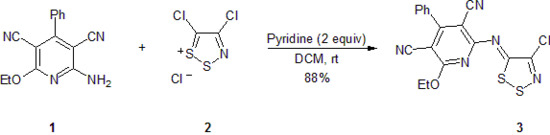
(Z)-2-(4-Chloro-5H-1,2,3-dithiazol-5-ylideneamino)-6-ethoxy-4-phenylpyridine-3,5-dicarbonitrile
Abstract
:
Experimental
(Z)-2-(4-Chloro-5H-1,2,3-dithiazol-5-ylideneamino)-6-ethoxy-4-phenylpyridine-3,5-dicarbonitrile (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Konstantinova, L.S.; Bol’shakov, O.I.; Obruchnikova, N.V.; Laborie, H.; Tanga, A.; Sopéna, V.; Lanneluc, I.; Picot, L.; Sablé, S.; Thiéry, V.; Rakitin, O.A. One-pot synthesis of 5-phenylimino, 5-thieno or 5-oxo-1,2,3-dithiazoles and evaluation of their antimicrobial and antitumor activity. Bioorg. Med. Chem. Lett. 2009, 19, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Thiery, V.; Rees, C.W.; Besson, T.; Cottenceau, G.; Pons, A.M. Antimicrobial activity of novel N-quinolinyl and N-naphthylimino-1,2,3-dithiazoles. Eur. J. Med. Chem. 1998, 33, 149–153. [Google Scholar] [CrossRef]
- Besson, T.; Rees, C.W.; Cottenceau, G.; Pons, A.M. Antimicrobial evaluation of 3,1-benzoxazin-4-ones, 3,1-benzothiazin-4-ones, 4-alkoxyquinazolin-2-carbonitriles and N-arylimino-1,2,3-dithiazoles. Bioorg. Med. Chem. Lett. 1996, 6, 2343–2348. [Google Scholar] [CrossRef]
- Mayer, R.; Foerster, E. Verfahren zur Herstellung von aromatisch oder heteroaromatisch substituierten Cyanthioformamiden. DD Pat. 212387, 1984. [Google Scholar]
- Christoforou, I.C.; Kalogirou, A.S.; Koutentis, P.A. The preparation of dicyano-1,3,4-thiadiazole and tricyanothiazole via 1,2,3-dithiazole chemistry. Tetrahedron 2009, 65, 9967–9972. [Google Scholar] [CrossRef]
- Appel, R.; Janssen, H.; Siray, M.; Knoch, F. Synthese und Reaktionen des 4,5-Dichlor-1,2,3-dithiazolium-chlorids. Chem. Ber. 1985, 118, 1632–1643. [Google Scholar] [CrossRef]
- English, R.F.; Rakitin, Ο.Α.; Rees, C.W.; Vlasova, O.G. Conversion of imino-1,2,3-dithiazoles into 2-cyanobenzothiazoles, cyanoimidoyl chlorides and diatomic sulfur. J. Chem. Soc. Perkin Trans. 1 1997, 201–206. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Rakitin, O.A.; Rees, C.W.; Sivadasan, S.; Torroba, T. New route to 2-cyanobenzimidazoles. Tetrahedron 1998, 54, 9639–9650. [Google Scholar] [CrossRef]
- Besson, T.; Rees, C.W.; Thiery, V. Convenient Synthesis of Dithiooxamides from N-Arylimino-1,2,3-dithiazoles. Synthesis 1999, 1345–1348. [Google Scholar] [CrossRef]
- Michaelidou, S.S.; Koutentis, P.A. The conversion of 2-(4-chloro-5H-1,2,3-dithiazolylidene-amino)benzonitriles into 3-aminoindole-2-carbonitriles using triphenylphosphine. Tetrahedron 2009, 65, 8428–8433. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. The reaction of 4,5-dichloro-1,2,3-dithiazolium chloride with dimethylsulfonium dicyanomethylide: an improved synthesis of (4-chloro-1,2,3-dithiazolylidene)-malononitrile. Tetrahedron 2009, 65, 6850–6854. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. The reaction of 4,5-dichloro-1,2,3-dithiazolium chloride with DMSO: An improved synthesis of 4-chloro-1,2,3-dithiazol-5H-one. Tetrahedron 2009, 65, 6855–6858. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. The degradation of 4,5-dichloro-1,2,3-dithiazolium chloride in wet solvents. Tetrahedron 2009, 65, 6859–6862. [Google Scholar] [CrossRef]
- Alvarez-Insúa, A.S.; Lora-Tamayo, M.; Soto, J.L. Synthesis of heterocyclic compounds. II. A simple one-step synthesis of pyridines from aldehydes and malononitrile. J. Heterocycl. Chem. 1970, 7, 1305–1309. [Google Scholar] [CrossRef]
- Harada, H.; Watanuki, S.; Takuwa, T.; Kawaguchi, K.; Okazaki, T.; Hirano, Y.; Saitoh, C. Medicine comprising dicyanopyridine derivative. Eur. Pat. 1302463A1, 2003. [Google Scholar]
- Christoforou, I.C.; Koutentis, P.A.; Michaelidou, S.S. 1,2,3-Dithiazole chemistry in heterocyclic synthesis. Arkivoc 2006, 7, 207–223. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Koutentis, P.A.; Michaelidou, S.S. (Z)-2-(4-Chloro-5H-1,2,3-dithiazol-5-ylideneamino)-6-ethoxy-4-phenylpyridine-3,5-dicarbonitrile. Molbank 2010, 2010, M690. https://doi.org/10.3390/M690
Koutentis PA, Michaelidou SS. (Z)-2-(4-Chloro-5H-1,2,3-dithiazol-5-ylideneamino)-6-ethoxy-4-phenylpyridine-3,5-dicarbonitrile. Molbank. 2010; 2010(3):M690. https://doi.org/10.3390/M690
Chicago/Turabian StyleKoutentis, Panayiotis A., and Sophia S. Michaelidou. 2010. "(Z)-2-(4-Chloro-5H-1,2,3-dithiazol-5-ylideneamino)-6-ethoxy-4-phenylpyridine-3,5-dicarbonitrile" Molbank 2010, no. 3: M690. https://doi.org/10.3390/M690