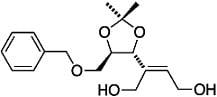
(E)-2-((4R,5R)-5-((Benzyloxy)methyl)-2,2-dimethyl-1,3-dioxolan-4-yl)but-2-ene-1,4-diol
Abstract
:Experimental
General
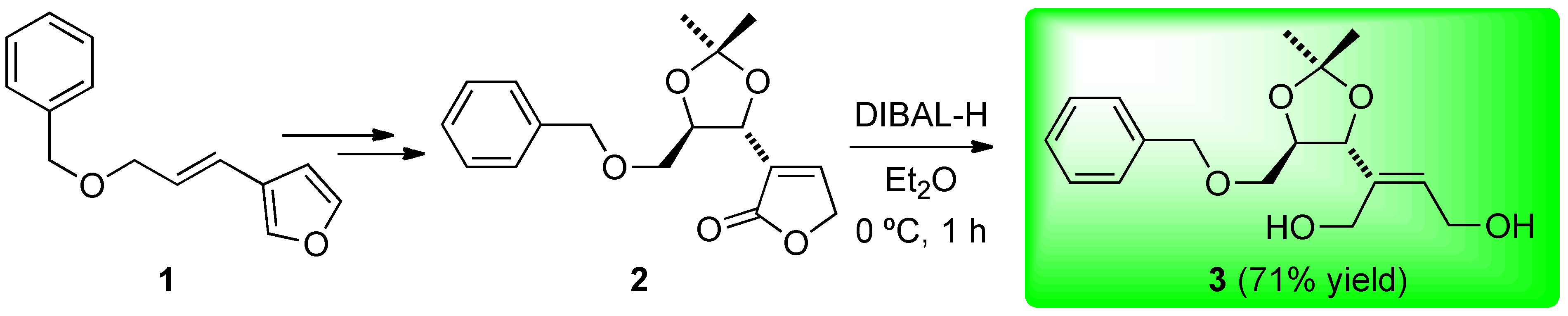
Synthesis of (E)-2-((4R,5R)-5-((benzyloxy)methyl)-2,2-dimethyl-1,3-dioxolan-4-yl)but-2-ene-1,4-diol (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
References and Notes
- Gasparski, C.M.; Herrinton, P.M.; Overman, L.E.; Wolfe, J.P. Synthesis of 3-acyltetrahydrofurans from formaldehyde acetals of allylic diols. Tetrahedron Lett. 2000, 41, 9431–9435. [Google Scholar] [CrossRef]
- Commeiras, L.; Santelli, M.; Parrain, J.-L. First total synthesis of (±)-taxifolial a and (±)-iso-caulerpenyne. Org. Lett. 2001, 3, 1713–1715. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Matsui, Y.; Ozawa, F.; Yoshifuji, M. Cyclodehydration of cis-2-butene-1,4-diol with active methylene compounds catalyzed by a diphosphinidenecyclobutene-coordinated palladium complex. J. Organomet. Chem. 2006, 691, 3151–3156. [Google Scholar] [CrossRef]
- Miura, T.; Takahashi, Y.; Murakami, M. Rhodium-catalysed substitutive arylation of cis-allylic diols with arylboroxines. Chem. Commun. 2007, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.A.; Rolla, G.A.; Cridland, A.P.; Gill, A.A. An improved synthesis of (2E,4Z)-6-(benzyloxy)-4-bromohexa-2,4-dien-1-ol. Tetrahedron 2007, 63, 9124–9128. [Google Scholar] [CrossRef] [Green Version]
- Musolino, M.G.; Apa, G.; Donato, A.; Pietropaolo, R.; Frusteri, F. Supported palladium catalysts for the selective conversion of cis-2-butene-1,4-diol to 2-hydroxytetrahydrofuran: Effect of metal particle size and support. Appl. Catal. A 2007, 325, 112–120. [Google Scholar] [CrossRef]
- Aponick, A.; Biannic, B. Gold-catalyzed dehydrative cyclization of allylic diols. Synthesis 2008, 3356–3359. [Google Scholar] [CrossRef]
- Commeiras, L.; Santelli, M.; Parrain, J.-L. total synthesis of (+) and (-)-furocaulerpin. Synlett 2002, 743–746. [Google Scholar] [CrossRef]
- Commeiras, L.; Parrain, J.-L. Concise enantioselective synthesis of furocaulerpin. Tetrahedron Asymmetry 2004, 15, 509–517. [Google Scholar] [CrossRef]
- Commeiras, L.; Bourdron, J.; Douillard, S.; Barbier, P.; Vanthuyne, N.; Peyrot, V. total synthesis of terpenoids isolated from caulerpale algae and their inhibition- of tubulin assembly. Synthesis 2006, 166–181. [Google Scholar]
- Commeiras, L.; Santelli, M.; Parrain, J.-L. On the construction of 2-substituted 1,4-diacetoxybutadiene moiety: Application to the synthesis of caulerpenyne. Tetrahedron Lett. 2003, 44, 2311–2314. [Google Scholar] [CrossRef]
