Abstract
The principle of substance stability – the feedback principle – is applicable to all biological systems. It boils down for different temporal hierarchies to the following: during the formation (self-assembly) of the most thermodynamically stable structures at the highest hierarchical level (j), e.g., the supramolecular level, in accordance with the second law, Nature spontaneously uses predominantly the (available for the given local part of the biological system) least thermodynamically stable structures belonging to a lower level, for example, the molecular level (j-1). The principle can be also applied to understructure hierarchical levels of any temporal hierarchy. It helps in understanding the causes of cancer formation and the origin of some other diseases.
"One of the principal objects of theoretical research in any department of knowledge is to find the point of view from which the subject appears in its greatest simplicity."J. Willard Gibbs [1]
“The aim of science is not things in themselves but the relations between things; outside these relations there is no reality knowable.”Henri Poincaré [2]
1. Introduction (Hierarchical Thermodynamics in Action)
For many decades, the opinion was widespread that natural open biological systems are far from an equilibrium state. It was also believed that far from equilibrium processes take place in these systems. Indeed, if this is true, then thermodynamics–thermostatics (or the thermodynamics of quasi-equilibrium systems and processes)–cannot be applied.
However, recently, the law of temporal hierarchies was formulated. This law substantiates the possibility of identifying (discerning) quasi-closed monohierarchical systems (subsystems) within open polyhierarchical biological systems (Appendix). It was also established that, as a rule, the processes of evolution in living natural systems are quasi-equilibrium processes. It was shown that models of living systems are analogues of models of equilibrium (quasi-equilibrium) chromatographic columns.
These facts allowed creation of the quasi-equilibrium thermodynamics of near to equilibrium quasi-closed systems. This thermodynamics is based on the statement that the functions of state (with a good approximation) at any moment of time in quasi-closed monohierarchical systems have a real physical meaning (sense).
Thus, classical thermodynamics in a linear approximation (the thermodynamics of near to equilibrium systems) at the phenomenological level can be used for the investigation of the origin of life, biological evolution, and the development and aging of organisms. The investigations are carried out in terms of kinetic (dynamical) linear thermodynamics.
It has been shown that the variation of the chemical composition of living beings in the course of ontogenesis and phylogenesis is a consequence of change in the mean specific value of the Gibbs function for supramolecular (intermolecular) interaction in formation of supramolecular structures of an organism's tissues, which tends to a minimum. More strictly speaking, this variation is connected with the trend of mean specific values of the Gibbs function related to a unit of volume or mass at all hierarchical levels to a minimum.
The principle of the substance stability–feedback has been formulated. It is applicable to any biological systems (belonging to different hierarchies). For instance, this principle explains the accumulation of a substance with a chemically high energy capacity by biological systems in the course of evolution and aging of living beings. This substance forces water out of these systems.
The arguments presented in the author’s works (in my view, well-substantiated) indicate that practically all concrete (detailed) recommendations relating to nutrition (and lifestyle) are individual. They should be formulated on the basis of general and anti-aging medicine (gerontology) and should take into account the findings of physicochemical dietetics. Nevertheless, the thermodynamic theory of biological evolution and the aging of living organisms (built on the foundation of classical science) provides an opportunity to formulate general concepts pertaining to nutrition and helping prolong healthy human life.
2. On the Principle of Substance Stability
The principle of substance stability describes the tendency (trend) of natural systems to local and general equilibria at all temporal and structural levels of the organization of matter [3,4,5,6,7]. It corresponds to the second law of the Clausius–Gibbs thermodynamics (thermostatics) and the Le Chatelier–Braun principle. The principle of substance stability is determined by the limited energetic potential (the Gibbs potential energy) of associated (interacting) elementary structures of every hierarchy. This principle appears at all hierarchical levels (temporal and structural) of living matter. It is connected with the fact that we can observe its action at time scales corresponding to our capabilities.
The principle was formulated by the author, who would like to obtain an understanding and the greatest simplicity for the creation of thermodynamic models of our world. Such an approach was applied by classics of science, including J. Willard Gibbs [1] and Henri Poincaré [2].
The formulation of the principle of substance stability in the general form (applicable to temporal hierarchies) in accordance with the law of temporal hierarchies (see Appendix) was presented in the monograph Thermodynamic Theory of the Evolution of Living Beings [4]. However, this principle, together with the principle of structure stabilization, was used by the author in the first articles dedicated to the problems of the thermodynamics of biological evolution in 1977–1978 [3,8]. In those papers, the principle was presented in a form of figure for the explanation of the variation in the chemical composition of tissues during ontogenesis and phylogenies (evolution). It should be stressed that many well-known rules of human behavior and human traditions are in correspondence with this principle.
The application of the principle of stability of matter to the structures of adjacent hierarchies constitutes additional proof that quasi-equilibrium thermodynamics of quasi-closed systems can be applied to biological systems in the real world.
3. The Formulations of the Principle and their Explanation
Earlier, the author proposed different formulations of the principle of substance stability, which do not contradict each other [3,4,5,6,8,9,10].
The principle applied to molecular and supramolecular structures was named the principle of the stability of a chemical substance. Subsequently this principle was applied by the author to various hierarchies as a part of the theory of the evolution of life. It has been named the principle of stability of matter or the principle of substance stability – the feedback (Gladyshev’s) principle.
It boils down to the following: during the formation (self-assembly) of the most thermodynamically stable structures at the highest hierarchical level (j), e.g., the supramolecular level, Nature, in accordance with the second law, spontaneously uses predominantly the (available for the given local part of the biological system) least thermodynamically stable structures belonging to a lower level, for example, the molecular level (j-1). The justice of the principle is proved on a quantitative basis as applied to the molecular and supramolecular structural levels of biological tissues.
I would like to present an illustration. The supramolecular structures of the tissues (a higher level of structure j, compared to the molecular level, j-1) in the course of ontogenesis and phylogenesis accumulate relatively unstable molecules (substances with a relatively high chemical energy capacity), for instance, fats, which force water out of these tissues. Similar phenomena occur in some molecular chromatographic columns (as a rule, in hydrophobic cells and columns) [4,6,8,11,12,13,14,15]. All chemists know about it. These columns accumulate substance with a high energy capacity. These facts do not surprise us, although open heterogeneous adsorbent (absorbent) – adsorbate systems, approaching supramolecular equilibria, on the whole, move away from chemical equilibrium with the environment.
