S/d
h where S is the standard error in d
h. The PDI value considered 0.1 for monodisperse system [
26], higher values than 0.1 denote polydispersity. The PDI value and the size of the micelle increase with concentration of the surfactants. With growing size the particles have ended up with increasing polydispersity.
Table 1.
The hydrodynamic diameter (dh), diffusion coefficient (D) and the polydispersity index (PDI) of micellar solution of Igepal at 298 K.
Table 1.
The hydrodynamic diameter (dh), diffusion coefficient (D) and the polydispersity index (PDI) of micellar solution of Igepal at 298 K.
| Surfactant | Concentration /mol.dm-3 | dh / nm | D × 106 | PDI × 101 | n |
| | 0.050 | 2.40 | 2.375 | 2.633 | 85 (85) |
| Igepal CO-210 | 0.075 | 2.45 | 2.326 | 2.702 | |
| | 0.103 | 2.60 | 2.270 | 2.785 | |
| | 0.050 | 3.00 | 1.900 | 2.803 | 72 (75) |
| Igepal CO-520 | 0.075 | 3.10 | 1.836 | 2.863 | |
| | 0.109 | 3.20 | 1.774 | 2.896 | |
| | 0.050 | 4.10 | 1.341 | 3.011 | 54 (55) |
| Igepal CO-720 | 0.075 | 4.30 | 1.279 | 3.091 | |
| | 0.105 | 4.50 | 1.222 | 3.218 | |
The combined profile of PDI with d
h and D with concentration of the surfactants is presented in
Fig 1. The variation of PDI with d
h values follow the linear relation (1). As D and d
h have inverse relationship, one should expect a schematic / regular decrease in D with increasing d
h. The dependence of D on [Igepal] for different system follow the relation (2).
where A, B, A
1 and B
1 are appropriate constants. Their values were collected in
Table 2 according to
Fig.1. The values of A and A
1 refer to the PDI and D value respectively in absence of Igepal, which should be equal in all cases but the difference may be due to the nature of the medium.
Figure 1.
Plot of a) diffusion coefficient (D) vs. concentration and b) polydispersity indices (PDI) vs. hydrodynamic diameter (dh) of Igepal CO-210 (⌷—⌷), Igepal CO-520 (●—●) and Igepal CO-720 (∇—∇).
Figure 1.
Plot of a) diffusion coefficient (D) vs. concentration and b) polydispersity indices (PDI) vs. hydrodynamic diameter (dh) of Igepal CO-210 (⌷—⌷), Igepal CO-520 (●—●) and Igepal CO-720 (∇—∇).
Table 2.
