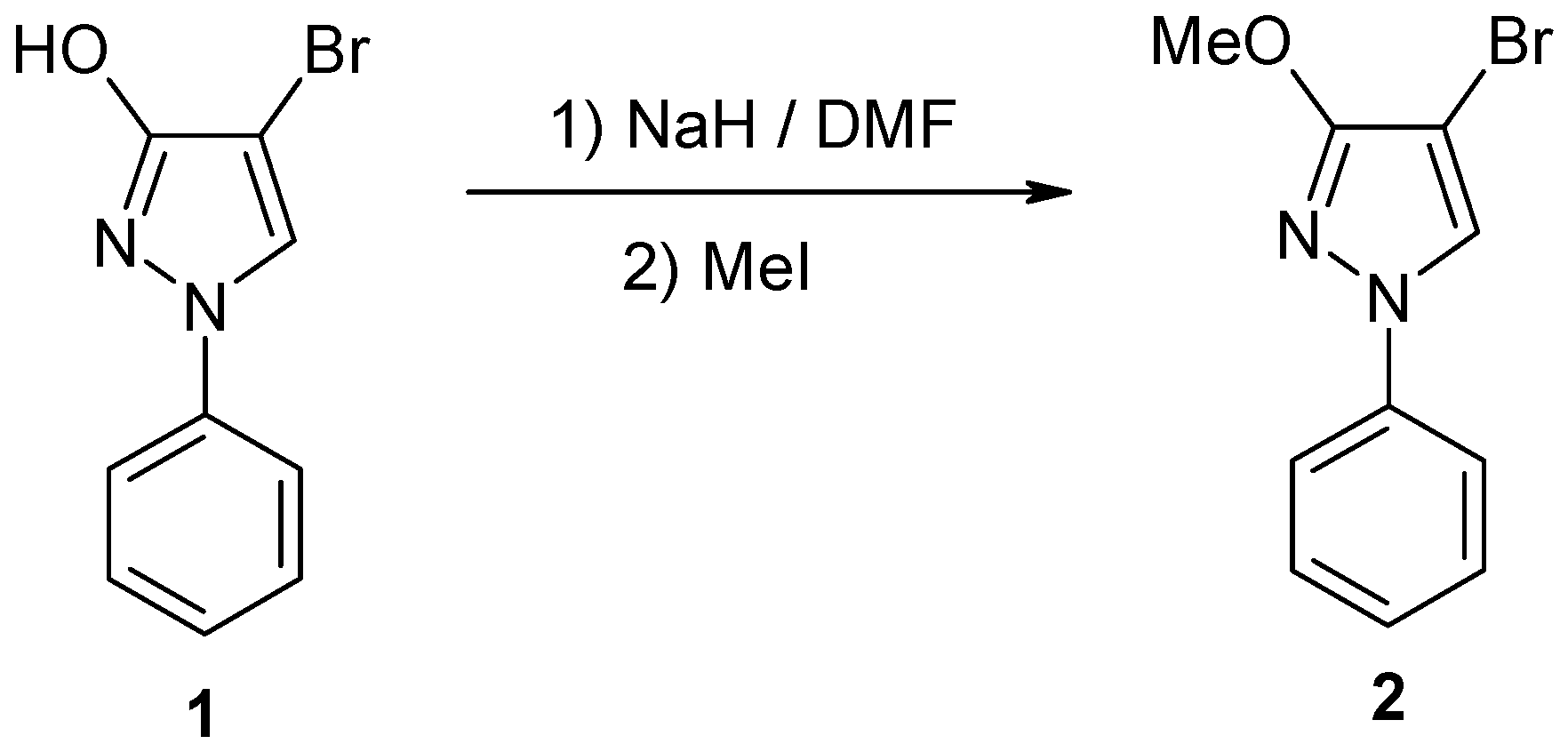
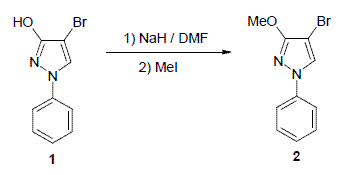4-Bromo-3-methoxy-1-phenyl-1H-pyrazole
Abstract
:Experimental
4-Bromo-3-methoxy-1-phenyl-1H-pyrazole (2)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Inoue, A.; Kitagawa, K.; Shinokubo, H.; Oshima, K. Selective halogen-magnesium exchange reaction via organomagnesium ate complex. J. Org. Chem. 2001, 66, 4333–4339, and references cited herein. [Google Scholar] [CrossRef] [PubMed]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. Convenient synthesis of acetylenes. Catalytic substitutions of acetylenic hydrogen with bromo alkenes, iodo arenes, and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar]
- Miyaura, N.; Suzuki, A. Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst. J. Chem. Soc. Chem. Commun. 1979, 19, 866–867. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compunds. Chem. Rev. 1995, 95, 2457–2583. [Google Scholar] [CrossRef]
- Ellis, G.P. Synthesis of Fused Heterocycles; John Wiley & Sons: New York, NY, USA, 1987; pp. 477–484. [Google Scholar]
- Riego, E.; Bayó, N.; Cuevas, C.; Albericio, F.; Álvarez, M. A new approach to 3-hydroxyquinoline-2-carboxylic acid. Tetrahedron 2005, 61, 1407–1411. [Google Scholar] [CrossRef]
- Arbačiauskienė, E.; Vilkauskaitė, G.; Eller, G.A.; Holzer, W.; Šačkus, A. Pd-catalyzed cross-coupling reactions of halogenated 1-phenylpyrazol-3-ols and related triflates. Tetrahedron 2009, 65, 7817–7824. [Google Scholar] [CrossRef] and the references cited herein.
- Ellis, G.P. Synthesis of Fused Heterocycles; John Wiley & Sons: New York, NY, USA, 1987; pp. 128, 190, 352. [Google Scholar]
- O’Brien, D.F.; Gates, J.W., Jr. Some reactions of 3-hydroxy-1-phenylpyrazole. J. Org. Chem. 1966, 31, 1538–1542. [Google Scholar] [CrossRef]
- Koenig, H.; Goetz, N.; Klein, U.; Eller, K. Process for producing N-substituted 3-hydroxypyrazoles WO 9703969, 1997. Chem. Abstr. 1997, 126, 199566. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kleizienė, N.; Arbačiauskienė, E.; Holzer, W.; Šačkus, A. 4-Bromo-3-methoxy-1-phenyl-1H-pyrazole. Molbank 2009, 2009, M639. https://doi.org/10.3390/M639
Kleizienė N, Arbačiauskienė E, Holzer W, Šačkus A. 4-Bromo-3-methoxy-1-phenyl-1H-pyrazole. Molbank. 2009; 2009(4):M639. https://doi.org/10.3390/M639
Chicago/Turabian StyleKleizienė, Neringa, Eglė Arbačiauskienė, Wolfgang Holzer, and Algirdas Šačkus. 2009. "4-Bromo-3-methoxy-1-phenyl-1H-pyrazole" Molbank 2009, no. 4: M639. https://doi.org/10.3390/M639