Antibody-mediated Prevention of Fusarium Mycotoxins in the Field
Abstract
:1. Introduction
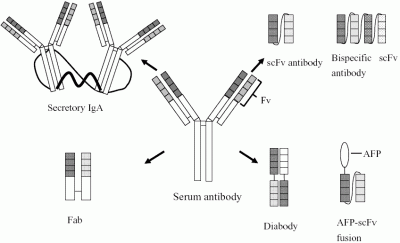
2. Antibodies specific for Fusarium mycotoxins and mycotoxin-producing fungi
3. Antibody expression in plants
4. Antibody-mediated prevention of Fusarium mycotoxins in field and postulated mechanisms
5. Conclusions
Acknowledgments
References
- Bennett, JW; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar]
- Murphy, PA; Hendrich, S; Landgren, C; Bryant, CM. Food mycotoxins: An update. J. Food Sci. 2006, 71, R51–R65. [Google Scholar]
- Chen, LF; Bai, GH; Desjardins, AE. Recent Advances in Wheat Head Scab Research in China; ; US Department of Agriculture; National Agricultural Library Internet Publication: Beltsville, MD, USA, Published online, 2000.
- Liu, S; Anderson, JA. Marker assisted evaluation of Fusarium head blight resistant wheat germplasm. Crop Sci. 2003, 43, 760–766. [Google Scholar]
- Windels, CE. Economic and social impacts of Fusarium head blight: Changing farms and rural communities in the Northern Great Plains. Phytopathology 2000, 90, 17–21. [Google Scholar]
- Dahleen, LS; Okubara, PA; Blechl, AE. Transgenic approaches to combat Fusarium head blight in wheat and barley. Crop Sci. 2001, 41, 628–637. [Google Scholar]
- Liu, DJ. Genome analysis in wheat breeding for disease resistance. Acta Bot. Sin. 2002, 44, 1096–1104. [Google Scholar]
- Anand, A; Zhou, T; Trick, HN; Gill, BS; Bockus, WW; Muthukrishnan, S. Greenhouse and field testing of transgenic wheat plants stably expressing genes for thaumatin-like protein, chitinase and glucanase against. Fusarium graminearum. J. Exp. Bot. 2003, 54, 1101–1111. [Google Scholar]
- Di, R; Tumer, NE. Expression of a truncated form of ribosomal protein L3 confers resistance to pokeweed antiviral protein and the Fusarium mycotoxin deoxynivalenol. Mol. Plant-Microbe. Interact. 2005, 18, 762–770. [Google Scholar]
- Mackintosh, CA; Lewis, J; Radmer, LE; Shin, S; Heinen, SJ; Smith, LA; Wyckoff, MN; Dill-Macky, R; Evans, CK; Kravchenko, S; Baldridge, GD; Zeyen, RJ; Muehlbauer, GJ. Overexpression of defense response genes in transgenic wheat enhances resistance to Fusarium head blight. Plant Cell Rep. 2007, 26, 479–488. [Google Scholar]
- Okubara, PA; Blechl, AE; McCormick, SP; Alexander, NJ; Dill-Macky, R; Hohn, TM. Engineering deoxynivalenol metabolism in wheat through the expression of a fungal trichothecene acetyltransferase gene. Theor. Appl. Genet. 2002, 106, 74–83. [Google Scholar]
- Hiatt, EE; Hill, NS; Hiatt, EN. Monoclonal antibodies incorporated into Neotyphodium coenophialum fungal cultures: Inhibition of fungal growth and stability of antibodies. Fungal Genet. Biol. 2001, 33, 107–114. [Google Scholar]
- Maiti, IB; Kolattukudy, PE. Prevention of fungal infection of plants by specific inhibition of cutinase. Science 1979, 205, 507–508. [Google Scholar]
- Boonrod, K; Galetzka, D; Nagy, PD; Conrad, U; Krczal, G. Single-chain antibodies against a plant viral RNA-dependent RNA polymerase confer virus resistance. Nat. Biotechnol. 2004, 22, 856–862. [Google Scholar]
- Nickel, H; Kawchuk, L; Twyman, RM; Zimmermann, S; Junghans, H; Winter, S; Fischer, R; Prüfer, D. Plantibody-mediated inhibition of the Potato leafroll virus P1 protein reduces virus accumulation. Virus Res. 2008, 136, 140–145. [Google Scholar]
