Synthesis and Physical Characterization of 2-((E)-1-(3-((E)-1-(2-hydroxyphenyl)ethylideneamino)-2-methylphenylimino)ethyl)phenol
Abstract
:Introduction:

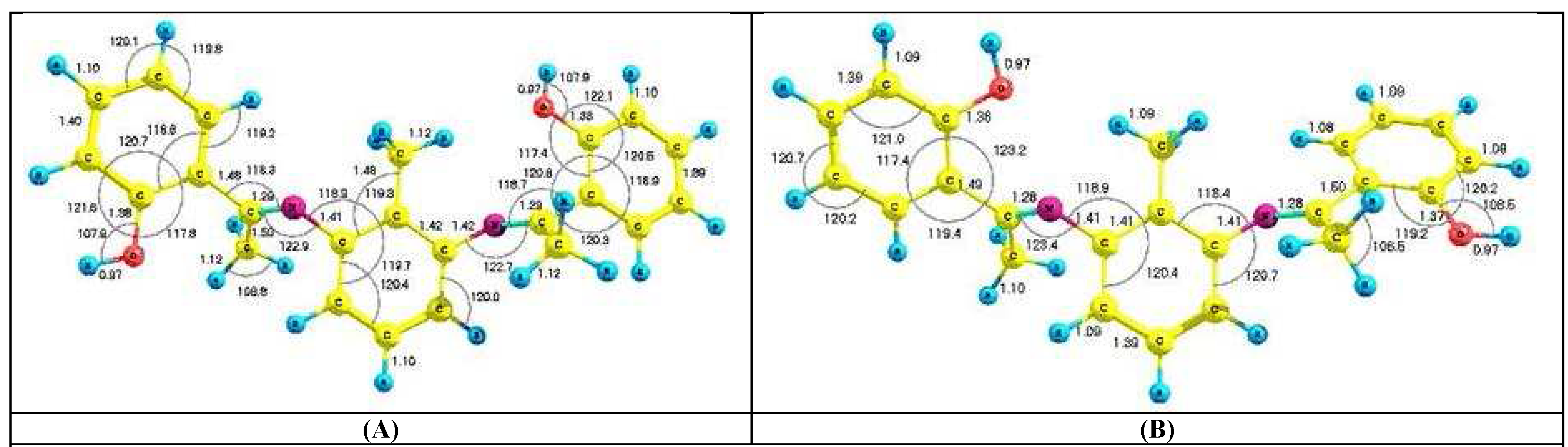
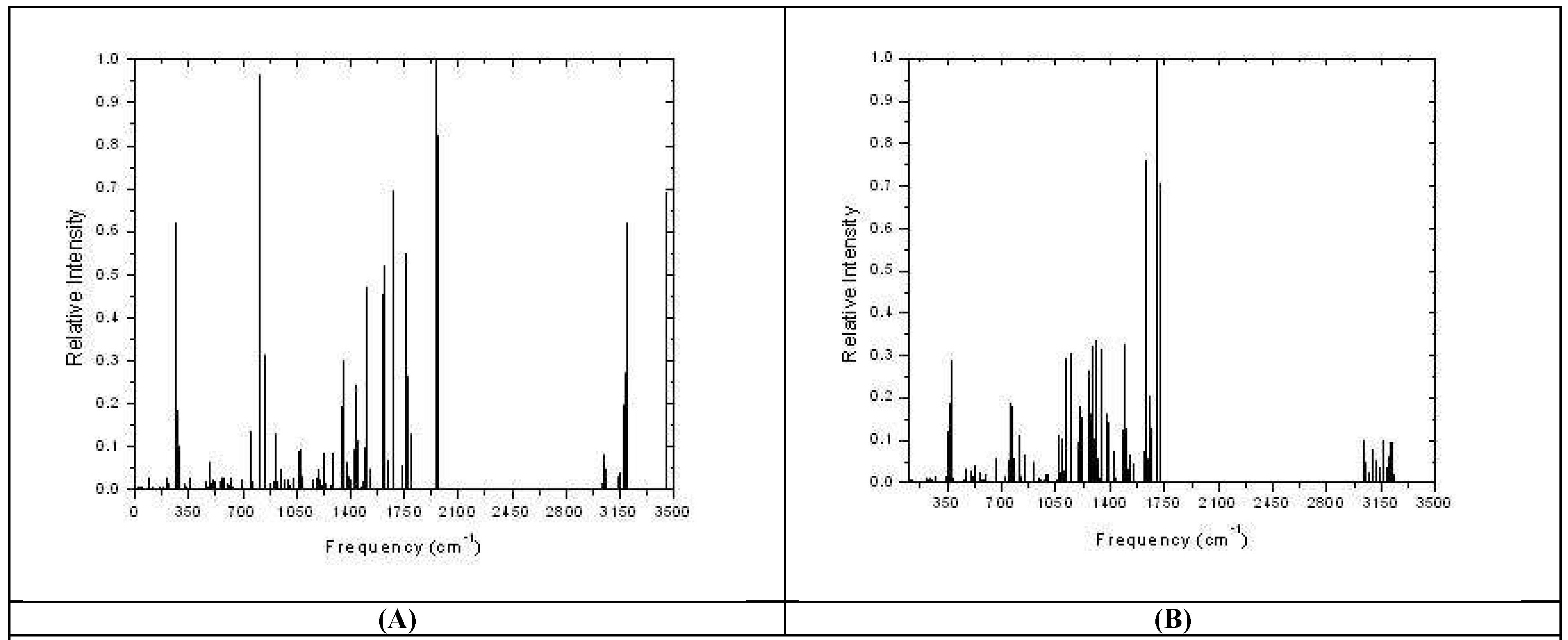
Results and Discussion:
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References:
- a) Jarrahpour, A. A.; Shekarriz, M.; Taslimi, A. Molecules 2004, 9, 29–38. b) Hakimelahi, G. H.; Jarrahpour, A. A. Helv. Chim Acta. 1989, 72(7), 1501–5. c) Hakimelahi, G. H.; Jarrahpour, A. A. J. Sci. R. Iran 1990, 1(5), 353–354. d) Venturini, A.; Gonzalez, J. J. Org. Chem. 2002, 67, 9089.
- Taggi, A. E.; Hafez, A. M.; Wack, H.; Young, B.; Ferraris, D.; Lectka, T. J. Am. Chem. Soc. 2002, 124, 6626. [PubMed]
- JaEl-masry, A. H.; Fahmy, H. H.; Abdelwahed, S. H. A. Molecules 2000, 5, 1429.
- Singh, W. M.; Dash, B. C. Pesticides 1988, 22(11), 33.
- Wang, P.H.; Keck, J. G.; Lien, E. J.; Lai, M. M. C. J. Med. Chem. 1990, 33(2), 608. [PubMed]
- Das, A.; Trousdale, M. D.; Ren, S.; Lien, E. J. Antiviral Res. 1999, 44(3), 201.
- Desai, S. B.; Desai, P. B.; Desai, K. R. Hetrocycl. Commun. 2001, 7(1), 83.
- Das, B. P.; Choudhury, R. T.; Das, K. G.; Choudhury, D. N.; Choudhury, B. Chem. Environ. Res. 1994, 3(1&2), 19.
- Samadhiya, S.; Halve, A. Orient. J .Chem. 2001, 17(1), 119.
- Mazloum Ardakani, M.; Salavati-Niasari, M.; Jamshidpoor, M. Sensors and Actuators 2004, B101, 302.
- Majumder, S.; Panda, G. S.; Choudhuri, S. K. Eur. J. Med. Chem. 2003, 38, 893. [PubMed]
- John, R. P.; Sreekanth, A.; Kurup, M. R. P.; Mobin, S. M. Polyhedron 2002, 21, 2515–2521.