In this environment there are precisely those chemical substances that penetrate the column. The removal from chemical equilibrium with the environment is the consequence of the trend toward a minimum of the specific supramolecular component of the Gibbs function – the Gibbs free energy (e.g., for biological tissue),  im.
im.
 im.
im. Now, for best understanding, I would like to present a detailed illustration of the thermodynamic model of ontogenesis and the aging of living organisms. In some cases I will repeat the same statements.
4. Thermodynamic Model of Ontogenesis and the Aging of Living Organisms
The thermodynamic trend of the evolution of biological systems is easiest to identify in studying an organism’s evolutionary development (ontogenesis).
It is demonstrated that the motive force of ontogenesis (as well as phylogenesis) is the trend toward a minimum of the specific supramolecular component of the Gibbs function of the ith organism (biological tissue),  [4,6,8,10,11,13,16,17]. The symbol «−» means that we consider the mean value (relating to the macrovolume), and the symbol «~ » stresses that the system is heterogeneous.
[4,6,8,10,11,13,16,17]. The symbol «−» means that we consider the mean value (relating to the macrovolume), and the symbol «~ » stresses that the system is heterogeneous.
 [4,6,8,10,11,13,16,17]. The symbol «−» means that we consider the mean value (relating to the macrovolume), and the symbol «~ » stresses that the system is heterogeneous.
[4,6,8,10,11,13,16,17]. The symbol «−» means that we consider the mean value (relating to the macrovolume), and the symbol «~ » stresses that the system is heterogeneous.This trend is presented in the lower part of Figure 1.
Scales A and B are different (Δ  ch is much greater than Δ
ch is much greater than Δ  im). The time axis set by the second law of thermodynamics is scaleless. Jagged lines plotted onto the curves emphasize the fact that fluctuations of environmental parameters (temperature, pressure, diet, physical fields, time of day, season, etc.) change the levels Δ
im). The time axis set by the second law of thermodynamics is scaleless. Jagged lines plotted onto the curves emphasize the fact that fluctuations of environmental parameters (temperature, pressure, diet, physical fields, time of day, season, etc.) change the levels Δ  ch and Δ
ch and Δ  im. Organisms adapt to these fluctuations only within the limits of the adaptive zone (the range of tolerance).
im. Organisms adapt to these fluctuations only within the limits of the adaptive zone (the range of tolerance).
 ch is much greater than Δ
ch is much greater than Δ  im). The time axis set by the second law of thermodynamics is scaleless. Jagged lines plotted onto the curves emphasize the fact that fluctuations of environmental parameters (temperature, pressure, diet, physical fields, time of day, season, etc.) change the levels Δ
im). The time axis set by the second law of thermodynamics is scaleless. Jagged lines plotted onto the curves emphasize the fact that fluctuations of environmental parameters (temperature, pressure, diet, physical fields, time of day, season, etc.) change the levels Δ  ch and Δ
ch and Δ  im. Organisms adapt to these fluctuations only within the limits of the adaptive zone (the range of tolerance).
im. Organisms adapt to these fluctuations only within the limits of the adaptive zone (the range of tolerance).The use of the average integral value  im (or Δ
im (or Δ  im) obliges one to refer to a new branch of physical chemistry, supramolecular thermodynamics [3,4,8], which studies complex supramolecular structures without any detailed analysis at the molecular level. This approach does not contradict the methods of phenomenological thermodynamics and is, perhaps, currently the only effective approach to the study of the thermodynamic aspects of evolution, aging, and behavior of living systems.
im) obliges one to refer to a new branch of physical chemistry, supramolecular thermodynamics [3,4,8], which studies complex supramolecular structures without any detailed analysis at the molecular level. This approach does not contradict the methods of phenomenological thermodynamics and is, perhaps, currently the only effective approach to the study of the thermodynamic aspects of evolution, aging, and behavior of living systems.
 im (or Δ
im (or Δ  im) obliges one to refer to a new branch of physical chemistry, supramolecular thermodynamics [3,4,8], which studies complex supramolecular structures without any detailed analysis at the molecular level. This approach does not contradict the methods of phenomenological thermodynamics and is, perhaps, currently the only effective approach to the study of the thermodynamic aspects of evolution, aging, and behavior of living systems.
im) obliges one to refer to a new branch of physical chemistry, supramolecular thermodynamics [3,4,8], which studies complex supramolecular structures without any detailed analysis at the molecular level. This approach does not contradict the methods of phenomenological thermodynamics and is, perhaps, currently the only effective approach to the study of the thermodynamic aspects of evolution, aging, and behavior of living systems.As ontogenesis progresses, one can observe the growth of the energy capacity of the biological mass (top part of Figure 1), that is, its specific chemical component  ch or Δ
ch or Δ  ch (as well as the change in the specific enthalpy of the chemical component), which is a secondary effect. According to the second law, the thermodynamics of supramolecular interactions (or supramolecular thermodynamics) “benefits” by the accumulation in a biological system of chemical substances with a high energy capacity (the reference is to the chemical component Δ
ch (as well as the change in the specific enthalpy of the chemical component), which is a secondary effect. According to the second law, the thermodynamics of supramolecular interactions (or supramolecular thermodynamics) “benefits” by the accumulation in a biological system of chemical substances with a high energy capacity (the reference is to the chemical component Δ  ch), which oust water from this system. This can be explained by the fact that substances with a relatively high energy capacity have a heightened capacity for participation in the formation of supramolecular structures (the principle of the stability of a chemical substance).
ch), which oust water from this system. This can be explained by the fact that substances with a relatively high energy capacity have a heightened capacity for participation in the formation of supramolecular structures (the principle of the stability of a chemical substance).
