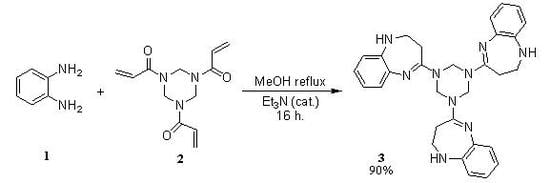
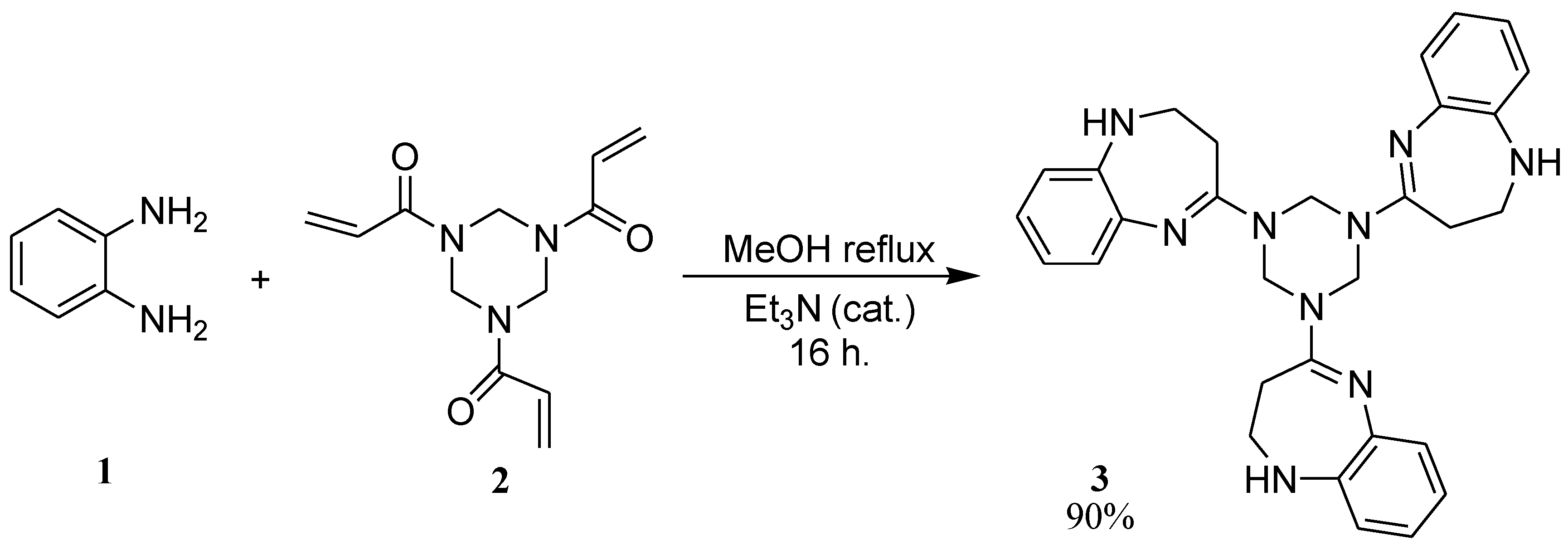
1,3,5-Tris-(2,3-dihydro-1H-1,5-benzodiazepin-4-yl)-1,2,3,4,5,6-hexahydro-s-triazine
Abstract
:Synthesis of 1,3,5-tris-(2,3-dihydro-1H-1,5-benzodiazepin-4-yl)-1,2,3,4,5,6-hexahydro-s-triazine (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Orlov, V.D.; Quiroga, J.; Kolos, N.N. Aromatic derivatives of 1H-2,3-dihydropyrazolo[4,5-b]-1,5-diazepine. Khim. Geterotsikl. Soedin. 1987, 363–369, Chem. Abstr. 1987, 107, 217603. [Google Scholar] [CrossRef]
- Insuasty, B.; Abonía, R.; Quiroga, J. The reaction of ketones with ortho-diamines. I. The reaction of aromatic α,β-unsaturated ketones with 4,5-dimethyl-1,2-phenylenediamine. An. Quim. 1992, 88, 718–721. [Google Scholar]
- Orlov, V.D.; Kolos, N.N.; Quiroga, J.; Kaluski, Z.; Figas, E.; Potekhin, A. Reaction of substituted 4,5-diaminopyrazoles with chalcones and acetylarenes. Chem. Heterocycl. Compd. 1992, 506–510. [Google Scholar]
- Insuasty, B.; Ramos, M.; Quiroga, J.; Sánchez, A.; Nogueras, M.; Hanold, N.; Meier, H. The reaction of aromatic, α,β-unsaturated ketones with 4,5-diamino-1,6-dihydropyrimidin-6-ones. J. Heterocycl. Chem. 1994, 31, 61–64. [Google Scholar] [CrossRef]
- Insuasty, B.; Ramos, M.; Moreno, R.; Quiroga, J.; Sánchez, A.; Nogueras, M.; Hanold, N.; Meier, H. Reaction of 4,5-diamino-1,6-dihydropyrimidin-6-ones with two equivalents of chalcones. J. Heterocycl. Chem. 1995, 32, 1229–1233. [Google Scholar] [CrossRef]
- Insuasty, B.; Abonía, R.; Quiroga, J.; Meier, H. Cyclocondensation reaction of 1,2-diamino-4-methylbenzene and p-substituted acetophenones. J. Heterocycl. Chem. 1993, 30, 229–231. [Google Scholar] [CrossRef]
