Results and Discussion
The chemical shifts of nitrogen (N) and
para (with respect to the amino group) carbon (C-4) atoms in the NMR spectra of anilines are collected in
Table 1. π-Electron density at the
para carbon atom in aromatic compounds depends on the twist angle of the substituent [
14] and thus, its chemical shift can be used to calculate these angles for the amino groups in anilines.
13C-NMR chemical shifts for unsubstituted aniline were found to be quite sensitive to the orientation of the NH
2 group [
15]. This relation was also confirmed by both theoretical (GIAO, Gauge-Independent Atomic Orbital) and experimental results [
15]. The amino group in aniline itself is tilted away from the ring plane by 42° [
15]. The chemical shift values increase (deshielding effect) as the amino group is moved away from the planar orientation with the largest changes appearing at the
ipso,
ortho and
para positions [
15].
13C-NMR spectra show that
N-phenylaziridine is the least conjugated among
N-phenyl cyclic polymethyleneimines [
16]. It has been found that
p-substituent in
N-phenylaziridines only slightly affects electron distribution in aziridine ring [
17]. This proves that
nN-π
Ar conjugation in these compounds is insignificant. The chemical shifts of
para carbon atom in the spectra
N,N-dimethyl- and
N,N-diethylanilines and 1-phenylaziridine, 1-phenylpyrrolidine and 1-phenylpiperidine correlate well with other known measures of benzene ring-nitrogen resonance such as oscillator strength of the UV bands and exaltation of molar refraction [
16,
18].
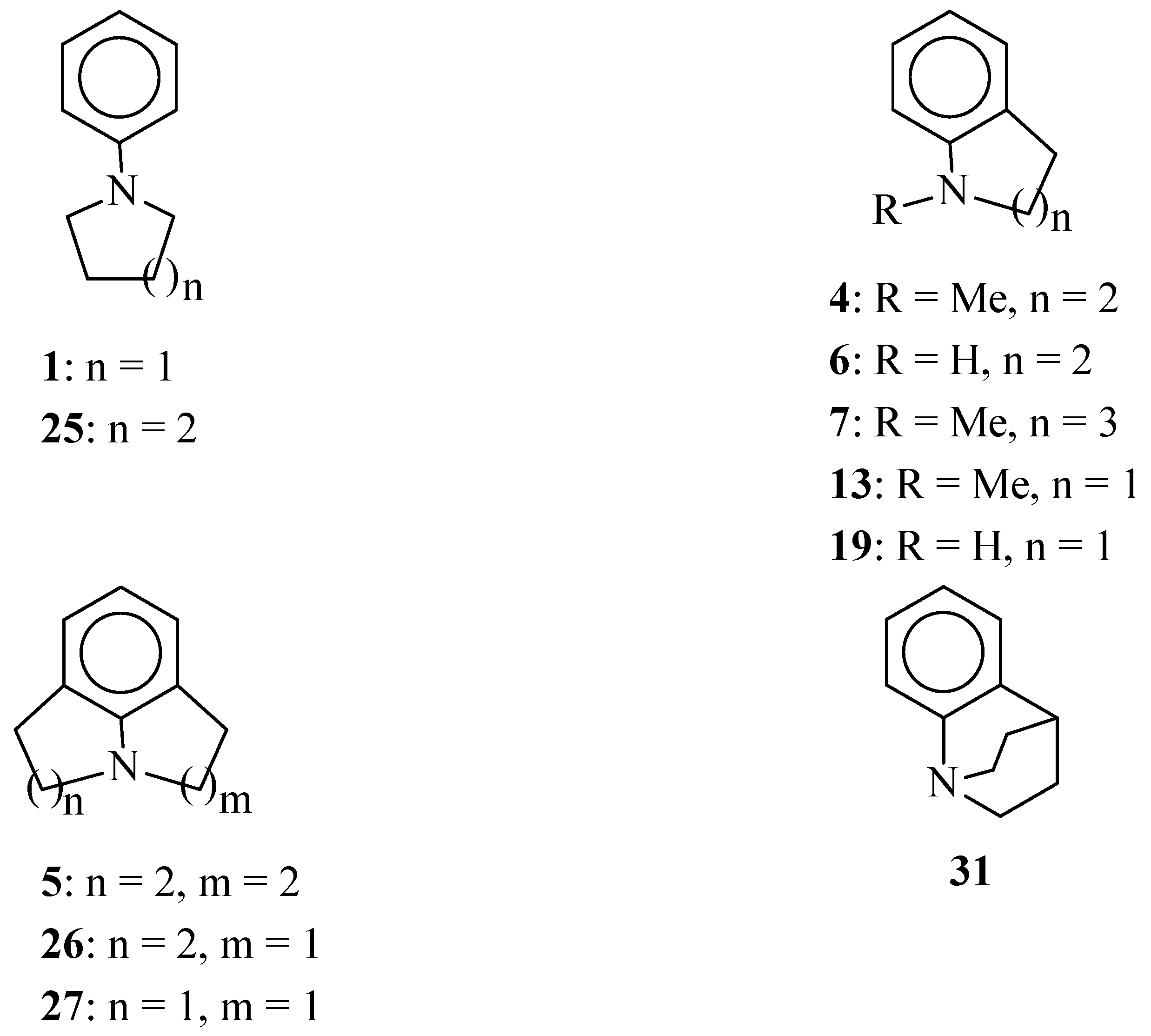
Scheme 1.
Structures of selected amines.
Scheme 1.
Structures of selected amines.
Numerous papers have been published on correlations of NMR chemical shifts with Hammett σ values [
19,
20,
21]. σ
p values for the NMe
2 and NH
2 [
8] as well as these for other non-amine substituents [
22] are known [
8]. Since in chloroform solutions δ(