 ch or Δ
ch or Δ  ch (as well as the change in the specific enthalpy of the chemical component), which is a secondary effect. According to the second law, the thermodynamics of supramolecular interactions (or supramolecular thermodynamics) “benefits” by the accumulation in a biological system of chemical substances with a high energy capacity (the reference is to the chemical component Δ
ch (as well as the change in the specific enthalpy of the chemical component), which is a secondary effect. According to the second law, the thermodynamics of supramolecular interactions (or supramolecular thermodynamics) “benefits” by the accumulation in a biological system of chemical substances with a high energy capacity (the reference is to the chemical component Δ  ch), which oust water from this system. This can be explained by the fact that substances with a relatively high energy capacity have a heightened capacity for participation in the formation of supramolecular structures (the principle of the stability of a chemical substance).
ch), which oust water from this system. This can be explained by the fact that substances with a relatively high energy capacity have a heightened capacity for participation in the formation of supramolecular structures (the principle of the stability of a chemical substance). 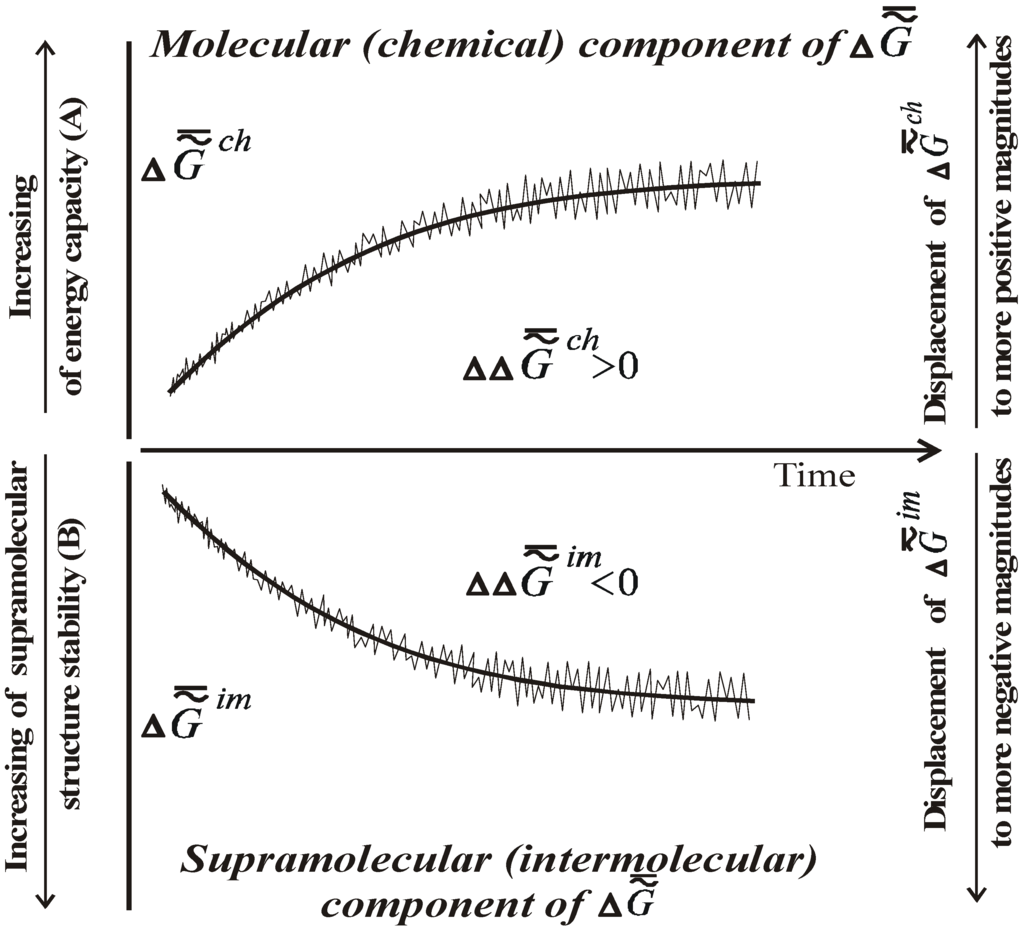
Figure 1.
Scheme of changes in the specific chemical energy capacity of the biological mass (biological tissue) (Δ  ch ) or the specific heat of combustion (A) and thermodynamic stability of its supramolecular structures during ontogenesis of living beings (Δ
ch ) or the specific heat of combustion (A) and thermodynamic stability of its supramolecular structures during ontogenesis of living beings (Δ  im) (B).
im) (B).
 ch ) or the specific heat of combustion (A) and thermodynamic stability of its supramolecular structures during ontogenesis of living beings (Δ
ch ) or the specific heat of combustion (A) and thermodynamic stability of its supramolecular structures during ontogenesis of living beings (Δ  im) (B).
im) (B).
The chemical composition of the supramolecular structures of biosystems changes slowly over temporal segments commensurate with the duration of adaptive processes and ontogenesis (as well as in phylogenies and during protracted stages of biological evolution as a whole). As biological tissue ages, supramolecular structures become more stable thermodynamically (needless to say, the reference is to the stability of the supramolecular structures themselves, not that of the chemical substance forming them: the energy capacity of the latter rises, while its stability decreases).
The selection of thermodynamically more stable supramolecular structures (structural stabilization of the phase) is determined by the thermodynamic factor: it is postulated that the time of retention, or retention time (the term was borrowed from chromatography), of molecules (macromolecules) at the supramolecular phase t imret is connected with the value of the Gibbs function of the formation of supramolecular structures:
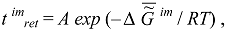 where A is a coefficient, R is the gas constant, and T is temperature.
where A is a coefficient, R is the gas constant, and T is temperature.