The value of A, B of equation (1) and A1, B1 of equation (2), CMC, surface area of the head group and free energy change for micelle formation of Igepal.
Table 2.
The value of A, B of equation (1) and A1, B1 of equation (2), CMC, surface area of the head group and free energy change for micelle formation of Igepal.
| Surfactant | A | B | A1 × 106 | B1 × 106 | CMC × 104
/ mol. dm-3 | σ/nm2 | -ΔG0
/ kJ .mol-1 |
| Igepal CO-210 | 0.0938 | 0.0712 | 2.4743 | 1.9815 | 4.534 | 0.25 | 19.041 |
| Igepal CO- 520 | 0.1412 | 0.0465 | 2.0037 | 2.1365 | 4.729 | 0.45 | 18.942 |
| Igepal CO- 720 | 0.0881 | 0.0517 | 1.4471 | 2.1760 | 4.973 | 0.96 | 18.867 |
Aggregation Number
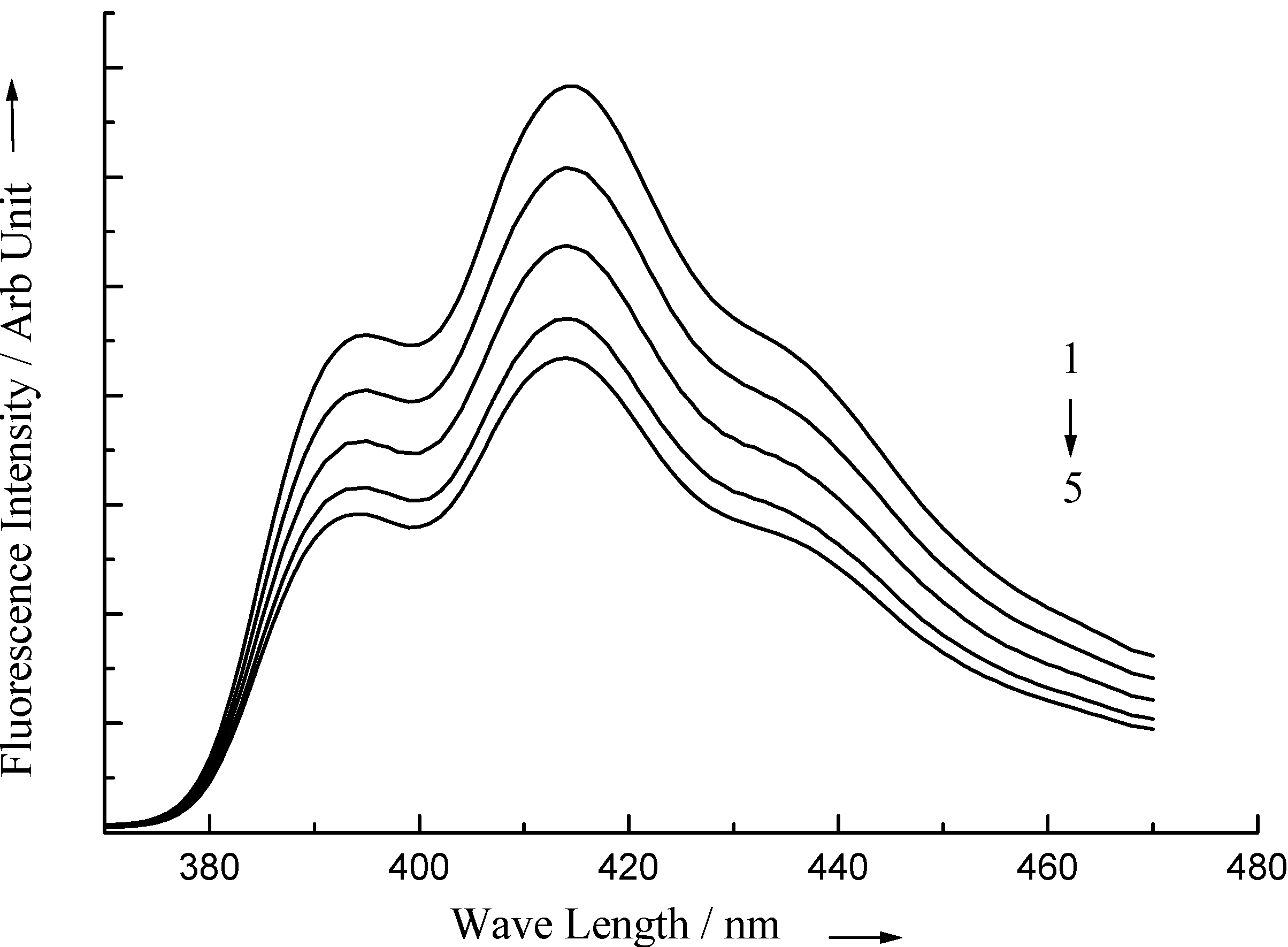
CPCl and other surfactants do not affect the absorption and fluorescence spectra of 1-AS and ST. The fluorescence intensity of 1-AS and ST decreases in the presence of quencher CPCl The quenching process is affected by the presence of surfactants. Several sets of fluorescence spectra of 1-AS in micellar solution of Igepal are illustrated in
Fig. 2. In the determination of aggregation number by FQ method, two probes, Safranine T and 1-AS have been used. To verify the n value two sets of experiments with these two different probes have been done. The measured ratio of the fluorescence intensity (F/F
0) in the presence of Q to that in the absence of Q is related to the micellar concentration by the expression [
27]:
On the basis of pseudophase model the effective micellar concentrations
where [S] is the stoichiometric concentration of the surfactant and n is the micellar aggregation number. For a fixed concentration of the surfactant the fluorescence intensity decreases with increasing concentration of the quencher. However the fluorescence lifetime remains constant confirming static quenching. From equations above, we obtain
where free monomer = cmc. Since [S]>> cmc, neglecting the latter, equation becomes
Figure 2.
Fluorescence spectra of 1-AS in Igepal in presence of 1) 0.1 m.mol.dm-3, 2) 0.2 m.mol.dm-3, 3) 0.3 m.mol.dm-3, 4) 0.4 m.mol.dm-3 and 5) 0.5 m.mol.dm-3 CPCl.
Figure 2.
Fluorescence spectra of 1-AS in Igepal in presence of 1) 0.1 m.mol.dm-3, 2) 0.2 m.mol.dm-3, 3) 0.3 m.mol.dm-3, 4) 0.4 m.mol.dm-3 and 5) 0.5 m.mol.dm-3 CPCl.