13C-4) values are 117.68, 116.38, 120.72, 125.38, 128.36, 126.43, 126.82, 132.84 and 134.72 ppm for aniline,
N,N-dimethylaniline, anisole, toluene, benzene, chlorobenzene, bromobenzene, benzonitrile and nitrobenzene [
23], respectively, the dependence between the substituent constant and the chemical shift of
para carbon atom is σ
p = 0.089 δ(
13C-4) – 11.22. This equation can be used to calculate the substituent constants for other groups (
Table 1). It is known that different
N,N-dialkylamino and polymethyleneimino, N(CH
2)
n, groups reveal similar inductive effects [
11].
Table 1.
Selected 15N- and 13C- chemical shifts [ppm] in the NMR spectra of substituted anilines for 0.1-0.2 M solutions in chloroform-d1 and substituent constants for the amino groups
Table 1.
Selected 15N- and 13C- chemical shifts [ppm] in the NMR spectra of substituted anilines for 0.1-0.2 M solutions in chloroform-d1 and substituent constants for the amino groups
| Compound | Amino group and additional substituents | δ(15N) | δ(13C4) | σp | σR | |
|---|
| 1 | N(CH2)4 | -308.7 | 115.20 | -0.92 | -1.02 | -0.57 |
| 2 | N(n-Bu)2 | -312.4 | 115.13 | -0.91 | -1.01 | -0.57 |
| 3 | NEt2 | -309.7 | 115.32 | -0.91 | -1.01 | -0.57a |
| 4 | N(Me)[2-(CH2)3] | -319.0 | 115.33 | -0.91 | -1.01 | -0.57 |
| 5 | N[2-(CH2)2][6-(CH2)3] | -318.5 | 115.61 | -0.89 | -0.99 | -0.56 |
| 6 | NH[2-(CH2)3] | -320.8 | 115.90 | -0.86 | -0.98 | -0.55 |
| 7 | N(Me)[2-(CH2)4] | -330.9 | 116.03 | -0.85 | -0.95 | -0.55 |
| 8 | NMe2 | -337.7 | 116.38 | -0.82b | -0.92 | -0.54a,c |
| 9 | N(Me)Et | -325.0 | 116.86 | -0.77 | -0.87 | -0.52 |
| 10 | NHMe | -329.2 | 116.98 | -0.76 | -0.88 | -0.52a |
| 11 | NHEt | -310.4 | 117.02 | -0.76 | -0.88 | -0.52a |
| 12 | 1-NH2,2,6-Me2 | -331.2 | 117.34 | -0.73 | -0.87 | -0.51 |
| 13 | N(Me)[2-(CH2)2] | -316.2 | 117.53 | -0.71 | -0.81 | -0.51 |
| 14 | 1-NH2,2,6-Et2 | -333.2 | 117.54 | -0.71 | -0.85 | -0.51 |
| 15 | 1-NH2,2-Me,6-Et | -332.4 | 117.67 | -0.70 | -0.84 | -0.50 |
| 16 | NH2 | -325.5 | 117.68 | -0.70d | -0.84 | -0.50 |