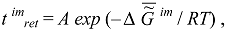
The molecules retained longest (which are products of biosynthesis or have entered the biosystem from the external environment) located in the supramolecular phase promote the selection of their own kind, which also changes the composition (and chemical nature) of the phase of supramolecular structures. As was noted above, this change is a consequence of the operation of the thermodynamic factor, although in this case it manifests itself through kinetics (Equation (4.1)). Thus, at the phase of supramolecular structures, there occurs an accumulation of molecules (absorption and adsorption) whose self-assembly is the most advantageous in thermodynamic terms (these molecules have a heightened affinity with the phase of supramolecular structures). In the presence of a matrix synthesis mechanism, these molecules have advantages in reduplication (multiplication). As a result, the specific Gibbs function of the formation of supramolecular structures Δ  im (or the specific value of the Helmholtz function, which practically coincides with it in the condensed phase) increases in absolute value, becoming more negative as biological tissue evolves (ages).
im (or the specific value of the Helmholtz function, which practically coincides with it in the condensed phase) increases in absolute value, becoming more negative as biological tissue evolves (ages).
 im (or the specific value of the Helmholtz function, which practically coincides with it in the condensed phase) increases in absolute value, becoming more negative as biological tissue evolves (ages).
im (or the specific value of the Helmholtz function, which practically coincides with it in the condensed phase) increases in absolute value, becoming more negative as biological tissue evolves (ages).For clarity, I would like to say that, in formulating the principle of substance stability, I consider two cases (in the figure 1, the index i at  and
and 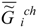 are omitted).
are omitted).
 and
and 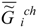 are omitted).
are omitted). Case 1. In this case, I am speaking about the tendency of the specific value of the Gibbs function of the complex system “investigated system – environment”  (1) to a minimum. This tendency is connected with the evolutional variation of the chemical composition of the system. The chemical composition of the system changes over a long time scale (during ontogenesis and phylogenesis – evolution).
(1) to a minimum. This tendency is connected with the evolutional variation of the chemical composition of the system. The chemical composition of the system changes over a long time scale (during ontogenesis and phylogenesis – evolution).
 (1) to a minimum. This tendency is connected with the evolutional variation of the chemical composition of the system. The chemical composition of the system changes over a long time scale (during ontogenesis and phylogenesis – evolution).
(1) to a minimum. This tendency is connected with the evolutional variation of the chemical composition of the system. The chemical composition of the system changes over a long time scale (during ontogenesis and phylogenesis – evolution). Case 2. In this case, the relatively supramolecular (and chemical - molecular) structural stability of the investigated systems is compared at each concrete moment of time (at a certain moment of time of ontogenesis and phylogenesis). Here the specific values of the Gibbs function of structure formation  (2) for the investigated systems are compared. In this case, an internal supramolecular equilibrium in each of the investigated systems of constant chemical and supramolecular composition is established and the value of
(2) for the investigated systems are compared. In this case, an internal supramolecular equilibrium in each of the investigated systems of constant chemical and supramolecular composition is established and the value of  (2) reaches a minimum. Here one can speak of the relatively thermodynamic stability of the structure of an investigated system.
(2) reaches a minimum. Here one can speak of the relatively thermodynamic stability of the structure of an investigated system.
 (2) for the investigated systems are compared. In this case, an internal supramolecular equilibrium in each of the investigated systems of constant chemical and supramolecular composition is established and the value of
(2) for the investigated systems are compared. In this case, an internal supramolecular equilibrium in each of the investigated systems of constant chemical and supramolecular composition is established and the value of  (2) reaches a minimum. Here one can speak of the relatively thermodynamic stability of the structure of an investigated system.
(2) reaches a minimum. Here one can speak of the relatively thermodynamic stability of the structure of an investigated system.Thus, the index i (in  ) in case 1 is related to the system of variable composition (the long time scale). In case 2, this index is related to the systems of constant composition (the short time scale). These statements are valid for all temporal hierarchies of the living world. In the figures of such type, the index i may be omitted.
) in case 1 is related to the system of variable composition (the long time scale). In case 2, this index is related to the systems of constant composition (the short time scale). These statements are valid for all temporal hierarchies of the living world. In the figures of such type, the index i may be omitted.
 ) in case 1 is related to the system of variable composition (the long time scale). In case 2, this index is related to the systems of constant composition (the short time scale). These statements are valid for all temporal hierarchies of the living world. In the figures of such type, the index i may be omitted.
) in case 1 is related to the system of variable composition (the long time scale). In case 2, this index is related to the systems of constant composition (the short time scale). These statements are valid for all temporal hierarchies of the living world. In the figures of such type, the index i may be omitted.On account of the principle of substance stability, the Gibbs functions of all hierarchical levels (during ontogenesis and phylogenesis – evolution) are shifted slowly to less negative values (figure 2).
It is very important to remember that the principle of substance stability is the qualitative (semi-quantitative) principle. Precise mathematical formulation of the principle may be impeded, because the measurement of the absolute values of thermodynamic functions is not possible.
The assessment of the value of Δ  im can be performed with the use of the approximate Gibbs– Helmholtz–Gladyshev equation [6,9,18], which can be used for the investigation of quasi-closed systems of variable composition. For the ith systems (substances) we have
im can be performed with the use of the approximate Gibbs– Helmholtz–Gladyshev equation [6,9,18], which can be used for the investigation of quasi-closed systems of variable composition. For the ith systems (substances) we have
 where Δ
where Δ  is the specific Gibbs function (specific Gibbs free energy) of the formation of the condensed phase of the substance (matter) i ,
is the specific Gibbs function (specific Gibbs free energy) of the formation of the condensed phase of the substance (matter) i , 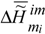 and
and 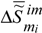 are the changes in the specific enthalpy and entropy during self-assembly,
are the changes in the specific enthalpy and entropy during self-assembly, 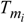 is the melting or freezing point (mean value), and T0 is the standard temperature (e.g., 25°C) at which the calculation of values of Δ
is the melting or freezing point (mean value), and T0 is the standard temperature (e.g., 25°C) at which the calculation of values of Δ  is done. It follows from Equation (4.2) that, in a certain approximation, there should be a correlation between Δ
is done. It follows from Equation (4.2) that, in a certain approximation, there should be a correlation between Δ  (calculated for the standard temperature) or the indicator of the nutrition product’s anti-aging (gerontological) value GPGi [6,19] and, for example, the congealing (pouring, freezing, or melting) point of the fats or oils [15,9,6]. Note that the GPGi indicator is proportionate to the value of Δ
(calculated for the standard temperature) or the indicator of the nutrition product’s anti-aging (gerontological) value GPGi [6,19] and, for example, the congealing (pouring, freezing, or melting) point of the fats or oils [15,9,6]. Note that the GPGi indicator is proportionate to the value of Δ  .
