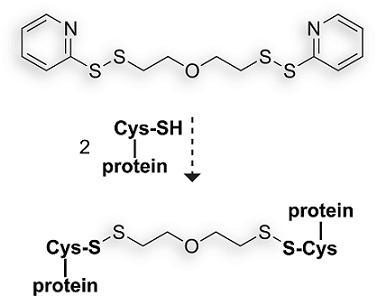
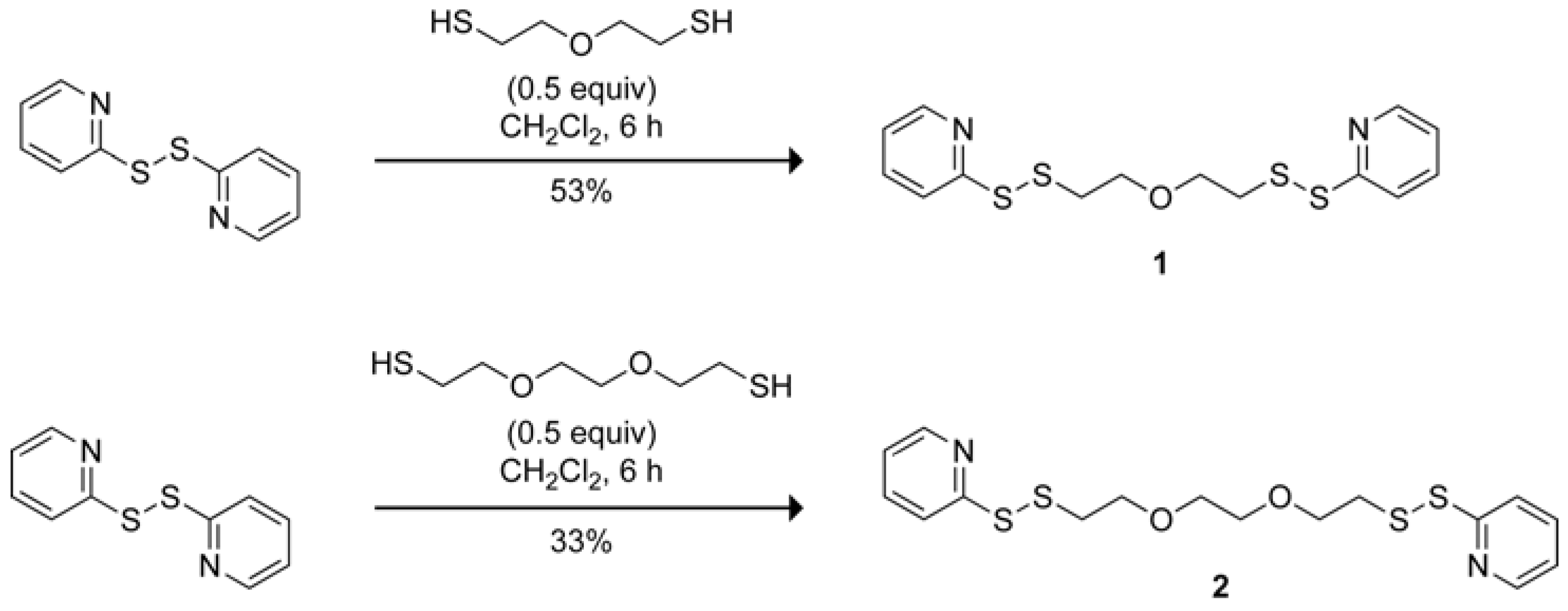
1,9-Bis(2-pyridyl)-1,2,8,9-tetrathia-5-oxanonane
Abstract
:Synthesis
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References and Notes
- Klink, T.A.; Woycechowsky, K.J.; Taylor, K.M.; Raines, R.T. Contribution of disulfide bonds to the conformational stability and catalytic activity of ribonuclease A. Eur. J. Biochem. 2000, 267, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Pecher, P.; Arnold, U. The effect of additional disulfide bonds on the stability and folding of ribonuclease A. Biophys. Chem. 2009, 141, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Beeby, M.; O’Connor, B.D.; Ryttersgaard, C.; Boutz, D.R.; Perry, L.J.; Yeates, T.O. The genomics of disulfide bonding and protein stabilization in thermophiles. PLoS Biol. 2005, 3, 1549–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-S.; Raines, R.T. Dibromobimane as a fluorescent crosslinking reagent. Anal. Biochem. 1995, 225, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Kotch, F.W.; Raines, R.T. Self-assembly of synthetic collagen triple helices. Proc. Natl. Acad. Sci. USA 2006, 103, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Ottl, J.; Moroder, L. Disulfide-bridged heterotrimeric collagen peptides containing the collagenase cleavage site of collagen type I. Synthesis and conformational properties. J. Am. Chem. Soc. 1999, 121, 653–661. [Google Scholar] [CrossRef]
- Pakula, A.A.; Simon, M.I. Determination of transmembrane protein structure by disulfide cross-linking: The Escherichia coli Tar receptor. Proc. Natl. Acad. Sci. USA 1992, 89, 4144–4148. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.; Jasti, J.; Beich-Frandsen, M.; Gouaux, E. Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell 2006, 127, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Messmore, J.M.; Holmgren, S.K.; Grilley, J.E.; Raines, R.T. Sulfur shuffle: Modulating enzymatic activity by thiol–disulfide interchange. Bioconjugate Chem. 2000, 11, 408–413. [Google Scholar] [CrossRef]
- Park, C.; Raines, R.T. Adjacent cysteine residues as a redox switch. Protein Eng. 2001, 14, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Grassetti, D.R.; Murray, J.F., Jr. Determination of sulfhydryl groups with 2,2′- or 4,4′-dithiopyridine. Arch. Biochem. Biophys. 1967, 119, 41–49. [Google Scholar] [CrossRef]
- Riddles, P.W.; Blakeley, R.L.; Zerner, B. Ellman’s reagent: 5,5′-Dithiobis(2-nitrobenzoic acid)—a reexamination. Anal. Biochem. 1979, 94, 75–81. [Google Scholar] [CrossRef]
- Zalipsky, S. Functionalized poly(ethylene glycol) for preparation of biologically relevant conjugates. Bioconjugate Chem. 1995, 6, 150–165. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kalia, J.; Raines, R.T. 1,9-Bis(2-pyridyl)-1,2,8,9-tetrathia-5-oxanonane. Molbank 2009, 2009, M642. https://doi.org/10.3390/M642
Kalia J, Raines RT. 1,9-Bis(2-pyridyl)-1,2,8,9-tetrathia-5-oxanonane. Molbank. 2009; 2009(4):M642. https://doi.org/10.3390/M642
Chicago/Turabian StyleKalia, Jeet, and Ronald T. Raines. 2009. "1,9-Bis(2-pyridyl)-1,2,8,9-tetrathia-5-oxanonane" Molbank 2009, no. 4: M642. https://doi.org/10.3390/M642