| 17 | 1-NH2,2-Me | -328.1 | 117.86 | -0.68 | -0.82 | -0.50 |
| 18 | 1-NHMe,2-Me | -327.1 | 117.86 | -0.68 | -0.80 | -0.50 |
| 19 | NH[2-(CH2)2] | -314.3 | 117.89 | -0.68 | -0.80 | -0.50 |
| 20 | 1-NMe2,2-Me | -347.3 | 118.23 | -0.65 | -0.75 | -0.49 |
| 21 | 1-NH2,2-Et | -329.4 | 118.36 | -0.64 | -0.78 | -0.48 |
| 22 | 1-NH2,2-t-Bu | -322.9 | 118.37 | -0.64 | -0.78 | -0.48 |
| 23 | 1-NH2,2-i-Pr | -329.4 | 118.49 | -0.63 | -0.77 | -0.48 |
| 24 | N(i-Pr)2 | -299.1 | 118.56 | -0.62 | -0.72 | -0.48 |
| 25 | N(CH2)5 | -313.2 | 118.91 | -0.59 | -0.69 | -0.47a |
| 26 | N[2-(CH2)2][6-(CH2)3] | -307.8 | 119.06 | -0.58 | -0.68 | -0.46 |
| 27 | N[2-(CH2)2][6-(CH2)2] | - | 120.0e | -0.49 | -0.59 | -0.44 |
| 28 | 1-NH2,2,6-(i-Pr)2 | -370.8 | 123.97 | -0.14 | -0.28 | -0.33 |
| 29 | 1-NMe2,2,6-Me2 | -364.8 | 124.72 | -0.07 | -0.17 | -0.31 |
| 30 | 1-NMe2,2,6-(i-Pr)2 | -370.2 | 126.26 | 0.07 | -0.03 | -0.27 |
| 31 | 1-N(CH2CH2)2CH-2f | -339.0g | 127.00h | 0.13 | 0.03 | -0.25 |
Available σ
I values are equal to 0.14, 0.12, 0.10 and 0.10 for
p-NH
2,
p- NHMe,
p-NMe
2 and
p‑NEt
2, respectively [
24]. This shows that the resonance effect of the N atom in anilines considerably predominates over its inductive effect.
Values of σ
R (resonance substituent constants) collected in
Table 1 were obtained by subtraction of σ
I from σ
p (σ
R = σ
p - σ
I). σ
I values used to calculate the σ
R constants are equal to 0.14 for
p-NH
2 with or without
ortho-substituent(s), 0.12 for
p-NHR (R = alkyl and polymethylene bridge in indoline or 1,2,3,4-tetrahydroquinoline) with or without
ortho-substituent(s), 0.10 for
p-NR
2 (R = alkyl and/or polymethylene bridge in 1-methylindoline, 1-methyl-1,2,3,4-tetrahydroquinoline, 1-methyl-2,3-benzohexamethyleneimine, lilolidine,
i.e. 1,2,5,6-tetrahydro-4
H-pyrrolo[3,2,1-ij]quinoline, julolidine,
i.e. 2,3,6,7-tetrahydro-1
H,5
H-pyrido[3,2,1-ij]quinoline, and benzoquinuclidine,
i.e. 3,4-dihydro-2
H-1,4-ethanoquinoline, with or without
ortho-substituent(s).