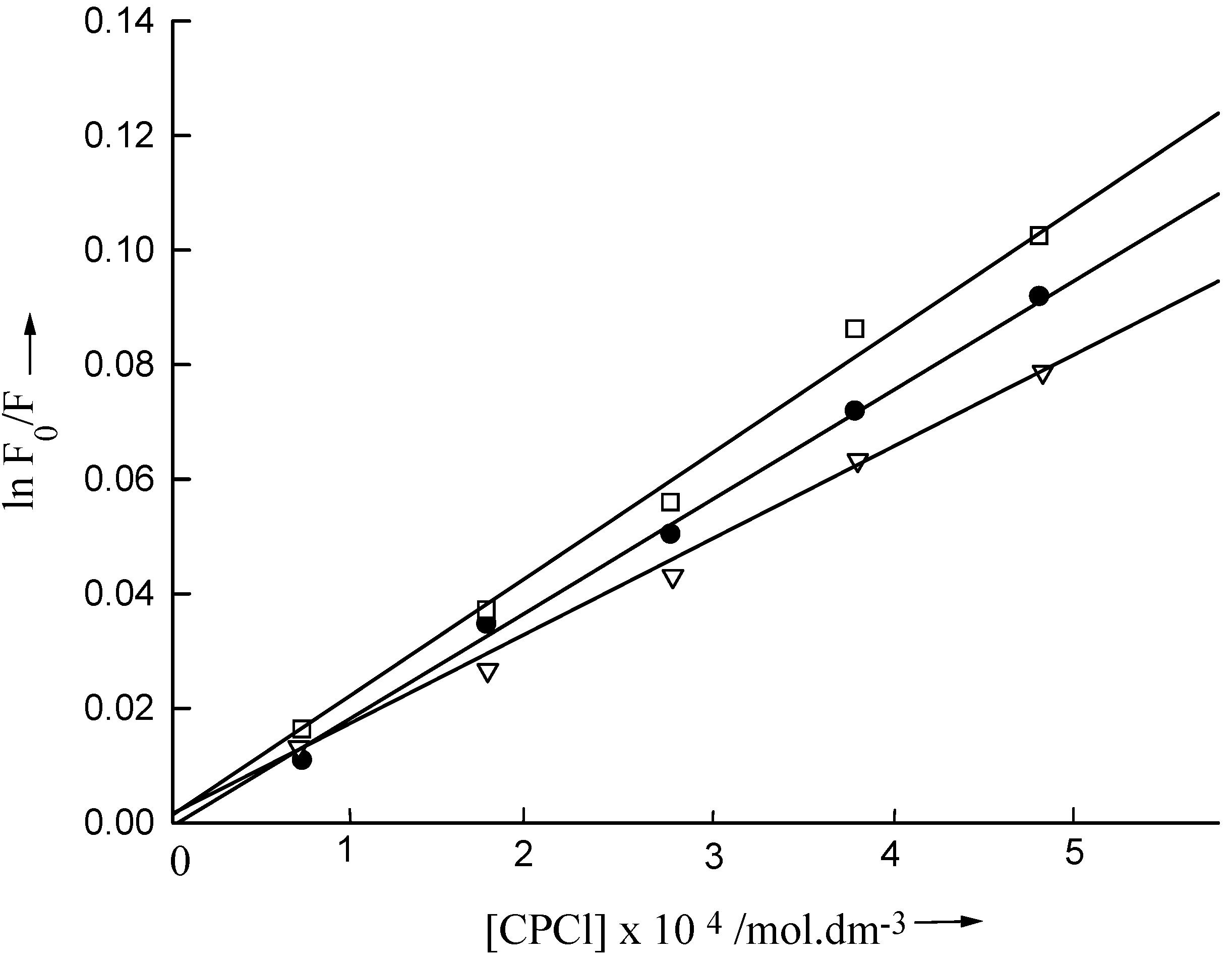
For the determination of n, [S] was kept constant and [Q] was varied. lnF
0/F was plotted against [Q] to determine n from the slopes of the linear curves passing through the origin (
Fig. 3). Using both the probes, obtained n values agree well with each other and are presented in
Table 1. In the Igepal series there is a regular decrease in the aggregation number on going from CO-210 to CO-720. The different number of polar components (CH
2-CH
2-O) attached with the same non-polar part in Igepal molecule is considered to be responsible for this systematic change. The aggregation number (n) bears a relation with the number of EO groups. The trend of the aggregation number with CMC in Igepal is also regular [
28]. In other words lower cmc causing tendency to form micelles with large aggregation number at lower concentration since the solubility of the amphiphiles having lesser number of EO groups will be lower than those with greater number EO groups.