- Commeiras, L.; Valls, R.; Santelli, M.; Parrain, J.-L. Efficient synthesis of (+/-)-dihydrorhipocephalin, a bioactive terpenoid from Caribbean marine algae of the Genera Penicillus and Udotea. Synlett 2003, 1719–1721. [Google Scholar]
- Gross, H.; Konig, G.M. Terpenoids from marine organisms: unique structures and their pharmacological potential. Phytochem. Rev. 2006, 5, 115–141. [Google Scholar] [CrossRef]
- Maimone, T.J.; Baran, P.S. Modern synthetic approaches to terpenes. Nat. Chem. Biol. 2007, 3, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Gademann, K.; Portmann, C. Secondary metabolites from cyanobacteria: Complex structures and powerful bioactivities. Curr. Org. Chem. 2008, 12, 326–341. [Google Scholar] [CrossRef]
- Rezanka, T.; Siristova, L.; Sigler, K. Antiviral Sesqui-, Di- and Sesterterpenes. Anti-Infect. Agents Med. Chem. 2009, 8, 169–192. [Google Scholar] [CrossRef]
- Paul, M.; Domingo, R. Synthesis of 1H-Cyclopropa[b]naphthalenes via Trapping of o-benzoquinodimethanes. Helv. Chim. Acta 1985, 68, 975–980. [Google Scholar]
- Myriam, S.; Narciso, C.; Manuel, R.-C.; Ester, I.; Tore, D.; Denis, T.; Albert, B.; Michel, R. Isoprenoid biosynthesis in Escherichia coli via the methylerythritol phosphate pathway: Enzymatic conversion of methylerythritol cyclodiphosphate into a phosphorylated derivative of (E)-2-methylbut-2-ene-1,4-diol. Tetrahedron Lett. 2002, 43, 1413–1415. [Google Scholar]
- Yoshio, I.; Kohji, W.; Tsuneaki, H. A new synthesis of trans-2-Substituted-2-butene-1,4-diols from 2-Butyne-1,4-diol via nucleophilic addition of gragnard reagents. Chem. Lett. 1984, 5, 765–768. [Google Scholar]
- Ceñal, J.P.; Carreras, C.R.; Tonn, C.E.; Padrón, J.I.; Ramírez, M.A.; Díaz, D.D.; García-Tellado, F.; Martín, V.S. Acid-mediated highly regioselective oxidation of substituted furans: A simple and direct entry to substituted butenolides. Synlett 2005, 1575–1578. [Google Scholar]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Carreras, C.R.; García, C.E.; Martín, V.S.; Tonn, C.E.; Díaz, D.D.; Ceñal, J.P. (E)-2-((4R,5R)-5-((Benzyloxy)methyl)-2,2-dimethyl-1,3-dioxolan-4-yl)but-2-ene-1,4-diol. Molbank 2010, 2010, M676. https://doi.org/10.3390/M676
Carreras CR, García CE, Martín VS, Tonn CE, Díaz DD, Ceñal JP. (E)-2-((4R,5R)-5-((Benzyloxy)methyl)-2,2-dimethyl-1,3-dioxolan-4-yl)but-2-ene-1,4-diol. Molbank. 2010; 2010(2):M676. https://doi.org/10.3390/M676
Chicago/Turabian StyleCarreras, Carlos R., Celina E. García, Víctor S. Martín, Carlos E. Tonn, David Díaz Díaz, and Juan Pedro Ceñal. 2010. "(E)-2-((4R,5R)-5-((Benzyloxy)methyl)-2,2-dimethyl-1,3-dioxolan-4-yl)but-2-ene-1,4-diol" Molbank 2010, no. 2: M676. https://doi.org/10.3390/M676