Analysis of the δ(
13C-4) values in
Table 1 shows that σ
p substituent constants for different dialkylamino groups change in the following order: N(
n-Bu)
2° NEt
2 < NMe
2 < N(Me)Et < N(
i-Pr)
2. Thus, in general, those containing longer alkyls are stronger electron-donors. On the other hand, dialkylamino groups including secondary alkyl,
e.g. i-propyl, are weaker donors. This may be caused by the strong steric interaction of the N-
i-propyl group with
ortho-hydrogen atoms. It seems interesting that σ
p-N(Me)Et° σ
p-NHMe = σ
p-NHEt.
Substitution at the
ortho carbon in
N,N-dimethylaniline causes NMe
2 group to twist out of the ring plane, so the nitrogen valences become more pyramidal [
28]. Thus,
ortho-substitution decreases the donor properties of the amino group. Comparison of δ(
13C-4) values for different
ortho-substituted anilines,
o-R-C
6H
4-NH
2 (
17 and
21-
23 in
Table 1) with that of the aniline itself (
16), shows that σ
p substituent constants depend on R in the following order: H < Me < Et °
t-Bu <
i-Pr. On the other hand, substitution in both
ortho positions (
12,
14 and
15) increases the donor properties of the amino (NH
2) group as compared to parent aniline
16. 2,6-Di-
i-propyloaniline (
28) is an exception: the amino group in this compound seems to have very weak electron-donor properties. This may be caused by the strong steric interaction of the
ortho-
i-propyl and NH
2 groups. When comparing different 2,6-R,R'-substituted anilines
12,
14-
17,
21-
23 and
28 one can see that σ
p substituent constant of the amino group change in the following order: R,R' = Me,Me < Et,Et < Me,Et = H,H < H,Me < H,Et = H,
t-Bu ° H,
i-Pr <<
i-Pr,
i-Pr. Due to strong steric interactions, the amino group in 2,
N,N-trimethylaniline (
20) is a weak electron-donor (calculations based on the band intensities show
ortho-methyl group in ethyl N,N,3-trimethyl-4-aminobenzoate to produce a 56 % steric inhibition to resonance [
32]). These interactions are much stronger in 2,6-dialkyl-
N,N-dimethylanilines
29 and
30. It is noteworthy that the twist angles in 2,6-diethyl- and 2,6-di-
i-propyl-
N,N-dimethylanilines were found to be equal to 77 and 88°, respectively [
33].
The length of the polymethylene bridge between N and Cortho in anilines 4, 7, 13, 6 and 19 has a considerable effect on the electronic properties of the N atom in these compounds. Thus, it is the strongest donor in 1,2,3,4-tetrahydroquinolines 4 and 6. The amino nitrogen atom in indolines 13 and 19 is much weaker donor. This tendency is also seen in δ(13C-4) values for julolidine (5) and lilolidine (26). The N atom in 1,2,4,5-tetrahydropyrrolo[3,2,1-hi]indole (27) is a weak electron-donor.
The nitrogen atom in benzoquinuclidine (
31) clearly reveals electron-acceptor properties. Studies of molecular structures of benzoquinuclidine and its 6-cyano derivative in the crystal state confirm that there is no
nN-π
Ar conjugation in these compounds (the aromatic π and nitrogen electron lone pair orbitals are orthogonal) [
31,
34]. Thus, there is no resonance interaction between the amino nitrogen atom and the benzene ring in benzoquinuclidine [
35].
