Effect of Several New and Currently Available Oxime Cholinesterase Reactivators on Tabun-intoxicated Rats
Abstract
:1. Introduction
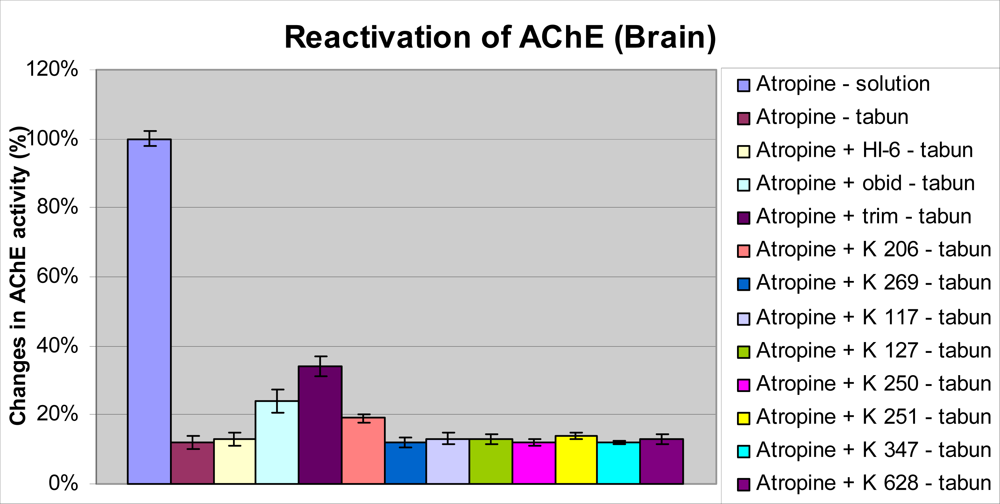
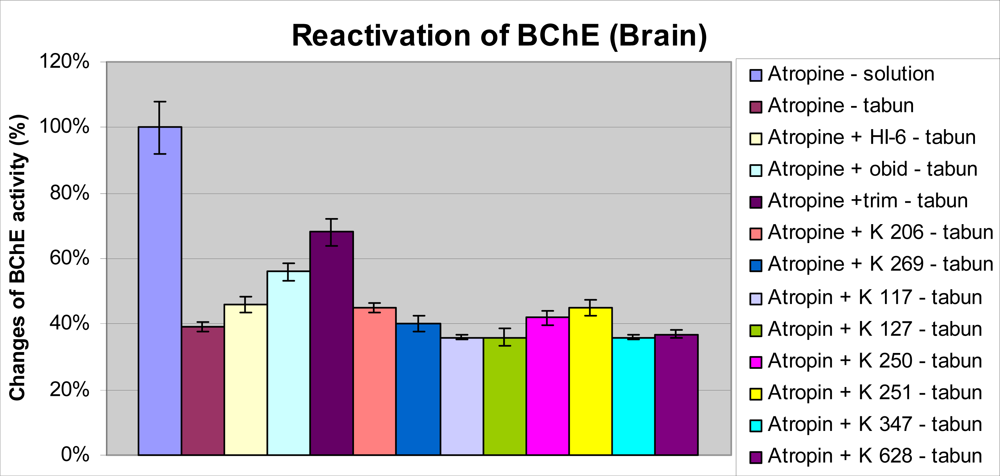
2. Results and Discussion
3. Experimental Section
3.1. Chemicals
3.2. Animals
3.3. Intoxication and sample collection
3.4. Biochemical examinations
3.5. Statistical evaluation
4. Conclusions
Acknowledgments
References and Notes
- Bajgar, J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis and treatment. Adv. Clin. Chem 2004, 38, 151–216. [Google Scholar]
- Marrs, T. C. Organophosphate poisoning. Pharmacol. Ther 1993, 58, 51–66. [Google Scholar]
- Dawson, R. M. Review of oximes available for treatment of nerve agent poisoning. J. Appl. Toxicol 1994, 14, 317–331. [Google Scholar]
- Koplovitz, I; Steward, JR. A comparison of the efficacy of HI6 and 2-PAM against soman, tabun, sarin, and VX in the rabbit. Toxicol. Lett 1994, 70, 269–279. [Google Scholar]
- Kuca, K; Jun, D; Bajgar, J. Currently used cholinesterase reactivators against nerve agent intoxication: comparison of their effectivity in vitro. Drug Chem. Toxicol 2007, 30, 31–40. [Google Scholar]
- Wilson, IB; Sondheimer, F. A specific antidote against lethal alkyl phosphate intoxication. V. Antidotal properties. Arch. Biochem. Biophys 1957, 69, 468–474. [Google Scholar]
- Musilek, K; Lipka, L; Racakova, V; Kuca, K; Jun, D; Dohnal, V; Dolezal, M. New methods in synthesis of acetylcholinesterase reactivators and evaluation of their potency to reactivate cyclosarin-inhibited AChE. Chem. Papers 2006, 60, 48–51. [Google Scholar]
- Musilek, K; Kuca, K; Jun, D; Dohnal, V; Kim, TH; Jung, YS; Dolezal, M. Synthesis of reactivators of phosphorylated acetylcholinesterase of bis-pyridiniumdialdoxime type with a 3-oxapentane connecting chain and their testing in vitro on a model of the enzyme inhibited by chlorpyrifos and methylchlorpyrifos. Ces. Slov. Farm 2006, 15, 115–119. [Google Scholar]
- Musilek, K; Holas, O; Jun, D; Dohnal, V; Gunn-Moore, F; Opletalova, V; Dolezal, M; Kuca, K. Monooxime reactivators of acetylcholinesterase with (E)-but-2-ene linker: Preparation and reactivation of tabun- and paraoxon-inhibited acetylcholinesterase. Bioorg. Med. Chem 2007, 15, 6733–6741. [Google Scholar]
- Musilek, K; Holas, O; Kuca, K; Jun, D; Dohnal, V; Opletalova, V; Dolezal, M. Synthesis of monooxime-monocarbamoyl bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against tabun- and paraoxon-inhibited acetylcholinesterase. J. Enzym. Inhib. Med. Chem 2008, 23, 70–76. [Google Scholar]