- Peschen, D; Li, HP; Fischer, R; Kreuzaler, F; Liao, YC. Fusion proteins comprising a Fusarium-specific antibody linked to antifungal peptides protect plants against a fungal pathogen. Nat. Biotechnol. 2004, 22, 732–738. [Google Scholar]
- Tavladoraki, P; Benvenuto, E; Trinca, S; de Martinis, D; Cattaneo, A; Galeffi, P. Transgenic plants expressing a functional single-chain Fv antibody are specifically protected from virus attack. Nature 1993, 366, 469–472. [Google Scholar]
- Zhang, MY; Zimmermann, S; Fischer, R; Schillberg, S. Generation and evaluation of movement protein-specific single-chain antibodies for delaying symptoms of Tomato spotted wilt virus infection in tobacco. Plant Pathol. 2008. [Google Scholar]
- Zimmermann, S; Schillberg, S; Liao, YC; Fisher, R. Intracellular expression of TMV-specific single-chain Fv fragments leads to improved virus resistance in shape. Nicotiana tabacum. Mol. Breed. 1998, 4, 369–379. [Google Scholar]
- Jobling, SA; Jarman, C; Teh, MM; Holmberg, N; Blake, C; Verhoeyen, ME. Immunomodulation of enzyme function in plants by single-domain antibody fragments. Nat. Biotechnol. 2003, 21, 77–80. [Google Scholar]
- Suzuki, Y; Mizuno, T; Urakami, E; Yamaguchi, I; Asami, T. Immunomodulation of bioactive gibberellin confers gibberellin-deficient phenotypes in plants. Plant Biotechnol. J. 2008, 6, 355–367. [Google Scholar]
- Ma, JKC; Barros, E; Bock, R; Christou, P; Dale, PJ; Dix, PJ; Fischer, R; Irwin, J; Mahoney, R; Pezzotti, M; Schillberg, S; Sparrow, P; Stoger, E; Twyman, RM. Molecular farming for new drugs and vaccines. Current perspectives on the production of pharmaceuticals in transgenic plants. EMBO Rep. 2005, 6, 593–599. [Google Scholar]
- Köhler, G; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar]
- Barna-Vetró, I; Gyöngyösi, A; Solti, L. Monoclonal antibody-based enzyme-linked immunosorbent assay of Fusarium T-2 and zearalenone toxins in cereals. Appl. Environ. Microbiol. 1994, 60, 729–731. [Google Scholar]
- Bird, CB; Malone, B; Rice, LG; Ross, PF; Eppley, R; Abouzied, MM. Determination of total fumonisins in corn by competitive direct enzyme-linked immunosorbent assay: Collaborative study. J. AOAC Int. 2002, 85, 404–410. [Google Scholar]
- Hayashi, Y; Arie, T; Yoneyama, K; Yamaguchi, I. Characterization of the antigenic determinant on Fusarium oxysporum recognized by a genus-specific monoclonal antibody. J. Gen. Appl. Microbiol 1998, 44, 43–47. [Google Scholar]
- Nagayama, S; Kawamura, O; Ohtani, K; Ryu, JC; Latus, D; Sudheim, L; Ueno, Y. Application of an enzyme-linked immunosorbent assay for screening of T-2 toxin-producing Fusarium spp. Appl. Environ. Microbiol. 1988, 54, 1302–1303. [Google Scholar]
- Yoshizawa, T; Kohno, H; Ikeda, K; Shinoda, T; Yokohama, H; Morita, K; Kusada, O; Kobayashi, Y. A practical method for measuring deoxynivalenol, nivalenol, and T-2 + HT-2 toxin in foods by an enzyme-linked immunosorbent assay using monoclonal antibodies. Biosci. Biotechnol. Biochem. 2004, 68, 2076–2085. [Google Scholar]
- Liao, YC; Li, HP; Zhao, CS; Yao, MJ; Zhang, JB; Liu, JL. Plantibodies: A novel strategy to create pathogen-resistant plants. Biotechnol. Genet. Eng. Rev. 2006, 23, 253–272. [Google Scholar]