Molecular models show that due to the angle strain in the five-membered ring, the C
Ar-C
Ar-N valence angle in indoline (
19) and its
N-methyl derivative
13 is expected to be much smaller than 120°. Bicyclic structures in
N-methylindoline (
13) and
N-methyl-1,2,3,4-tetrahydroquinoline (
4) were shown to be planar [
36]. On the other hand, the seven-membered ring in
N-methyl-
homo-tetrahydroquinoline (
7) is highly puckered [
36]. The UV spectrum of this compound shows considerable difference from the spectra of two lower homologues, and this difference is certainly due to the conformational freedom of the seven-membered ring [
37].
Although large differences in basic properties were found for
N,N-diethylaniline and
N-phenylpyrrolidine, close similarity of their
13C-NMR spectra (
Table 1) shows that electron distributions in the benzene rings are nearly the same in these two compounds [
16]. 1-Pyrrolidino group (
1) was found to be a very powerful electron-donor [
38]. It is much stronger electron-donor than 1-piperidino group (
25). Decreased
nN-π
Ar interaction in 1-(
p-nitrophenyl)piperidine, which reflects the relative rigidity of the chair conformation of the six-membered piperidine ring, is also consistent with the downfield shift for signals of protons
2 and
6 in spectra of 1-(
p-nitrophenyl)pyrrolidine, 1-(
p-nitrophenyl)hexamethyleneamine and
N,N-dialkylanilines, where alkyl = Me, Et [
39]. Low ε
max value of the band at 425 nm in the spectrum of
N-(
p-nitrophenyl)piperidine, as compared to the spectra of
N-(
p-nitrophenyl)pyrrolidine and
N-(
p-nitrophenyl)hexamethyleneimine, proves there is a serious steric inhibition to resonance in its molecule. The angle of twist of the CNC plane with respect to that of the benzene ring in
N-(
p-nitrophenyl)piperidine is equal to 33° [
40]. Due to interaction between the nitrogen atom and phenyl ring, the resonance energy in
N-phenylcyclopolymethyleneimines changes in the following order of the ring size: n = 3 < 6 < 4 < 5 [
41]. Analysis of the chemical shift values for the ring protons in
1H-NMR spectra of aniline derivatives shows that donor strength of the amino substituents in the ground state of aromatic amines change in the following order [
11]: 1-pyrrolidino > dimethylamino >1-piperidino.
Of course, confusion may result from the above discussion having its source in the dispersion of the substituent constants available for the amino groups [
13]. One should be aware of differentiation between these parameters depending
e.g. on application (or not) of the correction for the tautomeric equilibria possible in solutions of such compounds [
13]. Thus, the amino substituent in
N,N,2,6-tetramethylaniline (
29) was found to be a very weak electron-donor. However, comparison of the δ(
13C-4) values for this compound (124.72 ppm) and unsubstituted benzene (128.5 ppm) shows that this cannot be true. We have found that
values for some amino groups known [
25,
26,
27] (
Table 1) are linearly related to the obtained NMR chemical shifts of C-4 (
Table 1): δ(
13C-4) = 36.401
+ 135.922 (R = 0.9931 for five correlation points). This equation was used by us to calculate the respective substituent constants for other amino substituents (
Table 1). It can be seen that all substituents studied are electron-donor by character but, as expected, their releasing properties are very much differentiated.
The obtained substituent constants seem worthy to be compared to the results of earlier studies (only some amino groups were studied by other authors). Substituent
constants for the amino and dimethylamino groups, based on fluorine chemical shift in the NMR spectra of the respective fluoroanilines, are equal to -0.48 and -0.53, respectively [
42,
43]. Due to the steric inhibition to resonance of the
ortho-methyl group in
N,N,2-trimethyl-4-fluoroaniline the
constant for the twisted
p-dimethylamino substituent in this compound is equal to -0.24 [
28].
19F-NMR chemical shifts of 4-amino-3,5-dimethylfluorobenzene and of its
N,N-dimethyl derivative were interpreted in terms of a strong steric inhibition to resonance in their molecules but they are insufficient to push these amino groups out of conjugation with the benzene ring [
44].