.
 im can be performed with the use of the approximate Gibbs– Helmholtz–Gladyshev equation [6,9,18], which can be used for the investigation of quasi-closed systems of variable composition. For the ith systems (substances) we have
im can be performed with the use of the approximate Gibbs– Helmholtz–Gladyshev equation [6,9,18], which can be used for the investigation of quasi-closed systems of variable composition. For the ith systems (substances) we have

 is the specific Gibbs function (specific Gibbs free energy) of the formation of the condensed phase of the substance (matter) i ,
is the specific Gibbs function (specific Gibbs free energy) of the formation of the condensed phase of the substance (matter) i , 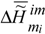 and
and 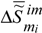 are the changes in the specific enthalpy and entropy during self-assembly,
are the changes in the specific enthalpy and entropy during self-assembly, 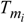 is the melting or freezing point (mean value), and T0 is the standard temperature (e.g., 25°C) at which the calculation of values of Δ
is the melting or freezing point (mean value), and T0 is the standard temperature (e.g., 25°C) at which the calculation of values of Δ  is done. It follows from Equation (4.2) that, in a certain approximation, there should be a correlation between Δ
is done. It follows from Equation (4.2) that, in a certain approximation, there should be a correlation between Δ  (calculated for the standard temperature) or the indicator of the nutrition product’s anti-aging (gerontological) value GPGi [6,19] and, for example, the congealing (pouring, freezing, or melting) point of the fats or oils [15,9,6]. Note that the GPGi indicator is proportionate to the value of Δ
(calculated for the standard temperature) or the indicator of the nutrition product’s anti-aging (gerontological) value GPGi [6,19] and, for example, the congealing (pouring, freezing, or melting) point of the fats or oils [15,9,6]. Note that the GPGi indicator is proportionate to the value of Δ  .
.The thermodynamic theory of ontogenesis can help explain the phenomenon of adaptation of living organisms to changes in various environmental factors [5,8,12]. Thus, a change in the temperature 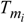 and chemical composition of food causes changes in the composition of the organism’s biological tissues.
and chemical composition of food causes changes in the composition of the organism’s biological tissues.
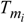 and chemical composition of food causes changes in the composition of the organism’s biological tissues.
and chemical composition of food causes changes in the composition of the organism’s biological tissues.Note that the scheme of ontogenesis presented (Fig. 1) is applicable to phylogenies and to biological evolution generally.
5. The Scheme of Application of the Principle to all Hierarchies of Living Matter
As was already noted, the principle of substance stability is evident at all hierarchical levels (temporal and structural) of living matter. A similar removal from “inferior” or “lower” equilibria takes place in all biological systems (belonging to different hierarchies). This corresponds to the quasi-equilibrium thermodynamics of quasi-closed systems. The second law of thermodynamics, as it was formulated by Gibbs [1,6,20,21], acts everywhere! We should take it into account in the case of specification of the scheme of changes in the specific Gibbs function of every hierarchy, precisely, every monohierarchy (see, for example, the figures in works [6,9,22].
The levels of the Gibbs function of formation of all monohierarchical systems during the emergence and degradation of living matter shift to lesser negative values.
Figure 2 presents the scheme of the change in the Gibbs function (the Gibbs free energy of formation of structures of the biological world). This scheme is a scheme of the cycle of the relative circulation of matter in nature. The cycle can be studied from the standpoint of hierarchical thermodynamics.
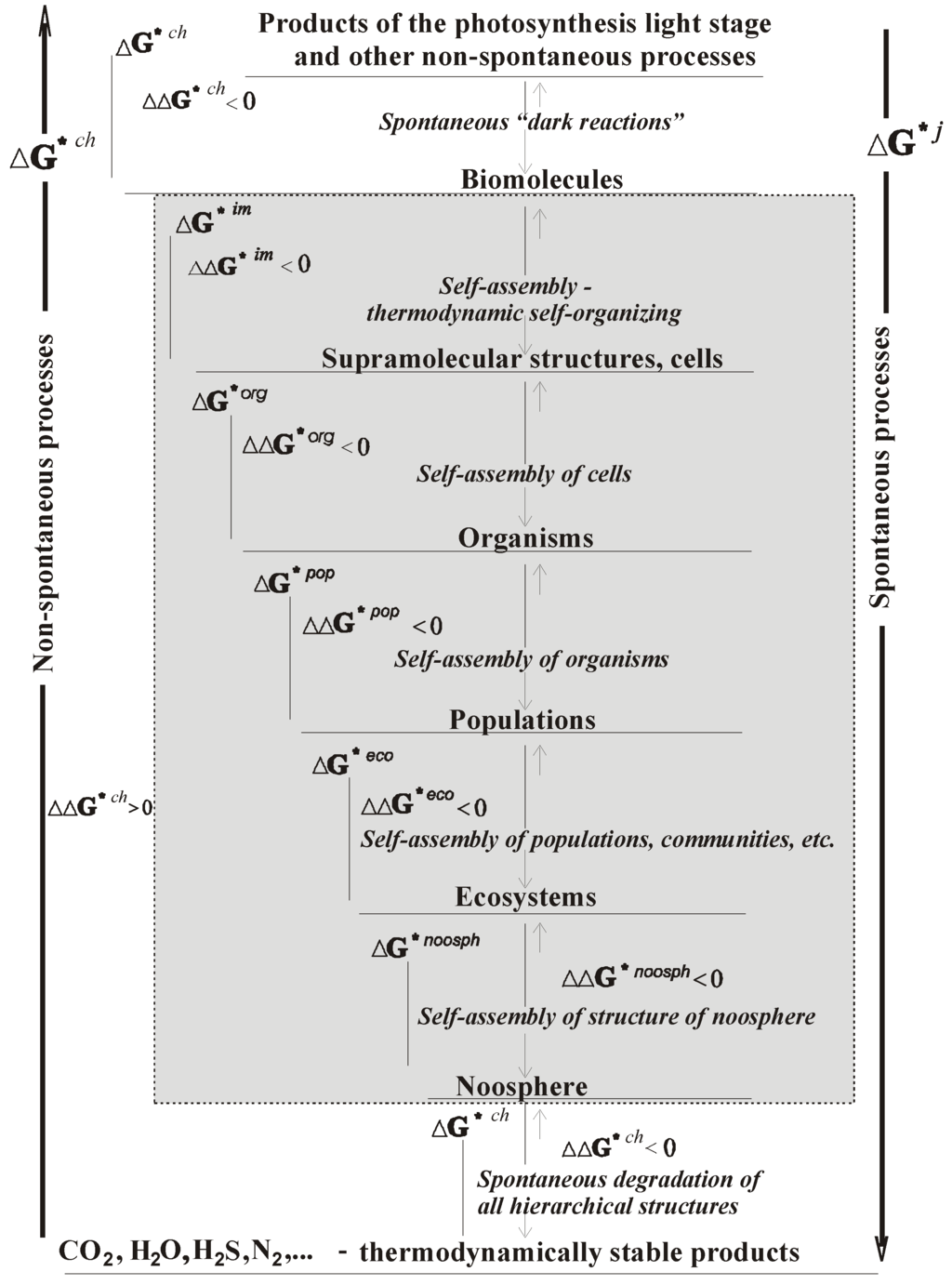
Figure 2.