- Musilek, K; Kucera, J; Jun, D; Dohnal, V; Opletalova, V; Kuca, K. Monoquaternary pyridinium salts with modified side chain-synthesis and evaluation on model of tabun- and paraoxon-inhibited acetylcholinesterase. Bioorg Med Chem 2008, 16, 8218–8223. [Google Scholar]
- Kim, TH; Kuca, K; Jun, D; Jung, YS. Design and synthesis of new bis-pyridinium oxime reactivators for acetylcholinesterase inhibited by organophosphorous nerve agent. Bioorg. Med. Chem. Lett 2005, 15, 2914–2917. [Google Scholar]
- Kuca, K; Jun, D; Kim, TH; Cabal, J; Jung, YS. In vitro evaluation of new acetylcholinesterase reactivators as causal antidotes against tabun and cyclosarin. Bull. Kor. Chem. Soc 2006, 27, 395–398. [Google Scholar]
- Shih, TM. Comparison of several oximes on reactivation of soman-inhibited blood, brain and tissue cholinesterase activity in rats. Arch. Toxicol 1993, 67, 637–646. [Google Scholar]
- Bieger, D; Wasserman, O. Ionization constants of cholinesterase-reactivating bis-pyridinium aldoximes. J. Pharm. Pharmacol 1967, 19, 844–847. [Google Scholar]
- Kuca, K; Jun, D; Musilek, K. Structural requirements of acetylcholinesterase reactivators. Mini Rev. Med. Chem 2006, 6, 269–277. [Google Scholar]
- Cabal, J; Kuca, K; Kassa, K. Specification of the structure of oximes able to reactivate tabun inhibited acetylcholinesterase. Pharmacol. Toxicol 2004, 95, 81–86. [Google Scholar]
- Kassa, J; Cabal, J. A comparison of the efficacy of a new asymmetric bispyridium oxime BI–6 with currently available oximes and H oximes against soman by in vitro and in vivo methods. Toxicology 1999, 132, 111–118. [Google Scholar]
- Kassa, J; Jun, D; Karasova, J; Bajgar, J; Kuca, K. A comparison of reactivating efficacy of newly developed oximes (K 074, K 075) and currently available oximes (obidoxime, HI–6) in soman, cyclosarin and tabun-poisoned rats. Chem. Biol. Interact 2008, 175, 425–427. [Google Scholar]
- Lorke, DE; Hasan, MY; Nurulain, SM; Shafiullah, M; Nagelkerke, N; Petroianu, GA. Effect of intrathecal pralidoxime administration upon survival of rats exposed to the organophosphate paraoxon. Neurotoxicology 2008, 29, 663–670. [Google Scholar]
- Lorke, DE; Kalasz, H; Petroianu, GA; Tekes, K. Entry of oximes into the brain: a review. Curr. Med. Chem 2008, 15, 743–753. [Google Scholar]
