Noncovalently Modified Carbon Nanotubes with Carboxymethylated Chitosan: A Controllable Donor-Acceptor Nanohybrid
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and materials
2.2. Preparation of cmCs/MWNTs nanohybrids
2.3. Characterization of functionalized-MWNTs
3. Results and Discussion
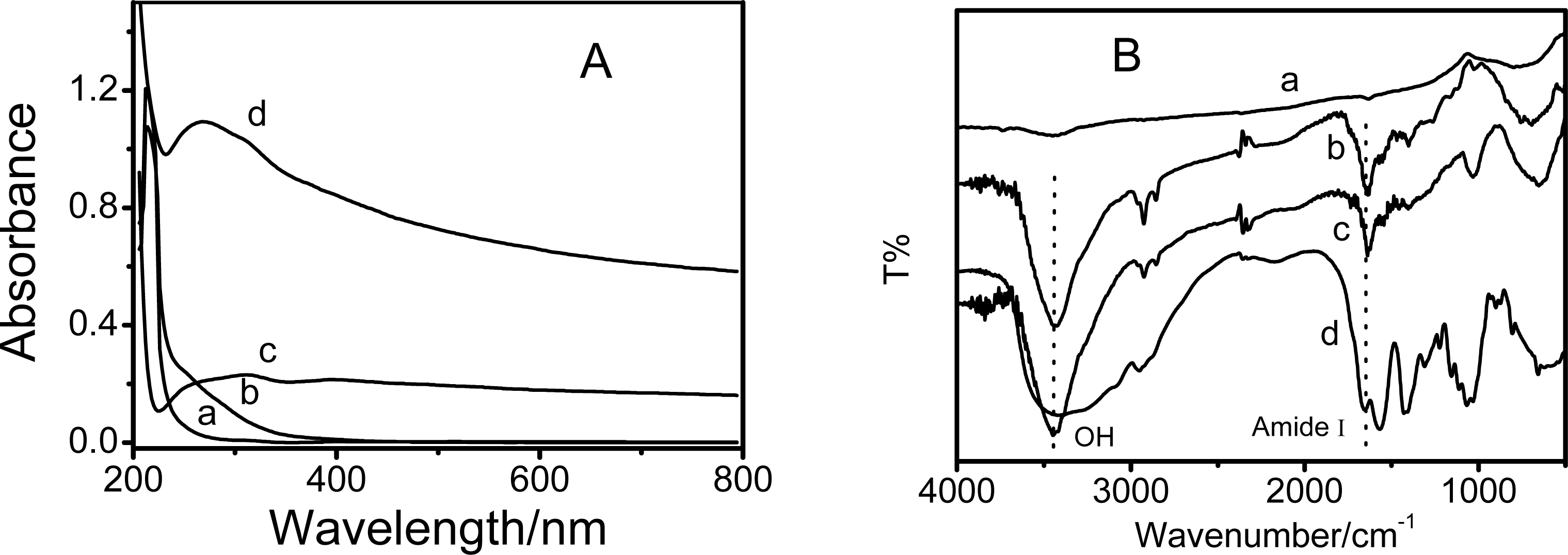
3.1. Preparation and characterization of cmCs/MWNTs nanohybrids
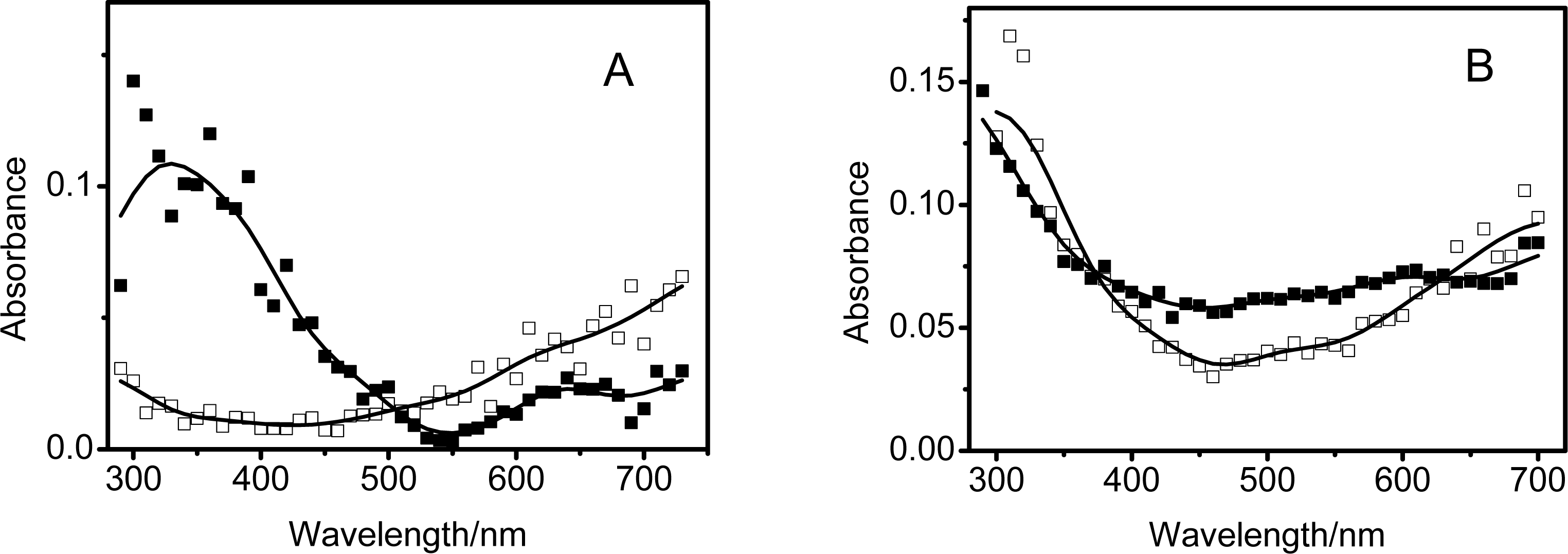
3.2. Photoinduced charge separation of cmCs/MWNTs nanobybrids
4. Conclusion
Acknowledgments
Appendix
Calculation of the percentages in cmCs/MWNTs composites:
References
- Hirsch, A. Functionalization of Single-Walled Carbon Nanotubes. Angew Chem Int Ed 2002, 41, 1853–1859. [Google Scholar]
- Tasis, D; Tagmatarchis, N; Bianco, A; Prato, M. Chemistry of Carbon Nanotubes. Chem Rev 2006, 106, 1105–1136. [Google Scholar]
- Kim, Y; Hong, S; Jung, S; Strano, MS; Choi, J; Baik, S. Dielectrophoresis of Surface Conductance Modulated Single-Walled Carbon Nanotubes Using Catanionic Surfactants. J Phys Chem B 2006, 110, 1541–1545. [Google Scholar]
- Star, A; Stoddart, JF; Steuerman, D; Diehl, M; Boukai, A; Wong, EW; Yang, X; Chung, SW; Choi, H; Heath, JR. Preparation and Properties of Polymer-Wrapped Single-Walled Carbon Nanotubes. Angew Chem Int Ed 2001, 40, 1721–1725. [Google Scholar]
- Pantarotto, D; Singh, R; McCarthy, D; Erhardt, M; Briand, JP; Prato, M; Kostarelos, K; Bianco, A. Functionalized Carbon Nanotubes for Plasmid DNA Gene Delivery. Angew Chem Int Ed 2004, 43, 5242–5246. [Google Scholar]
- Baskaran, D; Mays, JW; Zhang, XP; Bratcher, MS. Carbon Nanotubes with Covalently Linked Porphyrin Antennae: Photoinduced Electron Transfer. J Am Chem Soc 2005, 127, 6916–6917. [Google Scholar]
- Guldi, DM; Marcaccio, M; Paolucci, D; Paolucci, F; Tagmatarchis, N; Tasis, D; Vázquez, E; Prato, M. Single-Wall Carbon Nanotube–Ferrocene Nanohybrids: Observing Intramolecular Electron Transfer in Functionalized SWNTs. Angew Chem Int Ed 2003, 42, 4206–4209. [Google Scholar]
- Martin, RB; Qu, L; Lin, Y; Harruff, BA; Bunker, CE; Gord, JR; Allard, LF; Sun, YP. Functionalized Carbon Nanotubes with Tethered Pyrenes: Synthesis and Photophysical Properties. J Phys Chem B 2004, 108, 11447–11453. [Google Scholar]