Scheme of the change in the Gibbs function of formation of complex systems ΔG * I [6,8] during the emergence and degradation of chemical (ch) and supramolecular structures (im), as well as organisms (organism), populations (pop), communities (com), ecosystems (eco), and the noosphere (noosph). The index j is the type of structural hierarchy.
The long arrows in the center (the gray area) of the scheme at the bottom show the direction of thermodynamic self-organization (self-assembly) of the elementary structures of every (j-1)th monohierarchy, which are condensed to form the structures of the next (higher) jth monohierarchy. The short parallel arrows of the scheme show the displacement of Gibbs function to the back side – to lesser negative values during ontogenesis, phylogenesis, and evolution. This, as was already noted, is a consequence of the effect of the principle of substance stability!
Obviously, the motive force of the non-spontaneous processes of the cycle of matter, first of all, is connected with the Sun. In terms of “dark” spontaneous processes, the motive force of the self-assembly and evolution of biological structures at all hierarchical levels is “thermodynamic forces.”
In conformity with the principle of energy differentiation [3,4,8] (and the law of temporal hierarchies), the specific values of the Gibbs function of self-assembly (thermodynamic self-organization) at different hierarchical levels differ significantly. Thus, there exists the series
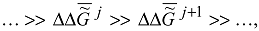 where ΔΔ
where ΔΔ  j and ΔΔ
j and ΔΔ  j+1 are the changes in the specific values of the Gibbs function of formation of structural hierarchies j and j + 1 calculated for a unit of volume or mass. In other words, the coordinate axes (the ordinate axes) of the scheme presented in Fig. 2 are of significantly different scale. The scale of this axis is the logarithmic scale (a rough estimation).
j+1 are the changes in the specific values of the Gibbs function of formation of structural hierarchies j and j + 1 calculated for a unit of volume or mass. In other words, the coordinate axes (the ordinate axes) of the scheme presented in Fig. 2 are of significantly different scale. The scale of this axis is the logarithmic scale (a rough estimation).
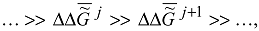
 j and ΔΔ
j and ΔΔ  j+1 are the changes in the specific values of the Gibbs function of formation of structural hierarchies j and j + 1 calculated for a unit of volume or mass. In other words, the coordinate axes (the ordinate axes) of the scheme presented in Fig. 2 are of significantly different scale. The scale of this axis is the logarithmic scale (a rough estimation).
j+1 are the changes in the specific values of the Gibbs function of formation of structural hierarchies j and j + 1 calculated for a unit of volume or mass. In other words, the coordinate axes (the ordinate axes) of the scheme presented in Fig. 2 are of significantly different scale. The scale of this axis is the logarithmic scale (a rough estimation).The Gibbs function of formation of molecules and supramolecular structures as complex systems often coincides, under the conditions of the Earth, with the Gibbs function of formation of the corresponding simple systems. In view of this, the asterisk in ΔG * i may be omitted.
Thus, the principle of the stability of matter or the principle of the stability of substance (feedback) is connected with the evolutional trend of mean specific values of the Gibbs function related to a unit of volume or mass at all hierarchical levels to a minimum.
For example, as was stated by the author in 1977–1978, biological objects become (primarily) chemically unstable (instable) during life. These objects age as a result of degradation [3]. Recently, Professor Leo Heiflick has noted this obvious circumstance. He wrote to the author: “I believe, as you do, that aging in biological material is caused by the thermodynamic instability of molecules. This instability increases at rates faster than the capacity for repair. I have described this in some recent papers ….” Here Professor Heiflick is speaking about the thermodynamic instability of chemical (molecular) substances.
The author applied the principle of substance stability to the structural hierarchies that function inside any temporal hierarchy. These structural hierarchies have been named “understructure hierarchies.” An illustration of the principle is provided by the selection of a sequence of nucleic acids including AU pairs in evolution, although these pairs are less stable from the standpoint of supramolecular thermodynamics than GC pairs. Hence, the selection of natural (AUGC) sequences takes into account not only the stability of the lower understructure supramolecular hierarchy, as was sometimes previously believed, but also the stability of the highest understructure supramolecular hierarchy, as well as tertiary, quaternary, and the highest supramolecular structures – nucleic acid–protein complexes.
There are some facts that call for application of the principle of substance stability to the hierarchy of cells. Thus, tumor cells have a lower ability for aggregation. As a result, they easily move in the body, which leads to the appearance of metastases. The cell membranes of tumor cells are, apparently, formed from supramolecular structures of increased stability. Hence, the supramolecular stability of cell aggregates formed with the participation of tumor cells should be lowered according to the principle in question. In order to increase the adhesive ability of the cells, the structure of membranes should be “diluted” and made less thermodynamically stable. Hence, it is clear why experimental anticancer diets propose the use of plant oils, fats of animals from cold seas, and other products containing residues of unsaturated low-melting-point fatty acids. The anti-tumor effect of aspirin can also be explained on the basis of such statements. These ideas agree with the recommendations made using the thermodynamic theory of aging [6,23] (in [23] there are many inadmissible errors in English, e.g. p. 437; in the Russian version of this journal there are no such errors).
The principle of substance stability allows us to understand the effect of the influence of some chemical substances on the supramolecular structures of nucleic acids [6]. As a result of the action of such substances, “sleeping” ancient genes (accumulated during the evolution of living beings) can awake. These genes can stimulate some types of cancer.