- Gyenge, M; Kalász, H; Petroianu, GA; Laufer, R; Kuca, K; Tekes, K. Measurement of K–27, an oxime-type cholinesterase reactivator by high-performance liquid chromatography with electrochemical detection from different biological samples. J. Chromatogr. A 2007, 1161, 146–151. [Google Scholar]
- Lorke, DE; Hasan, MY; Nurulain, SM; Sheen, R; Kuca, K; Petroianu, GA. Entry of two new asymmetric bispyridinium oximes (K-27 and K-48) into the rat brain: comparison with obidoxime. J. Appl. Toxicol 2007, 27, 482–490. [Google Scholar]
- Bartosova, L; Kuca, K; Kunesova, G; Jun, D. The acute toxicity of the acetylcholinesterase reactivators in mice in relation to their structure. Neurotoxicity Res 2006, 9, 291–296. [Google Scholar]
- Bajgar, J. Biological monitoring of exposure to nerve agents. Br. J. Ind. Med 1992, 49, 648–653. [Google Scholar]
- Ellman, GL; Coutney, DK; Andres, V; Featherstone, RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol 1961, 7, 88–95. [Google Scholar]
- Pohanka, M; Jun, D; Kuca, K. Improvement of acetylcholinesterase-based assay for organophosphates in way of identification by reactivators. Talanta 2008, 77, 451–454. [Google Scholar]
- Kassa, J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J. Toxicol. Clin. Toxicol 2002, 40, 803–816. [Google Scholar]




© 2008 by MDPI This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Karasova, J.Z.; Kassa, J.; Jung, Y.-S.; Musilek, K.; Pohanka, M.; Kuca, K. Effect of Several New and Currently Available Oxime Cholinesterase Reactivators on Tabun-intoxicated Rats. Int. J. Mol. Sci. 2008, 9, 2243-2252. https://doi.org/10.3390/ijms9112243
Karasova JZ, Kassa J, Jung Y-S, Musilek K, Pohanka M, Kuca K. Effect of Several New and Currently Available Oxime Cholinesterase Reactivators on Tabun-intoxicated Rats. International Journal of Molecular Sciences. 2008; 9(11):2243-2252. https://doi.org/10.3390/ijms9112243
Chicago/Turabian StyleKarasova, Jana Zdarova, Jiri Kassa, Young-Sik Jung, Kamil Musilek, Miroslav Pohanka, and Kamil Kuca. 2008. "Effect of Several New and Currently Available Oxime Cholinesterase Reactivators on Tabun-intoxicated Rats" International Journal of Molecular Sciences 9, no. 11: 2243-2252. https://doi.org/10.3390/ijms9112243
APA StyleKarasova, J. Z., Kassa, J., Jung, Y.-S., Musilek, K., Pohanka, M., & Kuca, K. (2008). Effect of Several New and Currently Available Oxime Cholinesterase Reactivators on Tabun-intoxicated Rats. International Journal of Molecular Sciences, 9(11), 2243-2252. https://doi.org/10.3390/ijms9112243



