Synthesis, Physical Characterization, Antibacterial and Antifungal activities of a novel bis(3-((E)-1-(2-hydroxyphenyl)ethylideneamino)phenyl)methanone
Abstract
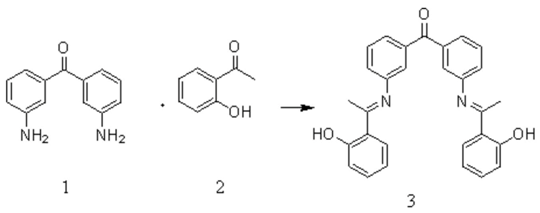
:Introduction
Results and Discussion:
Antibacterial and antifungal tests
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Boghaei, D.M.; Gharagozlou, M. Spectrochimica Acta Part A 61. 2005, 3061–3065. [Google Scholar] [PubMed]
- Zhang, J. X.; Zhou, Y.; Cai, G. J. J. Mol. Catal 1997, 11, 41–44.Holland, D.; Laidler, D.A.; Milner, D.J. J. Mol. Catal 1981, 11, 119–127.Chen, G. M.; Chen, F.; Zhou, C. Chem. Chin. Univ 1995, 16, 216–219.Qiu, M.; Liu, G.; Yiao, X. Chin. J.Catal 2001, 22, 77–80.Li, C.; Zhang, W.; Yao, X. Chin. J, Catal. 2000, 21, 77–80.Li, Z. N.; Liu, G.; Zheng, Z. Tetrahedron 2000, 56, 7187–7191.
- Gao, W. T.; Zheng, Z. Molecules 2002, 7, 511–516.
- Karmakar, R.; Choudhury, C. R.; Bravic, G.; Sutter, J. P.; Mitra, S. Polyhedron 2004, 23, 949–954.
- Frisch, M.J.; et al. GAUSSIAN 03, Revision A.1, M. J. Frisch, et. Al. Gaussian, Inc.: Pittsburgh PA, 2003. [Google Scholar]
- Foresman, J.B. Æ Frisch, , Exploring Chemistry with Electronic Structure Methods. In 2nd edition; Gaussian, INC: Pittsburgh, PA, 1996. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. July 2001. National Institute of Standards and Technology, Gaithersburg MD, 20899 (http://webbook.nist.gov)
- Jalbout, A.F.; Solimannejad, M.; Labonowski, J.K. Chem. Phys. Lett. 2003, 379, 503.
- Jalbout, A.F.; Jiang, Z.-Y.; Quasri, A.; Jeghnou, H.; Rhandour, A.; Dhamelincourt, M.C.; Dhamelincourt, P.; Mazzah, A. Vib. Spect. 2003, 33, 21.
- Jalbout, A.F.; Nazara, F.; Turker, L. J. Mol. Struct. (THEOCHEM) 2004, 627, 1, (Invited Review).
- Growth was measured in vitro using a liquid-phase method according to NCCLS guidelines from the American Society of Microbiology for 24 hours using various concentrations of drugs. Dei Cas, E.; Dujardin, L.; Ribeiro Pinto, M. E.; Ajana, F.; Fruit, J.; Poulain, D.; Camus, D.; Vernes, A.; Francois, N. Mycoses 1991, 34, 167–172. [PubMed]
- Sample Availability: Available from MDPI.

| Fitted Thermodynamic Equation (T/1000=t) | 100 K | 298.15 K | 1000 K | |
|---|---|---|---|---|
| Cp | 61.26809+ 1473.5495*t -123.39616*t2 -341.74085*t3 -0.0486*t-2 | 205.78 | 477.77 | 1077.52 |
| S | 53.05624*ln(t) + 1474.23257*t + 423.72982*t2/2 -484.29762*t3/3 -32.45372/(2*t2) -3357.13221 | 518.49 | 863.88 | 1807.1 |
| H | 89.57261*t + 1689.9106*t2/2 -308.96521*t3/3 -332.76221*t4/4 + 0.78161/t -370.051 | 13.13 | 80.56 | 670.35 |
| Sample CIP | Antimicrobial activity (MIC), µg/mL | |||
|---|---|---|---|---|
| S. cerevisiae (ATCC 28383) | S. aureus (4.83) | C. albicans (1180-79) | E. Coli (54127) | |
| 3 | >100 | 100 | >100 | >100 |
© 2006 MDPI. All rights reserved.
Share and Cite
Jalbout, A.F.; Jarrahpour, A.A.; Brunel, J.M.; Salmi, C.; Rezaei, S.; Trzaskowski, B. Synthesis, Physical Characterization, Antibacterial and Antifungal activities of a novel bis(3-((E)-1-(2-hydroxyphenyl)ethylideneamino)phenyl)methanone. Molbank 2006, 2006, M484. https://doi.org/10.3390/M484
Jalbout AF, Jarrahpour AA, Brunel JM, Salmi C, Rezaei S, Trzaskowski B. Synthesis, Physical Characterization, Antibacterial and Antifungal activities of a novel bis(3-((E)-1-(2-hydroxyphenyl)ethylideneamino)phenyl)methanone. Molbank. 2006; 2006(4):M484. https://doi.org/10.3390/M484
Chicago/Turabian StyleJalbout, A. F., A. A. Jarrahpour, J. M. Brunel, C. Salmi, S. Rezaei, and B. Trzaskowski. 2006. "Synthesis, Physical Characterization, Antibacterial and Antifungal activities of a novel bis(3-((E)-1-(2-hydroxyphenyl)ethylideneamino)phenyl)methanone" Molbank 2006, no. 4: M484. https://doi.org/10.3390/M484