The square roots of integrated absorbancies of the skeletal vibrations, involving the carbon-carbon stretching within the ring, in the 1600-1585 and 1500-1400 cm
-1 regions, are a good measure of the extent of resonance interaction between benzene ring and unsaturated substituent [
45]. It has been found that the resonance interactions between the N atom and benzene ring change in the following order: N(CH
2)
2 <N(CH
2)
5 <NMe
2 <N(CH
2)
3 <NEt
2 <N(CH
2)
4. The
values for such groups are equal to -0.38 for N(CH
2)
2, -0.47 for N(CH
2)
5, -0.52 for NHEt and NHMe, -0.53 for NHPr-
i and NMe
2, -0.54 for NHBu-
n, -0.55 for N(CH
2)
3, -0.57 for NEt
2 and -0.63 for N(CH
2)
4 [
25,
26,
27]. Other
values found for the NMe
2 group are equal to -0.54 (based on
19F NMR spectra) [
46], -0.52 (based on reactivity measurements) [
28] and -0.50 (evaluated by means of equation
= 2.0
) [
47].
Dipole moment of aniline (1.15 D [
48]) was found to be only slightly affected by the
ortho- and
N-methyl groups: its value is appreciably diminished only in 2,6,
N-trimethylaniline and especially in 2,
N,N-trimethyl- and 2,6,
N,N-tetramethylanilines [
46,
49]. Charge distribution in the molecules of aromatic amines shows the order of electron-donor strength of the amino groups to be (CH
2)
4N > Et
2N > (CH
2)
5N [
50,
51] and NEt
2 > N(CH
2)
4 > NMe
2 > N(CH
2)
5 [
50]. The calculated and measured dipole moments confirm the considerable twist of NMe
2 group in
ortho-methyl substituted
N,N-dimethyl-anilines [
52]. Thus, there is a strong steric inhibition to resonance in these molecules [
53]. Comparison of the experimentally observed and theoretically calculated dipole moments of 2,
N,N-trimethylaniline shows the hybridization of the nitrogen atom to be close to
sp3 [
14].
The sequence of the base strength of anilines of formula C
6H
5-NR
1R
2 in water changes in the following order [
54]: R
1/R
2 = H/H < H/Me < Me/Me < H/Et < H/
i-Pr < Et/Et. These results confirm that
N-alkyl groups cause an increase in basicity of aniline due to their +I effect. On the other hand,
N-alkylation may significantly change effectiveness of solvation of both amine and its conjugate acid. Alkyl groups in the
ortho positions in aniline always decrease its pK
a [
55]. On the other hand, substitution of the amino hydrogen atom(s) by alkyl(s) causes an increase of the base strength of aromatic amines.
Ortho alkyl groups in
N,N-dialkylanilines prevent the dialkylamino group from assuming coplanarity with the ring and hence decrease its resonance effect and increase the basicity of the compound. On the other hand, 2,
N-dimethylaniline is weaker and 2,
N,N-trimethylaniline is a stronger base than
N-methylaniline. Unexpectedly, 2,6,
N,N-tetramethylaniline has a lower pK
a than 2,
N,N-trimethylaniline [
55].