- Landquist, J.K. Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon: Oxford, UK, 1984; p. 166. [Google Scholar]
- Schutz, H. Benzodiazepines; Springer: Heidelberg, Germany, 1982. [Google Scholar]
- Insuasty, B.; Orozco, F.; Lizarazo, C.; Quiroga, J.; Abonía, R.; Hursthouse, M.; Nogueras, M.; Cobo, J. Synthesis of new indeno[1,2-e]pyrimido[4,5-b][1,4]diazepine-5,11-diones as potential antitumor agents. Bioorg. Med. Chem. 2008, 16, 8492–8500. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, B.; Orozco, F.; Quiroga, J.; Abonía, R.; Nogueras, M.; Cobo, J. Microwave induced synthesis of novel 8,9-dihydro-7H-pyrimido[4,5-b][1,4]diazepines as potential antitumor agents. Eur. J. Med. Chem. 2008, 43, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.M.; Ho, Y.C. Improved fixation of dyes on polyamide fibres. Part 1: Using 1,3,5-triacroylamino-hexahydro-s-triazine as a crosslinking agent. Dyes Pigm. 1995, 28, 171–192. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Insuasty, B.; Garcia, A.; Abonia, R.; Nogueras, M.; Cobo, J. 1,3,5-Tris-(2,3-dihydro-1H-1,5-benzodiazepin-4-yl)-1,2,3,4,5,6-hexahydro-s-triazine. Molbank 2010, 2010, M664. https://doi.org/10.3390/M664
Insuasty B, Garcia A, Abonia R, Nogueras M, Cobo J. 1,3,5-Tris-(2,3-dihydro-1H-1,5-benzodiazepin-4-yl)-1,2,3,4,5,6-hexahydro-s-triazine. Molbank. 2010; 2010(1):M664. https://doi.org/10.3390/M664
Chicago/Turabian StyleInsuasty, Braulio, Angelica Garcia, Rodrigo Abonia, Manuel Nogueras, and Justo Cobo. 2010. "1,3,5-Tris-(2,3-dihydro-1H-1,5-benzodiazepin-4-yl)-1,2,3,4,5,6-hexahydro-s-triazine" Molbank 2010, no. 1: M664. https://doi.org/10.3390/M664