- Verma, R; Boleti, E; George, AJT. Antibody engineering: Comparison of bacterial, yeast, insect and mammalian expression systems. J. Immunol. Methods 1998, 216, 165–181. [Google Scholar]
- Sidhu, SS; Li, B; Chen, Y; Fellouse, FA; Eigenbrot, C; Fuh, G. Phage-displayed antibody libraries of synthetic heavy chain complementarity determining regions. J. Mol. Biol. 2004, 338, 299–310. [Google Scholar]
- Winter, G; Griffiths, AD; Hawkins, RE; Hoogenboom, HR. Making antibodies by phage display technology. Annu. Rev. Immunol. 1994, 12, 433–455. [Google Scholar]
- Lee, CMY; Iorno, N; Sierro, F; Christ, D. Selection of human antibody fragments by phage display. Nat. Protoc. 2007, 2, 3001–3008. [Google Scholar]
- McKenzie, KM; Mee, JM; Rogers, CJ; Hixon, MS; Kaufmann, GF; Janda, KD. Identification and characterization of single chain anti-cocaine catalytic antibodies. J. Mol. Biol. 2007, 365, 722–731. [Google Scholar]
- Finlay, WJJ; Shaw, I; Reilly, JP; Kane, M. Generation of high-affinity chicken single-chain Fv antibody fragments for measurement of the Pseudonitzschia pungens toxin domoic acid. Appl. Environ. Microbiol. 2006, 72, 3343–3349. [Google Scholar]
- Shaw, I; O'Reilly, A; Charleton, M; Kane, M. Development of a high-affinity anti-domoic acid sheep scFv and its use in detection of the toxin in shellfish. Anal. Chem. 2008, 80, 3205–3212. [Google Scholar]
- Arbabi Ghahroudi, M; Desmyter, A; Wyns, L; Hamers, R; Muyldermans, S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997, 414, 521–526. [Google Scholar]
- Li, Y; Cockburn, W; Kilpatrick, JB; Whitelam, GC. High affinity scFvs from a single rabbit immunized with multiple haptens. Biochem. Biophys. Res. Commun. 2000, 268, 398–404. [Google Scholar]
- Chames, P; Coulon, S; Baty, D. Improving the affinity and the fine specificity of an anti-cortisol antibody by parsimonious mutagenesis and phage display. J. Immunol. 1998, 161, 5421–5429. [Google Scholar]
- van der Linden, RHJ; de Geus, B; Frenken, LGJ; Peters, H; Verrips, CT. Improved production and function of llama heavy chain antibody fragments by molecular evolution. J. Biotechnol. 2000, 80, 261–270. [Google Scholar]
- Qu, B; Li, HP; Zhang, JB; Xu, YB; Huang, T; Wu, AB; Zhao, CS; Carter, J; Nicholson, P; Liao, YC. Geographic distribution and genetic diversity of Fusarium graminearum and F. asiaticum on wheat spikes throughout China. Plant Pathol. 2008, 57, 15–24. [Google Scholar]
- Li, HP; Zhang, JB; Shi, RP; Huang, T; Fischer, R; Liao, YC. Engineering Fusarium head blight resistance in wheat by expression of a fusion protein containing a Fusarium-specific antibody and an antifungal peptide. Mol. Plant-Microbe Interact. 2008, 21, 1242–1248. [Google Scholar]
- Hiatt, A; Caffferkey, R; Bowdish, K. Production of antibodies in transgenic plants. Nature 1989, 342, 76–78. [Google Scholar]
- Düring, K; Hippe, S; Kreuzaler, F; Schell, J. Synthesis and self-assembly of a functional monoclonal antibody in transgenic. Nicotiana tabacum. Plant Mol. Biol. 1990, 15, 281–293. [Google Scholar]
- Wieland, WH; Lammers, A; Schots, A; Orzáez, DV. Plant expression of chicken secretory antibodies derived from combinatorial libraries. J. Biotechnol. 2006, 122, 382–391. [Google Scholar]