Figure 3.
Plot of ln F0/ F of 1-AS vs concentration of quencher in Igepal CO-210 (⌷—⌷), Igepal CO-520 (●—●) and Igepal CO-720 (∇—∇).
Figure 3.
Plot of ln F0/ F of 1-AS vs concentration of quencher in Igepal CO-210 (⌷—⌷), Igepal CO-520 (●—●) and Igepal CO-720 (∇—∇).
Discussion
The present fluorimetric procedure leads to fairly accurate aggregation number of the surfactants since using the same method and same probe the determined aggregation number of SDS, Tween series, Triton X 100 agreed well with the literature values. From earlier reports [
29,
30] it has been found that aggregation number of micelles depends on the concentration of the surfactant used in the medium. In this case we have considered the concentration of the Igepal hundred times stronger than their respective CMC values to calculate n. The location of the interaction is at the interface where the dye is adsorbed to generate the photophysical phenomenon. Safranine T and 1-AS are very soluble in water but exhibit poor solubility in hydrophobic medium. It therefore resides at the micellar interface. Since aqueous solution of quencher is used, they also interact at the micellar interface.
The free energy gain when a tail of a surfactant is transferred from water to the micellar core is much larger than on dimerization, this is the reason for the cooperativity in micelle formation. When many tails are in the core it can be assumed spherical. According to Israclachvili
et al. [
31], [
32] the preferred structure of aggregates formed by the amphiphilic molecules in aqueous media is determined by critical packing parameter, derived from simple geometrical consideration. The parameter is defined by v / a
0 ℓ
c where v is the volume of hydrocarbon chains being assumed to be fluid and incompressible, a
0 be the optimal head group area and ℓ
c the critical chain length which corresponds to the maximum effective length that the chain can assume. Considering the packing formation of the molecule in these systems the value of the parameter is nearly 0.175, which is below 0.33, hence corresponds to spherical micelle (critical chain length of hydrophobic portion becomes 1.773 nm and the volume of the hydrophobic chain portion upto benzene ring has been calculated as 69.59 x 10
-3 nm
3, a
0= 0.224 nm
2, considering the bond length of the respective bonds, hence v / a
0 ℓ
c= 0.175).
For spherical micelle the exposed surface area is proportional to n
2/3 where n is the aggregation number [
33]. Assuming the monomers are closely packed in a micelle and considering the d
h of the micelles determined from DLS and the aggregation number of the micelles obtained from the fluorescence quenching method, the surface area (σ) of the head group of the monomer have been calculated and the values are given in
Table 2. With increase in the number of EO group in the molecule the surface area of the head group increases. This increase in the area of the head group may be due to the difference in packing of the hydrophilic chains, which may exist as coiled form [
34]. The surface area of the head group of Igepal CO-210 having minimum number of EO groups in PEO part is close to that of the stearic acid [
35]. For the determination of aggregation number from surface area the relation is surface area
n
2/3 or surface area = k
′ n
2/3. The proportionality constant (k
′) value is approximately y/π where y is the number of EO groups in the PEO part of the surfactant. For the general value of k
′ experiment with more surfactants will be considered in future work. The surface area determined bears a direct relation with the number of EO groups in the molecule.
The repulsion between the head groups or the free energy of the corona limits the growth of the micelle and is responsible for the change of CMC with the length of the hydrophilic ethylene oxide part. In order to illustrate the effects of increasing size of EO block in Igepal, we use an expression for the free energy of the corona given by Semenov et al [
36] leading to the following expression for the free energy change per monomer for micelle formation:
where y is the number of EO groups in the PEO part. B
0, B
1 and B
2 are positive constants. The B parameter is selected to give reasonable values of the aggregation number and the CMC (
Table 2) for this Igepal series. This free energy function has been used to illustrate the behavior from y = 2 to 12 value for nonionic surfactants. The free energy of transfer of alkyl groups to water in process such as micelle formation, which depends on n
c and y, where n
c is the number of carbon atoms in the alkyl tail which is same here, will vary in the three cases. The B values depend on the nature of surfactants. For Igepal series the B values are B
0 = 7.84, B
1 = 0.02 and B
2 =0.052 with another nonionic surfactant, Brij series, (Brij 52, Brij 56, Brij 58) the B values obtained are B
0 = 14.05, B
1 = 0.04 and B
2 = 0.066.