The conclusions of hierarchical thermodynamic are excellent correspond to the conception of Libb Thims about the thermodynamics of “human molecules” [24].
I would like to note that the author’s conception of evolution and life, which were first put forth in 1977–1978, correspond to James Lovelock’s Gaia theory of life on the Earth, which he proposed in 1979. The basis of his well-known ecological theory is that the Earth is a self-regulating organism that adjusts to changes in order to maintain suitable conditions for life.
Some facts confirm the author’s point of view that feedback between all hierarchical levels of the biological world is based on hierarchical thermodynamics. These feedbacks can be schematically presented as a sequence: biosphere → ecosystems → populations’ → organisms’ → cells → supramolecular structures → proteins and some other macromolecules → DNA (RNA) [5,6,10,23]. Hence, the principle in question is applicable to all hierarchies (and understructure hierarchies), including the molecular and supramolecular structures for which it was first formulated.
I believe that the principle of substance stability in various forms can be extended to all hierarchies of matter [5,6,10].
6. Conclusion
Some investigators, it is possible, will call attention to the fact that much of what I am writing about is already known! Much, but not all. And this is not the point! The point is that, on the physical basis of hierarchical thermodynamics, a general physical theory, it was possible to reveal a generality (unity) that is characteristic of the living world (and not only the living one). Of course, this unity, the principle of substance stability, generalizing the simplicity of many thermodynamic quantitative models, corresponds to a philosophical method of cognition of the world that has developed over centuries: first, simplicity is revealed, and then from an aggregate of simplicities, unity is revealed [2,21]. However, if an aggregate of unities exists, it again may lead to a simplicity, which will embody a new unity [21].
The thermodynamic theory applied to the living world should not only explain all that is reliably known but also predict new knowledge.
Appendix
The law of temporal hierarchies (which some researchers have began to call Gladyshev’s law) can be presented as a series of strong inequalities. The direction of this series is towards increasing average life-spans of structures on going from lower to higher structures. In the simplest case, this law can be presented as
where t m (t ch) is the average life-span (duration of existence) of the organism’s molecules (chemical compounds) taking part in metabolism; t im (t supra) is the average life-span of any intermolecular (supramolecular) structures of the organism’s tissues renovated in the process of its growth and development; t organism is the average life-span of organisms in the population; and t pop is the average life-span of the populations. For the sake of simplicity and clarity, I consciously (intend) omit in the series of strong inequalities (A.1) the life-span of cells and some other complex supramolecular structures. Needless to say, this series (determined by the presence of metabolism in the world of living matter) accords well with reality and reflects the existence of temporal hierarchies in living systems. This rigorously substantiates the possibility of identifying (separating) monohierarchical quasi-closed systems (subsystems) belonging to different temporal (structural) hierarchies in open polyhierarchical biological systems. Note that each type (species) of organism is characterized by its own average life-span values for different-type hierarchies. However, series (A.1) is observed for each species of organism.
…<< t m << t im << t organism << t pop << …
The series of times of imagined relaxation of different-hierarchy structures postulated by the author in 1976 had an opposite direction as compared to series (A.1). Nevertheless, both these series give reason to make a conclusion on the possibility of identifying quasi-closed systems in open biological objects. I believe there is a profound link between the directions of these series of the times of imagined relaxation and the life-spans of different-hierarchy structures. The sources of this link can, I believe, be identified on a statistical basis for an ideal structural hierarchical model. In any case, I can perceive a simple route towards comprehending the existence of this link.
Thus, the law of temporal hierarchies makes it possible to identify, in open biological systems, quasi-closed thermodynamic systems (subsystems) and to study their development (ontogenesis) and evolution (phylogenesis) by studying the change in the specific (per unit of volume or mass) value of the Gibbs function of formation of the given higher hierarchical structure from structures of a lower level.
It was established that, in the process of ontogenesis (as well as phylogenesis and evolution generally), the specific value of the Gibbs function of formation of supramolecular structures of the tissues of an ith organism  tends toward a minimum:
tends toward a minimum:
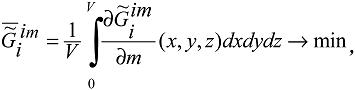 where V is the volume of the system; m is the mass of the identified microvolumes; x, y, and z are coordinates; the symbol “−” means that value
where V is the volume of the system; m is the mass of the identified microvolumes; x, y, and z are coordinates; the symbol “−” means that value  is specific (relating to the macrovolume); and the symbol “~” stresses the heterogeneous character of the system. For clarity, I would like to say once again that the correlation (A.2) shows the tendency of the specific value of the Gibbs function of the complex system “investigated system – environment”
is specific (relating to the macrovolume); and the symbol “~” stresses the heterogeneous character of the system. For clarity, I would like to say once again that the correlation (A.2) shows the tendency of the specific value of the Gibbs function of the complex system “investigated system – environment”  (1) to a minimum. This tendency is connected with the evolutional variation of the chemical composition of the system. The chemical composition of the system changes over a long time scale (during ontogenesis and phylogenesis – evolution).
(1) to a minimum. This tendency is connected with the evolutional variation of the chemical composition of the system. The chemical composition of the system changes over a long time scale (during ontogenesis and phylogenesis – evolution).
 tends toward a minimum:
tends toward a minimum:
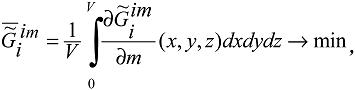
 is specific (relating to the macrovolume); and the symbol “~” stresses the heterogeneous character of the system. For clarity, I would like to say once again that the correlation (A.2) shows the tendency of the specific value of the Gibbs function of the complex system “investigated system – environment”
is specific (relating to the macrovolume); and the symbol “~” stresses the heterogeneous character of the system. For clarity, I would like to say once again that the correlation (A.2) shows the tendency of the specific value of the Gibbs function of the complex system “investigated system – environment”  (1) to a minimum. This tendency is connected with the evolutional variation of the chemical composition of the system. The chemical composition of the system changes over a long time scale (during ontogenesis and phylogenesis – evolution).