- Voss, A; Niersbach, M; Hain, R; Hirsch, HJ; Liao, YC; Kreuzaler, F; Fischer, R. Reduced virus infectivity in N. tabacum secreting a TMV-specific full-size antibody. Mol. Breed. 1995, 1, 39–50. [Google Scholar]
- De Wilde, C; Peeters, K; Jacobs, A; Peck, I; Depicker, A. Expression of antibodies and Fab fragments in transgenic potato plants: a case study for bulk production in crop plants. Mol. Breed. 2002, 9, 271–282. [Google Scholar]
- Yuan, Q; Hu, W; Pestka, JJ; He, SY; Hart, LP. Expression of a functional antizearalenone single-chain Fv antibody in transgenic Arabidopsis plants. Appl. Environ. Microbiol. 2000, 66, 3499–3505. [Google Scholar]
- Fischer, R; Schumann, D; Zimmermann, S; Drossard, J; Sack, M; Schillberg, S. Expression and characterization of bispecific single-chain Fv fragments produced in transgenic plants. Eur. J. Biochem. 1999, 262, 810–816. [Google Scholar]
- Kathuria, S; Sriraman, R; Nath, R; Sack, M; Pal, R; Artsaenko, O; Talwar, GP; Fischer, R; Finnern, R. Efficacy of plant-produced recombinant antibodies against HCG. Hum. Reprod. 2002, 17, 2054–2061. [Google Scholar]
- Hood, EE; Woodard, SL; Horn, ME. Monoclonal antibody manufacturing in transgenic plants — myths and realities. Curr. Opin. Biotechnol. 2002, 13, 630–635. [Google Scholar]
- Twyman, RM; Stoger, E; Schillberg, S; Christou, P; Fischer, R. Molecular farming in plants: host systems and expression technology. Trends Biotechnol. 2003, 21, 570–578. [Google Scholar]
- Gargouri-Bouzid, R; Jaoua, L; Rouis, S; Saïdi, MN; Bouaziz, D; Ellouz, R. PVY-resistant transgenic potato plants expressing an anti-Nia protein scFv antibody. Mol. Biotechnol. 2006, 33, 133–140. [Google Scholar]
- Vaquero, C; Sack, M; Chandler, J; Drossard, J; Schuster, F; Monecke, M; Schillberg, S; Fischer, R. Transient expression of a tumor-specific single-chain fragment and a chimeric antibody in tobacco leaves. Proc. Natl. Acad. Sci. USA 1999, 96, 11128–11133. [Google Scholar]
- Li, HP; Yao, MJ; Liao, YC. Heterologous expression and functional identification of a chitinase gene from wheat. J. Plant Physiol. Mol. Biol. 2005, 31, 589–593. [Google Scholar]
- Gadaleta, A; Giancaspro, A; Blechl, AE; Blanco, A. A transgenic durum wheat line that is free of marker genes and expresses 1Dy10. J. Cereal Sci 2008. [Google Scholar]
- Wright, M; Dawson, J; Dunder, E; Suttie, J; Reed, J; Kramer, C; Chang, Y; Novitzky, R; Wang, H; Artim-Moore, L. Efficient biolistic transformation of maize (Zea mays L.) and wheat (Triticum aestivum L.) using the phosphomannose isomerase gene, pmi, as the selectable marker. Plant Cell Rep. 2001, 20, 429–436. [Google Scholar]
- Conrad, U; Fiedler, U. Compartment-specific accumulation of recombinant immunoglobulins in plant cells: an essential tool for antibody production and immunomodulation of physiological functions and pathogen activity. Plant Mol. Biol. 1998, 38, 101–109. [Google Scholar]
- Ramírez, N; Oramas, P; Ayala, M; Rodríguez, M; Pérez, M; Gavilondo, J. Expression and long-term stability of a recombinant single-chain Fv antibody fragment in transgenic Nicotiana tabacum seeds. Biotechnol. Lett. 2001, 23, 47–49. [Google Scholar]
- McMullen, M; Jones, R; Gallenberg, D. Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar]