Benzoquinuclidine (
31,
Scheme 1), is a particular aromatic amine. As expected, it is relatively strong base, its pK
a value being the greatest one in a series of selected anilines measured in 50 % aqueous ethanol at 25°C [
56]: pK
a: C
6H
5-NH
2 < C
6H
5-NMe
2 <
o-Me-C
6H
4-NMe
2 < C
6H
5‑NHBu‑
t << C
6H
5-N(Me)Bu-
t < benzoquinuclidine. 1,2,4,5-Tetrahydropyrrolo[3,2,1-hi]indole (
27) was unexpectedly found to be stronger base (pK
a = 4.1, in 50 % aqueous ethanol) than julolidine (
5) (pK
a~3.6) [
57]. Perhaps, in this case the pK
a value also reflects both the conformational changes in the molecule and differences in steric hindrance to solvation [
58]. It has been found that linear correlation between the twist angle of the amino group and pK
a values of aniline derivatives is very rough due to peculiar solvation effects [
31]. Thus, the anomalies in the base strength attributed to the steric hindrance of solvation [
58] also enable evaluation of the conformation of aromatic amines [
26,
59]. Acidities of
p-aminobenzoic acids, R
2N-C
6H
4-CO
2H, and the rates of reduction of
p-nitroanilines, R
2N-C
6H
4-NO
2, show the following order of the electron donating effect for different amino substituents [
60]: N(CH
2)
5 < NMe
2 < N(CH
2)
4 < NEt
2 < N(
i-Pr)
2. Decreased mesomerism that results from the twisting of the NCC plane out of the benzene ring plane, is illustrated by the pK
a value for
p-(
N-piperidino)benzoic acid, which is lower as compared to other
p-aminobenzoic acids,
p-R
2N-C
6H
4-CO
2H [
39] (see also ref. [
60]) : pK
a: Et
2N > (CH
2)
4N > Me
2N > (CH
2)
5N.
The σ substituent constants for some amino groups defined on the basis of dissociation constants of
p-aminobenzoic acids,
p-R
2N-C
6H
4-CO
2H, are equal to -0.41 for N(CH
2)
5, -0.62 for NH
2, -0.69 for NMe
2 and N(CH
2)
4, and -0.71 for NEt
2 [
61]. The pK
a values of 4-amino-3,5-dimethylbenzoic acid and its
N,N-dimethyl derivative, as well as saponification rates for the respective ethyl esters, have been interpreted in terms of steric inhibition to resonance [
62].
Although benzoquinuclidine (
31) is an aromatic amine, there is no resonance interaction between the amino nitrogen and the benzene ring in its molecule. It has been proven by its unsuccessful diazo coupling with
p-nitrobenzenediazonium salts [
35]. The σ
Ro for the twisted -N< group in benzoquinuclidine is equal to -0.134 [
63] (as compared to -0.53 for NMe
2 group [
64]). On the other hand, another hindered amine,
i.e. 2,6-di-(
t-butyl)aniline, can be readily coupled with arenediazonium salts to give the respective
p-aminoazobenzenes [
35,
59,
65]. Due to steric inhibition to resonance in
N-
t-butyl-
N-methylaniline, this compound does not react with nitrous acid nor with ethyl nitrite to give the
para-nitroso derivative [
65]. The formation of 4'-nitro-4-(
N-
t-butyl-
N-methylamino)azobenzene from this compound and
p-nitrobenzenediazonium chloride proceeds extremely slowly with only 10 % yield [
65]. None of
N,N-dimethylanilines containing 2-
t-butyl and 2,6-dimethyl groups can be nitrosated [
65].
The nitrogen chemical shifts are influenced by the substituent
via polar [
66] and steric effects [
67] and thus can be useful to estimate the degree of
n-π interaction in anilines [
68]. The effect of
N-methylation on the
15N-NMR chemical shift of aniline is known for long and consistent with the present data (
8,
10 and
16 in
Table 1) [
69].
Ortho-substitution in
N,N-dimethylaniline causes a considerably large shift of its
15N-signal [
33]. This has been attributed to the torsional distortion of the NMe
2 group [
33], which results in a decreased electron delocalization in the molecule [
67]. The data in
Table 1 show that the changes of δ(
15N) may be both positive (cf.
10vs.
18;
16vs.
22;
19vs.
13) and negative (
4vs.
6;
8vs.
20,
29 and
30;
16vs.
12,
14,
15,
17,
21,
23 and
28). Thus, δ(
15N) cannot be used as a direct quantitative measure of electronic effects of the N atom in anilines, although δ(
15N) of
N-(
p-R-phenyl)aziridines qualitatively show, that the lone-pair electrons of aziridine nitrogen interact less effectively with benzene ring than those in the corresponding
N,N-dimethylanilines [
68].