(1) to a minimum. This tendency is connected with the evolutional variation of the chemical composition of the system. The chemical composition of the system changes over a long time scale (during ontogenesis and phylogenesis – evolution). Note that correlation (A.2) implies taking into account intermolecular interactions in all hierarchical structures of the biological tissue (intra- and extracellular interactions). This is fully justified since structural hierarchy does not always coincide with temporal hierarchy. Thus, some types of cells do not divide (according to current views) and, like organs, age along with the organism. However, for any supramolecular hierarchy (j-1) there exists some higher (j + х) hierarchy, so that t j-1<< t j +x, where t j-1 and t j +x are the average life-spans (lifetimes) of elementary structures of the corresponding structural hierarchies in a living system, х = 0, 1, 2, … , etc.
Acknowledgments
I am grateful to Prof. G. Arrhenius, Prof. N.N. Bogolyubov, Jr., Dr. K.G. Denbigh, Prof. E.T. Denisov, Prof. V.P. Kazakov, Prof. Y.S. Lipatov, Prof. Y.B. Monakov, Prof. A.A. Logunov, Dr. Shu-Kun Lin, Prof. V.V. Sychev, Prof. V.M. Frolov, for the support, systematic fruitful discussions, and valuable advice.
References
- Gibbs, J.W. The Collected Works of J. Willard Gibbs. Thermodynamics; Longmans, Green and Co.: New York, 1928; Volume V. 1, pp. 55–349. [Google Scholar]
- Poincaré, A. On Science; Nauka: Moscow, 1983. (in Russian) [Google Scholar]
- Gladyshev, G.P. On the Thermodynamics of Biological Evolution. J. Theor. Biology 1978, 75, 425–441, (Preprint of Institute of Chemical Physics of Academy of Sciences of the USSR, May 1977). [Google Scholar] [CrossRef]
- Gladyshev, G.P. Thermodynamic Theory of the Evolution of Living Beings; Nova Sci. Publ. Inc.: New York, 1997; p. 142. [Google Scholar]
- Gladyshev, G.P. On the principle of Substance Stability and Thermodynamic Feedback in Hierarchic System of Bioworld. Biology Bulletin 2002, 29, 1–4. [Google Scholar] [CrossRef]
- Gladyshev, G.P. Supramolecular Thermodynamics is a Key to Understanding Phenomenon of Life. What is Life from a Physical Chemist’s Viewpoint, Second Ed. ed; Regular and Chaotic Dynamics: Moscow–Izhevsk, 2003; p. 144. (in Russian) [Google Scholar]
- Gladyshev, G.P. Internet: http://www.endeav.org/evolut/age/evol.htm.
- Gladyshev, G.P. Thermodynamics and macrokinetics of natural hierarchical processes; Nauka: Moscow, 1988; p. 287. (in Russian) [Google Scholar]
- Gladyshev, G.P. Thermodynamic self-organization as a mechanism of hierarchical structures formation of biological matter. Progress in Reaction Kinetics and Mechanism 2003, 28, 157–188. [Google Scholar] [CrossRef]
- Gladyshev, G.P. Macrothermodynamics of Biological Evolution: Aging of Living Beings. International Journal of Modern Physics B. 2004, 18, 801–825. [Google Scholar] [CrossRef]
- Gladyshev, G.P. A Motive Force of Biological Evolution. Herald of Russian Academy of Science 1994, 64, 118–124. [Google Scholar]
- Gladyshev, G.P.; Kitaeva, D.K.; Ovcharenko, E.N. Why does the Chemical Composition of Living Things Adapt the Environment. J. Biol. Systems 1996, 4, 555. [Google Scholar]
- Gladyshev, G.P. Thermodynamic theory of biological evolution and aging. Experimental confirmations of theory. Entropy. 1999, 4, pp. 55–68, (http://www.mdpi.org/entropy/).
- Gladyshev, G.P. The Hierarchical Equilibrium Thermodynamics of Living Systems in Action. SEED Journal. 2002, 3, pp. 42–59, (http://www.library.utoronto.ca/see/pages).
- Gladyshev, G.P. Thermodynamics of biological evolution and aging. Electron. J. Math. Phys. Sci. 2002, Sem. 2, 1–15. [Google Scholar]
- Gladyshev, G.P. Thermodynamics of Aging. In 1998 AAAS Annual Meeting and Science Innovation Exhibition; American Association for the Advancement of Science: Philadelphia, 1998; pp. A-30, S-26. [Google Scholar]
- Gladyshev, G.P. On the Thermodynamics, Entropy and Evolution of Biological Systems: What is Life from a Physical Chemist's Viewpoint. Entropy 1999, 1, 9–20. [Google Scholar] [CrossRef]
- Kozlov, G.V.; Novikov, V.U. A cluster model for the polymer amorphous state. Physica-Uspekhi 2001, 44, 681–724. [Google Scholar] [CrossRef]
- Gladyshev, G.P. The method for measuring the gerontological value of bio-active substances and compositions, mainly foodstuffs and cosmetics. Canadian Patent 2,327,747, 2004. [Google Scholar]
- Gladyshev, Georgi P. The second law of thermodynamics and evolution. In Reports of 18-th International Conf. on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, NTNU, Trondheim, Norway, June 20–23 2005; 2005. (http://www.chem.ntnu.no/ecos2005/). [Google Scholar]
- Gladyshev, G.P. The Mathematical Physics and the Theory of Life. History of science and techniques 2005. [Google Scholar]
- Gladyshev, G.P. Macrothermodynamics of Biological Evolution and Aging of Living Beings: Physical Chemistry of Dietaries. In Proceedings of International Higher Education Academy of Sciences; 2003; 4, pp. 19–46. [Google Scholar]
- Gladyshev, G.P. Thermodynamics of Aging. Biology Bulletin 1998, 25, 433–441. [Google Scholar]
- Thims, Libb. The papers in the field of human thermodynamics. Institute of Human Thermodynamics: Chicago, 2005. Internet: http://www.humanthermodynamics.com/HT-history.html, http://en.wikipedia.org/wiki/Thermodynamic_evolution.
- Penrose, R. The Emperor’s New Mind. Concerning Computers, Minds and The Laws of Physics; Oxford University Press: Oxford, 1999; Possible relevance to brain plasticity; pp. 437–439. [Google Scholar]
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.