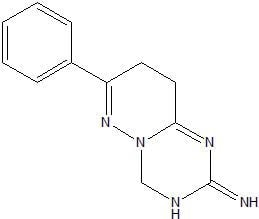
7-Phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine
Abstract
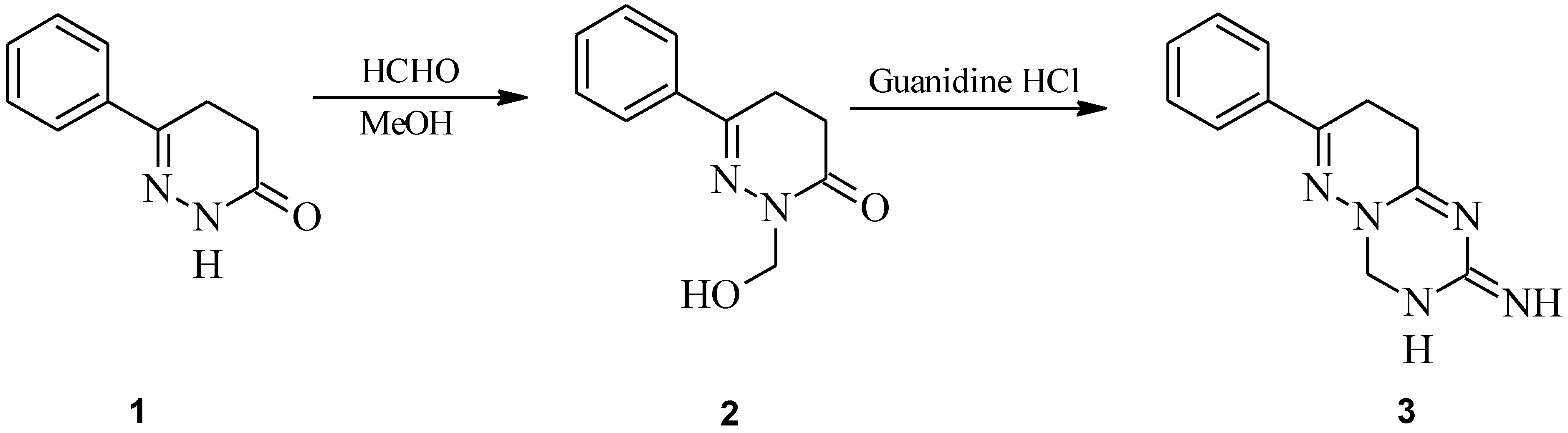
:
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Bristol, J.A.; Sircar, I.; Moss, W.H.; Evans, D.B.; Weishmaar, E. Cardiotonic agents. 1. 4,5-Dihydro-6-[4-(1H-imidazol-1-yl)phenyl]-3(2H)-pyridazinones: novel positive inotropic agents for the treatment of congestive heart failure. J. Med. Chem. 1984, 27, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- Sircar, I.; Duell, B.L.; Cain, M.H.; Bruke, S.E.; Bristol, J.A. Cardiotonic agents. 4. Synthesis and biological evaluation of N-substituted 2,4,4a,5-tetrahydro-3H-indeno[1,2-c]pyridazin-3-ones: rigid structures derived from CI-930 and analogs. J. Med. Chem. 1986, 29, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- Demirayak, S.; Karaburun, A.C.; Beis, R. Some pyrrole substituted aryl pyridazinone and phthalazinone derivatives and their antihypertensive activities. Eur. J. Med. Chem. 2004, 39, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Smolyar, N.N.; Yutilov, Y.M.; Gresko, S.V. Synthesis of 4-amino-6-(hetaryl)pyridazin-3-ones as analogs of pyridazine-based cardiotonic agents. Pharm. Chem. J. 2009, 43, 87–88. [Google Scholar] [CrossRef]
- Mirzoeva, S.; Sawkar, A.; Zasadzki, M.; Guo, L.; Velentza, A.V.; Ramstrom, H.; Haiech, J.; Van Eldik, L.J.; Watterson, D.M.; Dunlap, V.; Bourguignon, J.J. Discovery of a 3-Amino-6-phenyl-pyridazine Derivative as a New Synthetic Antineuroinflammatory Compound. J. Med. Chem. 2002, 45, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, E.; Coelho, A.; Ravina, E. Pyridazines. Part 34: Retro-ene-assisted palladium-catalyzed synthesis of 4,5-disubstituted-3(2H)-pyridazinones. Tetrahedron Lett. 2003, 44, 4459–4462. [Google Scholar] [CrossRef]
- Foks, H.; Wisterowicz, K.; Miszke, A.; Brozewicz, K.; Wisnlewska, K.; Dabrowska-Szponar, M. Synthesis, Fungicidal and Antibacterial Activity of New Pyridazine Derivatives. Heterocycles 2009, 78, 961–975. [Google Scholar] [CrossRef]
- Montero-Lastres, A.; Fraiz, N.; Laguna, R.; Cano, E.; Estevez, I.; Ravina, E. Pyridazines XVIII. 6-aryl-3(2H)-pyridazinones inhibit calcium influx in stimulated platelets. Biol. Pharm. Bull. 1999, 22, 1376–1379. [Google Scholar]