- Xu, XM; Parry, DW; Nicholson, P; Thomsett, MA; Simpson, D; Edwards, SG; Cooke, BM; Doohan, FM; Brennan, JM; Moretti, A; Tocco, G; Mule, G; Hornok, L; Giczey, G; Tatnell, J. Predominance and association of pathogenic fungi causing Fusarium ear blight in wheat in four European countries. Eur. J. Plant Pathol. 2005, 112, 143–154. [Google Scholar]
- Chen, PD; Liu, DJ. Transfer of scab resistance from Leymus racemosus, Roegneria ciliaris and R. kamoji into common wheat. Proceedings of the International Symposium on Wheat Improvement for Scab Resistance 2000, 62–67. [Google Scholar]
- Li, B; Liu, F; Xu, R; Huang, C; Cheng, F; Liu, J; Meng, J; Mou, J. Sumai3: Its development, genetic characteristics and applications in wheat breeding for Fusarium head blight resistance. Proceedings of the International Symposium on Wheat Improvement for Scab Resistance, Suzhou and Nanjing, China 2000, 187–193. [Google Scholar]
- Zwart, RS; Muylle, H; Van Bockstaele, E; Roldán-Ruiz, I. Evaluation of genetic diversity of Fusarium head blight resistance in European winter wheat. Theor. Appl. Genet. 2008. [Google Scholar]
- Shin, S; Mackintosh, CA; Lewis, J; Heinen, SJ; Radmer, L; Dill-Macky, R; Baldridge, GD; Zeyen, RJ; Muehlbauer, GJ. Transgenic wheat expressing a barley class II chitinase gene has enhanced resistance against Fusarium graminearum. J. Exp. Bot. 2008, 59, 2371–2378. [Google Scholar]
- Wu, AB; Li, HP; Zhao, CS; Liao, YC. Comparative pathogenicity of Fusarium graminearum isolates from China revealed by wheat coleoptile and floret inoculations. Mycopathologia 2005, 160, 75–83. [Google Scholar]
- Zhang, JB; Li, HP; Dang, FJ; Qu, B; Xu, YB; Zhao, CS; Liao, YC. Determination of the trichothecene mycotoxin chemotypes and associated geographical distribution and phylogenetic species of the Fusarium graminearum clade from China. Mycol. Res. 2007, 111, 967–975. [Google Scholar]
- Waldron, BL; Moreno-Sevilla, B; Anderson, JA; Stack, RW; Frohberg, RC. RFLP mapping of QTL for Fusarium head blight resistance in wheat. Crop Sci. 1999, 39, 805–811. [Google Scholar]
- Bohlmann, H. The best of both worlds in plant protection. Nat. Biotechnol. 2004, 22, 682–683. [Google Scholar]
- Lacadena, J; Martínez del Pozo, A; Gasset, M; Patiño, B; Campos-Olivas, R; Vázquez, C; Martínez-Ruiz, A; Mancheño, JM; Oñaderra, M; Gavilanes, JG. Characterization of the antifungal protein secreted by the mould Aspergillus giganteus. Arch. Biochem. Biophys. 1995, 324, 273–281. [Google Scholar]

© 2008 by MDPI This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, Z.-Q.; Li, H.-P.; Zhang, J.-B.; Glinka, E.; Liao, Y.-C. Antibody-mediated Prevention of Fusarium Mycotoxins in the Field. Int. J. Mol. Sci. 2008, 9, 1915-1926. https://doi.org/10.3390/ijms9101915
Hu Z-Q, Li H-P, Zhang J-B, Glinka E, Liao Y-C. Antibody-mediated Prevention of Fusarium Mycotoxins in the Field. International Journal of Molecular Sciences. 2008; 9(10):1915-1926. https://doi.org/10.3390/ijms9101915
Chicago/Turabian StyleHu, Zu-Quan, He-Ping Li, Jing-Bo Zhang, Elena Glinka, and Yu-Cai Liao. 2008. "Antibody-mediated Prevention of Fusarium Mycotoxins in the Field" International Journal of Molecular Sciences 9, no. 10: 1915-1926. https://doi.org/10.3390/ijms9101915