- Frisch, M.J.; et al. GAUSSIAN 03, Revision A.1; Frisch, M.J., et al., Eds.; Gaussian, Inc.: Pittsburgh PA, 2003. [Google Scholar]
- Foresman, J.B. Æ Frisch, Exploring Chemistry with Electronic Structure Methods, 2nd edition; Gaussian, INC: Pittsburgh, PA, 1996. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69, July 2001; National Institute of Standards and Technology: Gaithersburg, MD 20899.
- Jalbout, A.F.; Solimannejad, M.; Labonowski, J.K. Chem. Phys. Letts. 2003, 379, 503.
- Jalbout, A.F.; Jiang, Z.-Y.; Quasri, A.; Jeghnou, H.; Rhandour, A.; Dhamelincourt, M.C.; Dhamelincourt, P.; Mazzah, A. Vib. Spect. 2003, 33, 21.
- Jalbout, A.F.; Nazara, F.; Turker, L. J. Mol. Struct. (THEOCHEM) 2004, 627, 1, (Invited Review).
- Jalbout, A.F.; Adamowicz, L.; Solimmanejad, M. Chem. Phys. Letts. 2006, xx–xx.
- Sample Availability: Available from MDPI
| Fitted Thermodynamic Equation (T/1000=t) | 100 K | 298.15K | 1000 K | ||
| AM1 | Cp | -32.60241+ 1692.63626*t -907.85165*t2 + 150.38779*t3 +0.51679*t-2 | 179.04 | 398.44 | 904.39 |
| S | 53.732 *ln(t) + 1197.55633 *t + 17.76292 *t2/2 -370.35669 *t3/3 - 5533.6327 /(2*t2) + 153.29627 | 462.67 | 755.21 | 1541.55 | |
| ∆H | 465.16043 *t + 6885.20409 *t2/2 -13920.11871 *t3/3 + 7543.03025 *t4/4 – 7.7704 /t -1756.97223 | 11.50 | 68.42 | 560.53 | |
| B3LYP/6-31G* | Cp | -72.8544+ 1979.22049*t -1324.98024*t2 + 333.74311*t3 +0.56512*t-2 | 168.36 | 412.38 | 916.56 |
| S | 35.62635*ln(t) + 1302.38545*t + 32.60854*t2/2 -463.77548*t3/3 + 2344.59164/(2*t2) + 159.49792 | 439.53 | 731.65 | 1540.23 | |
| ∆H | -101.60395*t + 6628.71227*t2/2 -13286.08662*t3/3 + 7109.30838*t4/4 + 14.26015/t +702.59222 | 10.54 | 67.85 | 572.53 |
© 2006 MDPI. All rights reserved.
Share and Cite
Jarrahpour, A.A.; Jalbout, A.F.; Rezaei, S.; Trzaskowski, B. Synthesis and Physical Characterization of 2-((E)-1-(3-((E)-1-(2-hydroxyphenyl)ethylideneamino)-2-methylphenylimino)ethyl)phenol. Molbank 2006, 2006, M455. https://doi.org/10.3390/M455
Jarrahpour AA, Jalbout AF, Rezaei S, Trzaskowski B. Synthesis and Physical Characterization of 2-((E)-1-(3-((E)-1-(2-hydroxyphenyl)ethylideneamino)-2-methylphenylimino)ethyl)phenol. Molbank. 2006; 2006(1):M455. https://doi.org/10.3390/M455
Chicago/Turabian StyleJarrahpour, A. A., A. F. Jalbout, S. Rezaei, and B. Trzaskowski. 2006. "Synthesis and Physical Characterization of 2-((E)-1-(3-((E)-1-(2-hydroxyphenyl)ethylideneamino)-2-methylphenylimino)ethyl)phenol" Molbank 2006, no. 1: M455. https://doi.org/10.3390/M455