- Kaizerman, J.; Lucas, B.; McMinn, D.L.; Zamboni, R. Annelated pyridazines for the treatment of tumors driven by inappropriate hedgehog signaling. PCT Int. Appl. WO 2009035568, 2009. [Google Scholar]
- Ishida, A.; Honma, K.; Yato, M.; Nishiyama, S.; Okumura, F. The syntheses of several heterocyclic skeletons of pyridazine. Eur. Pat. Appl. EP 661273, 1995. [Google Scholar]
- Monge, A.; Parrado, P.; Font, M.; Fernández-Alvarez, E. Selective thromboxane synthetase inhibitors and antihypertensive agents. New derivatives of 4-hydrazinopyridazino[4,5-a]indole and related compounds. J. Med. Chem. 1987, 30, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Akahane, A.; Katayama, H.; Mitsunaga, T.; Kato, T.; Kinoshita, T.; Kita, Y.; Kusunoki, T.; Terai, T.; Yoshida, K.; Shiokawa, Y. Discovery of 6-oxo-3-(2-phenylpyrazolo[1,5-a]pyridin-3-yl)-1(6H)-pyridazinebutanoic acid (FK 838): A novel non-xanthine adenosine A1 receptor antagonist with potent diuretic activity. J. Med. Chem. 1999, 42, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.G.H.; Bethell, R.C.; Cammack, N.; Hancock, A.P.; Hann, M.M.; Green, D.V.S.; Lamont, R.B.; Noble, S.A.; Orr, D.C.; Payne, J.J.; Ramsay, M.V.J.; Shingler, A.H.; Smith, C.; Storer, R.; Williamson, C.; Willson, T. Synthesis and anti-HIV-1 activity of a series of imidazo[1,5-b]pyridazines. J. Med. Chem. 1993, 36, 3784–3794. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Mishra, R.; Shaharyar, M. Synthesis, characterization and antihypertensive activity of pyridazinone derivatives. Eur. J. Med. Chem. 2010, 45, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Siddiqui, A.A.; Mishra, R.; Shaharyar, M.; Husain, A.; Rashid, M.; Pal, M.; Yathirajan, H.S. 7-Phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine. Molbank 2011, 2011, M717. https://doi.org/10.3390/M717
Siddiqui AA, Mishra R, Shaharyar M, Husain A, Rashid M, Pal M, Yathirajan HS. 7-Phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine. Molbank. 2011; 2011(1):M717. https://doi.org/10.3390/M717
Chicago/Turabian StyleSiddiqui, Anees A., Ravinesh Mishra, M. Shaharyar, Asif Husain, Mohd. Rashid, Manoj Pal, and Hemmige S. Yathirajan. 2011. "7-Phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine" Molbank 2011, no. 1: M717. https://doi.org/10.3390/M717