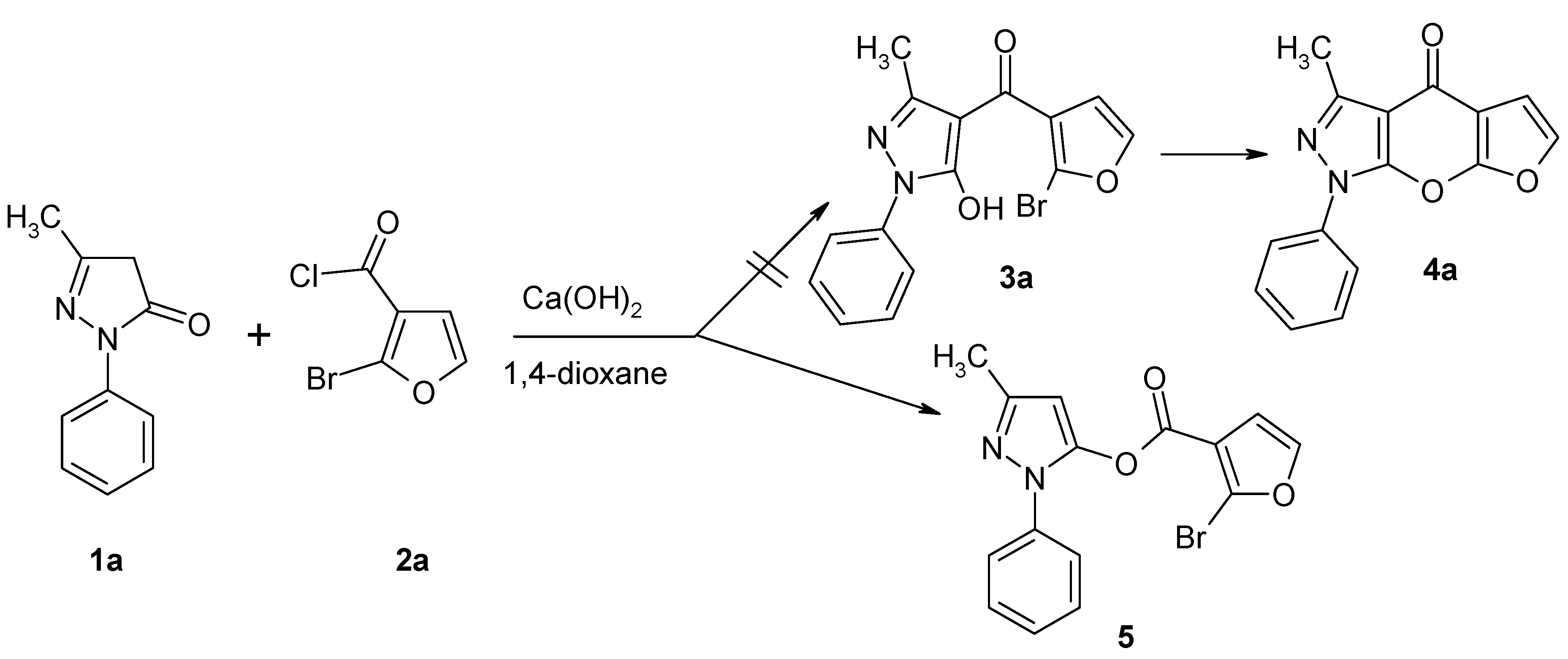
3-Methyl-1-phenyl-1H-pyrazol-5-yl 2-Bromo-3-furan-carboxylate
Abstract
:Experimental
3-Methyl-1-phenyl-1H-pyrazol-5-yl 2-bromo-3-furancarboxylate (5)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Eller, G.A.; Wimmer, V.; Haring, A.W.; Holzer, W. An Efficient Approach to Heterocyclic Analogues of Xanthone: A Short Synthesis of all possible Pyrido[5,6]pyrano[2,3-c]pyrazol-4(1H)-ones. Synthesis 2006, 24, 4219–4229. [Google Scholar] [CrossRef]
- Eller, G.A.; Haring, A.W.; Datterl, B.; Zwettler, M.; Holzer, W. Tri- and Tetracyclic Heteroaromatic Systems: Synthesis of Novel Benzo-, Benzothieno- and Thieno-Fused Pyrano[2,3-c]pyrazol-4(1H)-ones. Heterocycles 2007, 71, 87–104. [Google Scholar] [CrossRef]
- Eller, G.A.; Holzer, W. A Convenient Approach to Heterocyclic Building Blocks: Synthesis of Novel Ring Systems Containing a [5,6]Pyrano[2,3-c]pyrazol-4(1H)-one Moiety. Molecules 2007, 12, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Eller, G.A.; Datterl, B.; Holzer, W. Pyrazolo[4’,3’:5,6]pyrano[2,3-b]quinoxalin-4(1H)-one: Synthesis and Characterization of a Novel Tetracyclic Ring System. J. Heterocycl. Chem. 2007, 44, 1139–1144. [Google Scholar] [CrossRef]
- Eller, G.A.; Wimmer, V.; Holzer, W. Synthesis of Novel Polycyclic Ring Systems Containing two Pyrano[2,3-c]pyrazol-4(1H)-one Moieties. Khim. Geterotsikl. Soedin. 2007, 1251–1255. [Google Scholar] (Chem. Heterocycl. Comp. 2007, 43, 1060–1064.).
- Eller, G.A.; Habicht, D.; Holzer, W. Synthesis of a Novel Pentacycle: 8-Methyl-10-phenylpyrazolo[4’,3’:5,6]pyrano[3,2-c][1,10]phenanthrolin-7(10H)-one. Khim. Geterotsikl. Soedin. 2008, 884–890. [Google Scholar] (Chem. Heterocycl. Comp. 2008, 44, 709–714.).
- Jensen, B.S. Synthesis of 1-phenyl-3-methyl-4-acyl-5-pyrazolones. Acta Chem. Scand. 1959, 13, 1668–1670. [Google Scholar] [CrossRef]
- Maruoka, H.; Yamagata, K.; Okabe, F.; Tomioka, Y. Synthesis of 1-Acyl-1,2-dihydro-3H-pyrazol-3-ones via Lewis Acid-Mediated Rearrangement of 3-Acyloxypyrazoles. J. Heterocycl. Chem. 2006, 43, 859–865. [Google Scholar] [CrossRef]

© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Holzer, W.; Guo, C.; Schalle, K. 3-Methyl-1-phenyl-1H-pyrazol-5-yl 2-Bromo-3-furan-carboxylate. Molbank 2009, 2009, M603. https://doi.org/10.3390/M603
Holzer W, Guo C, Schalle K. 3-Methyl-1-phenyl-1H-pyrazol-5-yl 2-Bromo-3-furan-carboxylate. Molbank. 2009; 2009(3):M603. https://doi.org/10.3390/M603
Chicago/Turabian StyleHolzer, Wolfgang, Changbin Guo, and Karin Schalle. 2009. "3-Methyl-1-phenyl-1H-pyrazol-5-yl 2-Bromo-3-furan-carboxylate" Molbank 2009, no. 3: M603. https://doi.org/10.3390/M603