- Ehli, C; Rahman, GMA; Jux, N; Balbinot, D; Guldi, DM; Paolucci, F; Marcaccio, M; Paolucci, D; Melle-Franco, M; Zerbetto, F; Campidelli, S; Prato, M. Interactions in Single Wall Carbon Nanotubes/Pyrene/Porphyrin Nanohybrids. J Am Chem Soc 2006, 128, 11222–11231. [Google Scholar]
- Herranz, M; Martin, N; Campidelli, S; Prato, M; Brehm, G; Guldi, DM. Control over Electron Transfer in Tetrathiafulvalene-Modified Single-Walled Carbon Nanotubes. Angew Chem Int Ed 2006, 45, 4478–4482. [Google Scholar]
- Bertholon, I; Hommel, H; Labarre, D; Vauthier, C. Properties of Polysaccharides Grafted on Nanoparticles Investigated by EPR. Langmuir 2006, 22, 5485–5490. [Google Scholar]
- Chen, T; Small, DA; Wu, L; Rubloff, GW; Ghodssi, R; Vazquez-Duhalt, R; Bentley, WE; Payne, GF. Nature-Inspired Creation of Protein-Polysaccharide Conjugate and Its Subsequent Assembly onto a Patterned Surface. Langmuir 2003, 19, 9382–9386. [Google Scholar]
- Yang, H; Wang, SC; Merciera, P; Akins, DL. Diameter-selective dispersion of single-walled carbon nanotubes using a water-soluble, biocompatible polymer. Chem Commun 2006, 1425–1427. [Google Scholar]
- Saito, K; Troiani, V; Qiu, H; Solladie, N; Sakata, T; Mori, H; Ohama, M; Fukuzumi, S. Nondestructive Fromation of Supramolecular Nanohydrids of Single-Walled Carbon Nanotubes with Flexible Porphyrinic Polypeptides. J Phys Chem C 2007, 111, 1194–1199. [Google Scholar]
- Liu, Y; Gao, L; Sun, J. Noncovalent Functionalization of Carbon Nanotubes with Sodium Lignosulfonate and Subsequent Quantum Dot Decoration. J Phys Chem C 2007, 111, 1223–1229. [Google Scholar]
- Zhang, M; Gorski, W. Electrochemical Sensing Platform Based on the Carbon Nanotubes/Redox Mediators-Biopolymer System. J Am Chem Soc 2005, 127, 2058–2059. [Google Scholar]
- Zhang, M; Smith, A; Gorski, W. Carbon Nanotube-Chitosan System for Electrochemical Sensing Based on Dehydrogenase Enzymes. Anal Chem 2004, 76, 5045–5050. [Google Scholar]
- Hu, C; Chen, Z; Shen, A; Shen, X; Li, J; Hu, S. Water-soluble single-walled carbon nanotubes via noncovalent functionalization by a rigid, planar and conjugated diazo dye. Carbon 2006, 44, 428–434. [Google Scholar]
- Long, D; Wu, G; Chen, D; Yao, S. Spectroscopic Study the Interaction between Methyl Viologen and core-shell structure Polymer nanoparticles. Res Chem Intermediat 2005, 31, 613–623. [Google Scholar]
- Tanima, B; Susmita, M; Ajay, KS; Rakesh, KS; Amarnath, M. Preparation, characterization and biodistribution of ultrafine chitosan nanoparticles. Int J Pharm 2002, 243, 93–105. [Google Scholar]
- Masatoshi, S; Minoru, M; Hitoshi, S; Hiroyuki, S; Yoshihiro, S. Preparation and characterization of water-soluble chitin and chitosan derivatives. Carbohyd Polym 1998, 36, 49–59. [Google Scholar]
- Zhai, M; Kudoh, H; Wu, G; Wach, RA; Muroya, Y; Katsumura, Y; Nagasawa, N; Zhao, L; Yoshii, F. Laser Photolysis of Carboxymethylated Chitin Derivatives in Aqueous Solution. Part 1. Formation of Hydrated Electron and a Long-Lived Radica. Biomacromolecules 2004, 5, 453–457. [Google Scholar]
- Zhai, M; Kudoh, H; Wu, G; Wach, RA; Muroya, Y; Katsumura, Y; Nagasawa, N; Zhao, L; Yoshii, F. Laser Photolysis of Carboxymethylated Chitin Derivatives in Aqueous Solution. Part 2. Reaction of OH· and SO4·− Radicals with Carboxymethylated Chitin Derivatives. Biomacromolecules 2004, 5, 458–462. [Google Scholar]
- Sankararamakrishnan, N; Sanghi, R. Preparation and characterization of a novel xanthated chitosan. Carbohyd Polym 2006, 66, 160–167. [Google Scholar]
- Ding, W; Lian, Q; Samuels, RJ; Polk, MB. Synthesis and characterization of a novel derivative of chitosan. Polymer 2003, 44, 547–556. [Google Scholar]
- Wang, X; Liu, Y; Yu, G; Xu, C; Zhang, J; Zhu, D. Anisotropic Electrical Transport Properties of Aligned Carbon Nanotube Films. J Phys Chem B 2001, 105, 9422–9425. [Google Scholar]






Share and Cite
Long, D.; Wu, G.; Zhu, G. Noncovalently Modified Carbon Nanotubes with Carboxymethylated Chitosan: A Controllable Donor-Acceptor Nanohybrid. Int. J. Mol. Sci. 2008, 9, 120-130. https://doi.org/10.3390/ijms9020120
Long D, Wu G, Zhu G. Noncovalently Modified Carbon Nanotubes with Carboxymethylated Chitosan: A Controllable Donor-Acceptor Nanohybrid. International Journal of Molecular Sciences. 2008; 9(2):120-130. https://doi.org/10.3390/ijms9020120
Chicago/Turabian StyleLong, Dewu, Guozhong Wu, and Guanglai Zhu. 2008. "Noncovalently Modified Carbon Nanotubes with Carboxymethylated Chitosan: A Controllable Donor-Acceptor Nanohybrid" International Journal of Molecular Sciences 9, no. 2: 120-130. https://doi.org/10.3390/ijms9020120
APA StyleLong, D., Wu, G., & Zhu, G. (2008). Noncovalently Modified Carbon Nanotubes with Carboxymethylated Chitosan: A Controllable Donor-Acceptor Nanohybrid. International Journal of Molecular Sciences, 9(2), 120-130. https://doi.org/10.3390/ijms9020120



